Gelatin-Based Hydrogels through Homobifunctional Triazolinediones Targeting Tyrosine Residues
Abstract
1. Introduction
2. Results and Discussion
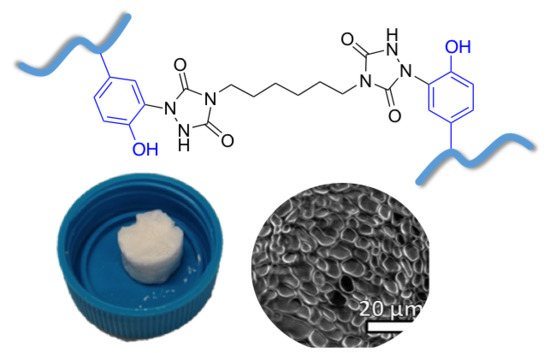
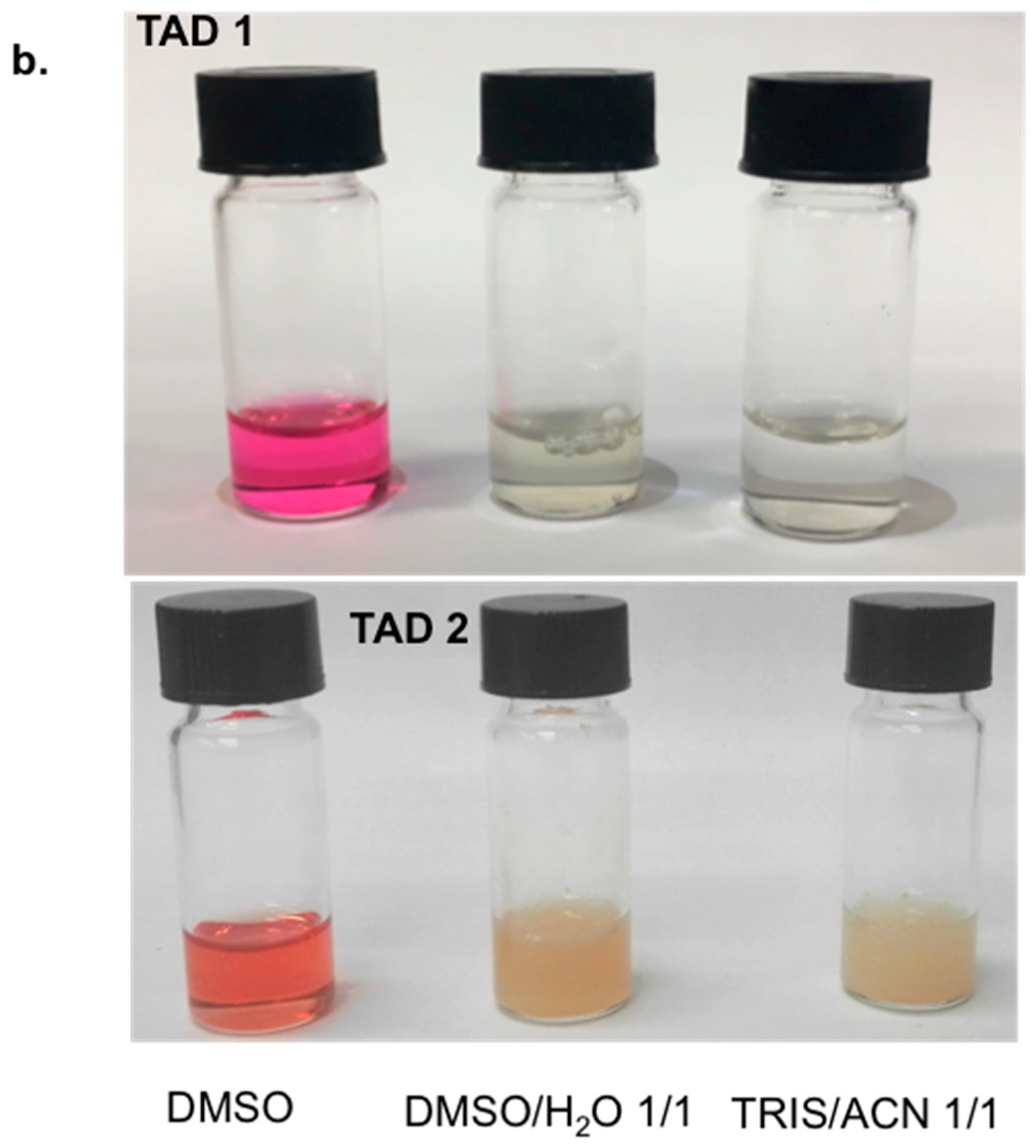
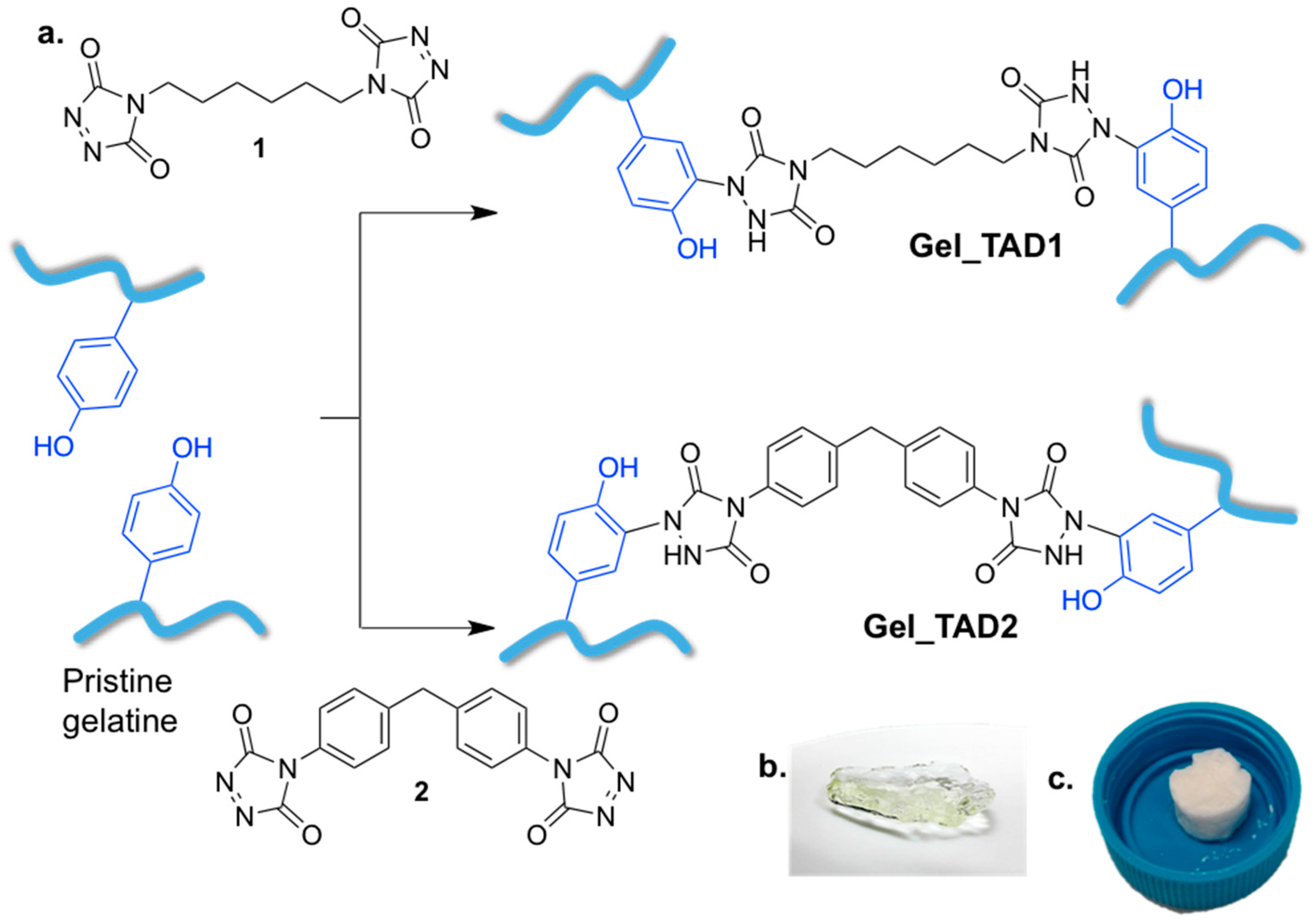
2.1. Cross-Linking of Gelatin
2.2. Characterization of Cross-Linked Gelatin
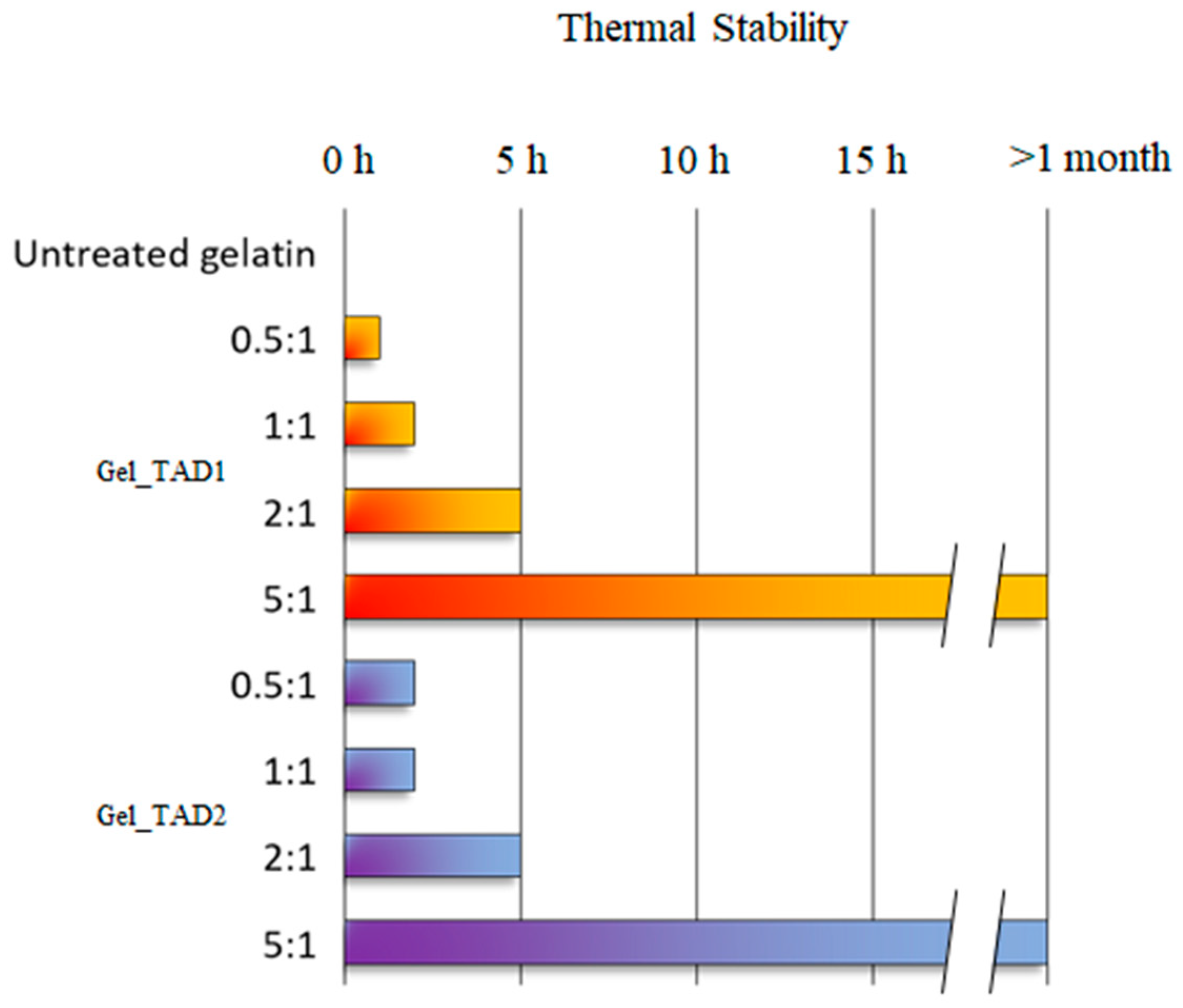
2.2.1. Thermal Stability
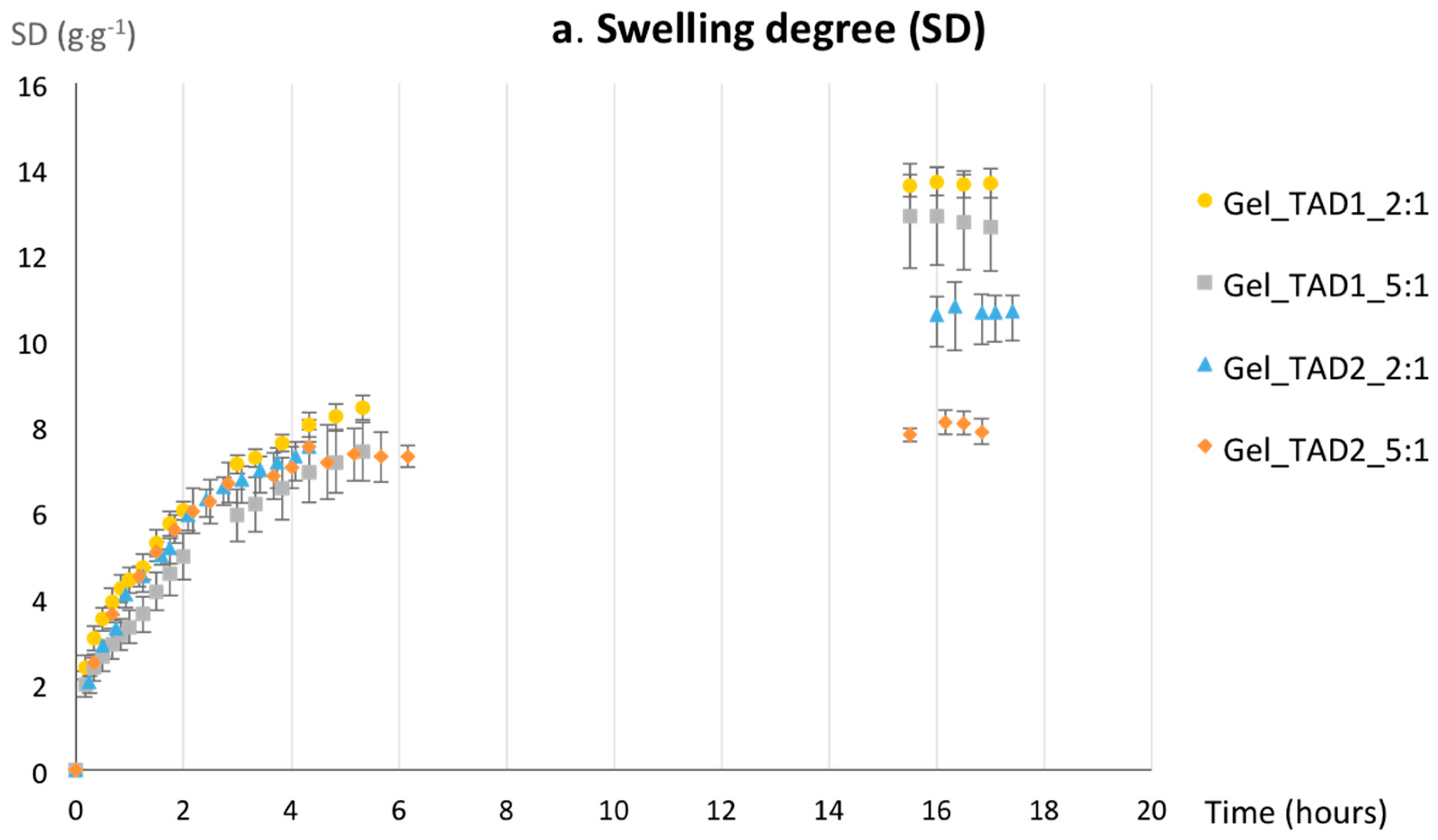
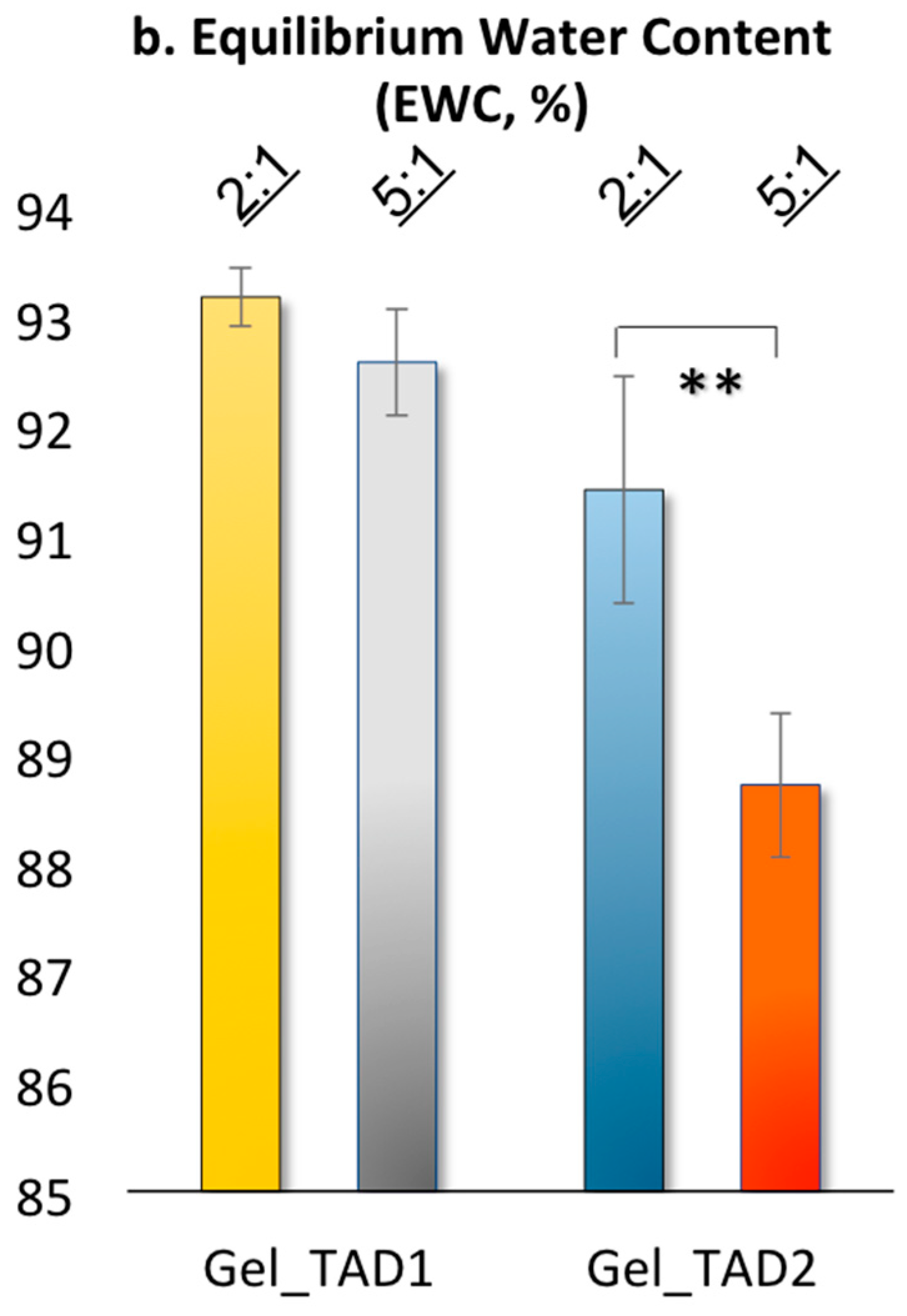
2.2.2. Swelling Properties
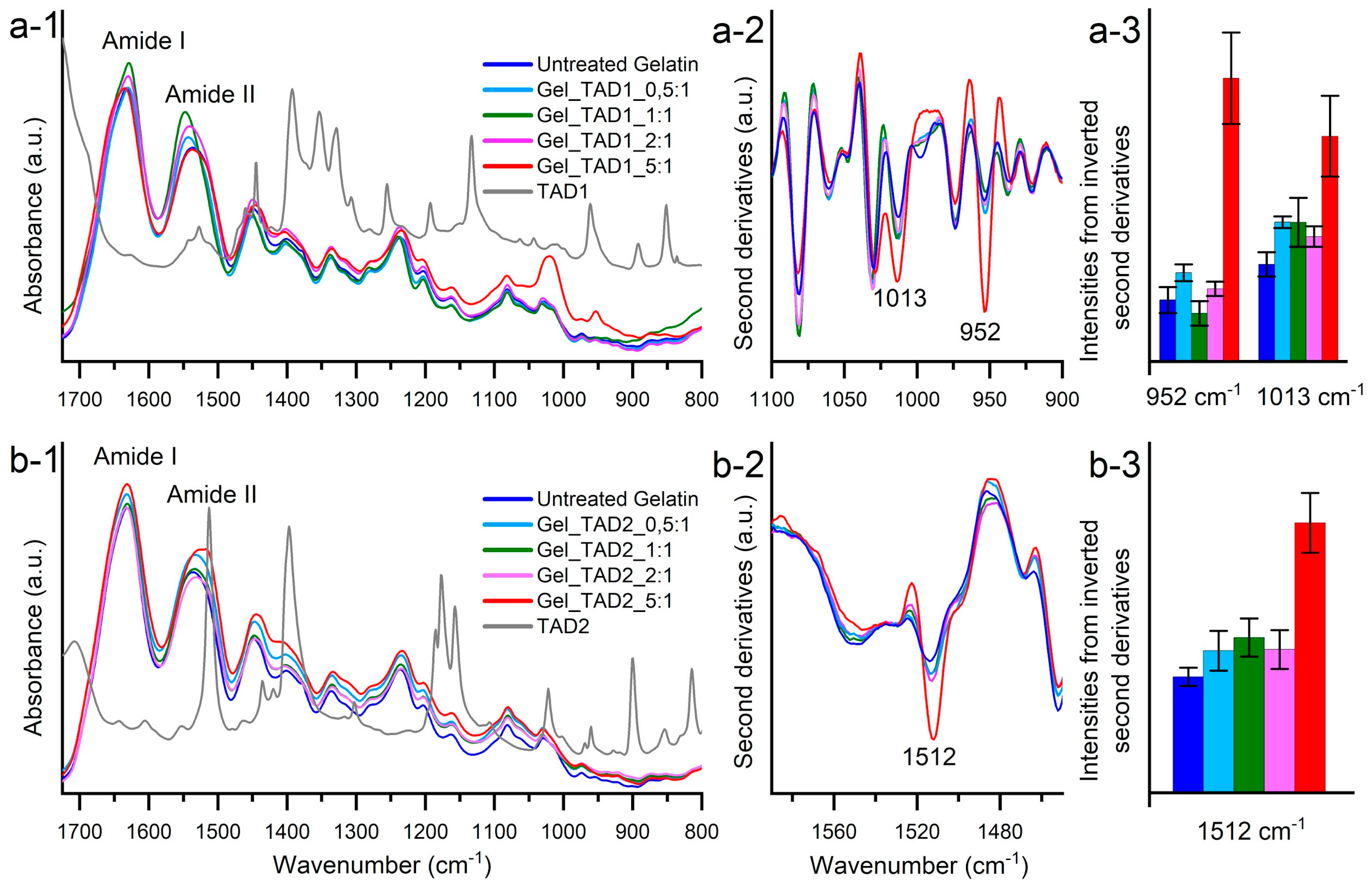
2.2.3. FT-IR Characterization
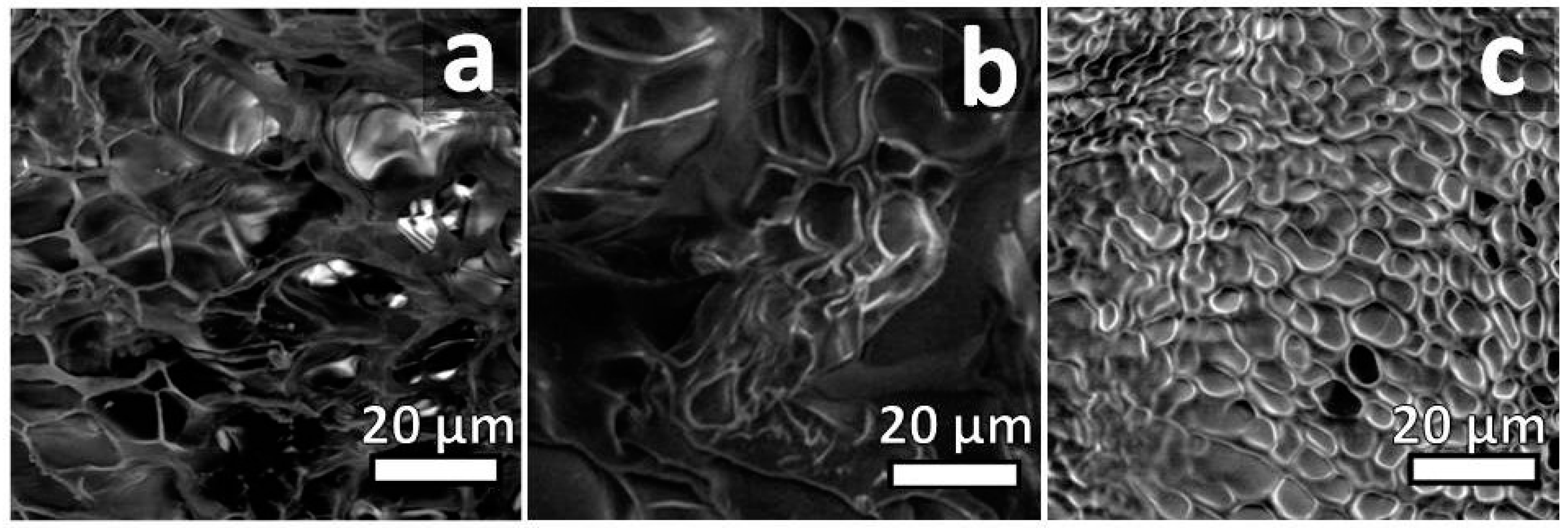
2.2.4. Scanning Electron Microscopy Micrographs
3. Materials and Methods
3.1. General
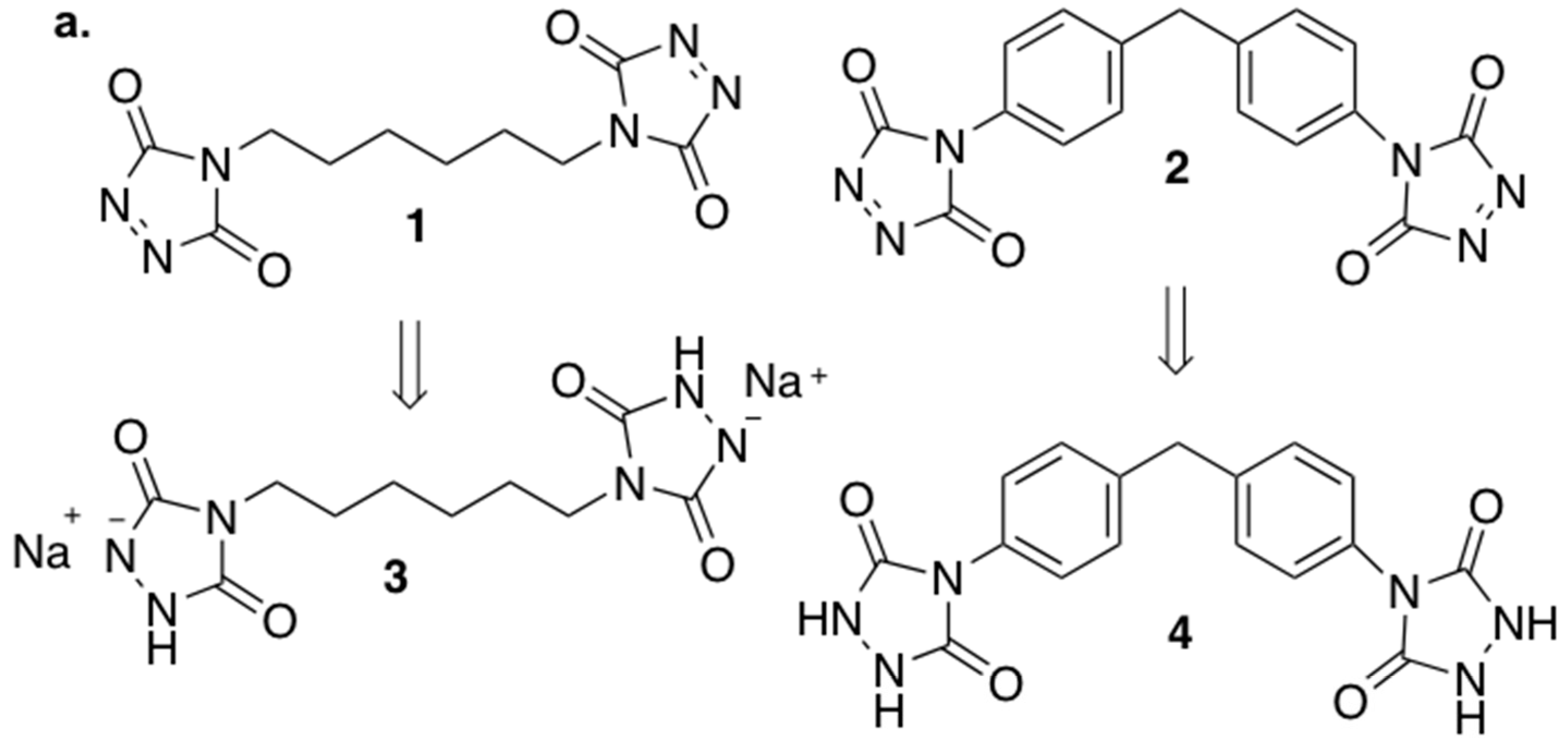
3.2. Synthesis of Cross-Linking Agents
3.3. Gelatin Cross-Linking
3.3.1. Preparation of TAD 1 Cross-Linked Hydrogel Gel_TAD1
3.3.2. Preparation of TAD 2 Cross-Linked Hydrogel Gel_TAD2
3.4. Thermal Stability Studies
3.5. Swelling Studies
3.6. SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, S.J.; Xiong, G.; Roguin, A.; Choong, C. Immobilization of Gelatin onto Poly(Glycidyl Methacrylate-Grafted Polycaprolactone Substrates for Improved Cell-Material Interactions. Biointerphases 2012, 7, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 2010, 30, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fu, X.B. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release 2010, 142, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef]
- Xiaomeng, L.; Jing, Z.; Naoki, K.; Guoping, C. Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype. Polymers 2017, 9, 309–322. [Google Scholar]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 16, 4706. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Sgambato, A.; Cipolla, L.; Russo, L. Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering. Gels 2016, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.G.; Gostynska, N.; Montesi, M.; Panseri, S.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. Investigation of different cross-linking approaches on 3D gelatin scaffolds for tissue engineering application: A comparative analysis. Int. J. Biol. Macromol. 2017, 95, 1199–1209. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Baslé, E.; Joubert, N.; Pucheault, M. Protein Chemical Modification on Endogenous Amino Acids. Chem. Biol. 2010, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Davis, B.G. Selective chemical protein modification. Nat. Commun. 2014, 5, 4740. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Lallana, E.; Fernandez-Trillo, F.; Sousa-Herves, A.; Riguera, R.; Fernandez-Megia, E. Click chemistry with polymers, dendrimers, and hydrogels for drug delivery. Pharm. Res. 2012, 29, 902–921. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Agten, S.M.; Dawson, P.E.; Hackeng, T.M. Oxime conjugation in protein chemistry: From carbonyl incorporation to nucleophilic catalysis. J. Pept. Sci. 2016, 22, 271–279. [Google Scholar] [CrossRef]
- Kölmel, D.K.; Kool, E.T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef]
- van Berkel, S.S.; van Eldijk, M.B.; van Hest, J.C. Staudinger ligation as a method for bioconjugation. Angew. Chem. Int. Ed. Engl. 2011, 50, 8806–8827. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.I.; Jung, N.; Biskup, M.; Schepers, U.; Bräse, S. Bioconjugation via azide-Staudinger ligation: An overview. Chem. Soc. Rev. 2011, 40, 4840–4871. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F.; Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne “click” chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef]
- Liu, X.; Miller, A.L.; Fundora, K.A.; Yaszemski, M.J.; Lu, L. Poly(ε-caprolactone) dendrimer cross-linked via metal-free click chemistry: Injectable hydrophobic platform for tissue engineering. ACS Macro Lett. 2016, 5, 1261–1265. [Google Scholar] [CrossRef]
- Pozsgay, V.; Vieira, N.E.; Yergey, A.A. Method for bioconjugation of carbohydrates using Diels-Alder cycloaddition. Org. Lett. 2002, 4, 3191–3194. [Google Scholar] [CrossRef]
- Willems, L.I.; Verdoes, M.; Florea, B.I.; van der Marel, G.A.; Overkleeft, H.S. Two-step labeling of endogenous enzymatic activities by Diels-Alder ligation. Chembiochem 2010, 11, 1769–1781. [Google Scholar] [CrossRef]
- Gregoritza, M.; Brandl, F.P. The Diels-Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef]
- Dondoni, A. The emergence of thiol-ene coupling as a click process for materials and bioorganic chemistry. Angew. Chem. Int. Ed. Engl. 2008, 47, 8995–9007. [Google Scholar] [CrossRef]
- Russo, L.; Battocchio, C.; Secchi, V.; Magnano, E.; Nappini, S.; Taraballi, F.; Gabrielli, L.; Comelli, F.; Papagni, A.; Costa, B.; et al. Thiol-ene mediated neoglycosylation of collagen patches: A preliminary study. Langmuir 2014, 30, 1336–1342. [Google Scholar] [CrossRef]
- Azagarsamy, M.A.; Anseth, K.S. Bioorthogonal Click Chemistry: An Indispensable Tool to Create Multifaceted Cell Culture Scaffolds. ACS Macro Lett. 2013, 2, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Themed Issue “Applications of click chemistry”. Chem. Soc. Rev. 2010, 4, 1221–1408.
- Occhetta, P.; Visone, R.; Russo, L.; Cipolla, L.; Moretti, M.; Rasponi, M. VA-086 methacrylate gelatine photopolymerizable hydrogels: A parametric study for highly biocompatible 3D cell embedding. J. Biomed. Mater. Res. A 2015, 103, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Sgambato, A.; Visone, R.; Occhetta, P.; Moretti, M.; Rasponi, M.; Nicotra, F.; Cipolla, L. Gelatin hydrogels via thiol-ene chemistry. Monatsh. Chem. 2016, 147, 587–592. [Google Scholar] [CrossRef]
- García-Astraina, C.; Gandinib, A.; Peñaa, C.; Algara, I.; Eceizaa, A.; Corcueraa, M.; Gabilondo, N. Diels–Alder “click” chemistry for the cross-linking of furfuryl-gelatin-polyetheramine hydrogels. RSC Adv. 2014, 4, 35578–35587. [Google Scholar] [CrossRef]
- Tamura, M.; Yanagawa, F.; Sugiura, S.; Takagi, T.; Sumaru, K.; Kanamori, T. Click-crosslinkable and photodegradable gelatin hydrogels for cytocompatible optical cell manipulation in natural environment. Sci. Rep. 2015, 9, 15060. [Google Scholar] [CrossRef] [PubMed]
- Piluso, S.; Vukićevića, R.; Nöchel, U.; Braun, S.; Lendlein, A.; Neffe, A.T. Sequential alkyne-azide cycloadditions for functionalized gelatin hydrogel formation. Eur. Polym. J. 2018, 100, 77–85. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Ryon Shin, S.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Inoue, M.; Sasaki, M.; Nakasu, A.; Takayanagi, M.; Taguchi, T. An antithrombogenic citric acid-crosslinked gelatin with endothelialization activity. Adv. Healthc. Mater. 2012, 1, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Shie, M.Y.; Lin, J.H.; Chen, Y.W.; Yao, C.H.; Chen, Y.S. Biodegradable Bisvinyl Sulfonemethyl-crosslinked Gelatin Conduit Promotes Regeneration after Peripheral Nerve Injury in Adult Rats. Sci. Rep. 2017, 7, 17489. [Google Scholar] [CrossRef]
- Madhurakkat Perikamana, S.K.; Lee, J.; Lee, Y.B.; Shin, Y.M.; Lee, E.J.; Mikos, A.G.; Shin, H. Materials from Mussel-Inspired Chemistry for Cell and Tissue Engineering Applications. Biomacromolecules 2015, 16, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Applegate, M.B.; Omenetto, F.G.; Kaplan, D.L. Dityrosine Cross-Linking in Designing Biomaterials. ACS Biomater. Sci. Eng. 2016, 2, 2108–2121. [Google Scholar] [CrossRef]
- Kodadek, T.; Duroux-Richard, I.; Bonnafous, J.C. Techniques: Oxidative cross-linking as an emergent tool for the analysis of receptor-mediated signalling events. Trends Pharmacol. Sci. 2005, 26, 210–217. [Google Scholar] [CrossRef] [PubMed]
- De Bruycker, K.; Billiet, S.; Houck, H.A.; Chattopadhyay, S.; Winne, J.M.; Du Prez, F.E. Triazolinediones as Highly Enabling Synthetic Tools. Chem. Rev. 2016, 116, 3919–3974. [Google Scholar] [CrossRef]
- Ban, H.; Gavrilyuk, J.; Barbas, C.F. Tyrosine bioconjugation through aqueous ene-type reactions: A click-like reaction for tyrosine. J. Am. Chem. Soc. 2010, 132, 1523–1525. [Google Scholar] [CrossRef]
- Ban, H.; Nagano, M.; Gavrilyuk, J.; Hakamata, W.; Inokuma, T.; Barbas, C.F. Facile and stabile linkages through tyrosine: Bioconjugation strategies with the tyrosine-click reaction. Bioconj. Chem. 2013, 24, 520–532. [Google Scholar] [CrossRef]
- Vandewalle, S.; De Coen, R.; De Geest, B.G.; Du Prez, F.E. Tyrosine-Triazolinedione Bioconjugation as Site-Selective Protein Modification Starting from RAFT-Derived Polymers. ACS Macro Lett. 2017, 6, 1368–1372. [Google Scholar] [CrossRef]
- Al-Momani, E.; Israel, I.; Buck, A.K.; Samnick, S. Improved synthesis of [18F]FS-PTAD as a new tyrosine-specific prosthetic group for radiofluorination of biomolecules. Appl. Rad. Isot. 2015, 104, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.M.; Ahmed, I.; Vigovskaya, A.; Fruk, L. Clickable tyrosine binding bifunctional linkers for preparation of DNA-protein conjugates. Bioconj. Chem. 2013, 24, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Hanay, S.B.; Ritzen, B.; Brougham, D.; Dias, A.A.; Heise, A. Exploring Tyrosine-Triazolinedione (TAD) Reactions for the Selective Conjugation and Cross-Linking of N-Carboxyanhydride (NCA) Derived Synthetic Copolypeptides. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Culbertson, B.M.; McGrath, J.E. Polymer Science and Technology; Plenum Press: New York, NY, USA, 1985; pp. 8–11. [Google Scholar]
- Zolfigol, M.A.; Mallakpour, S.E.; Madrakian, E.; Ghaemi, E. Oxidation of urazoles to their corresponding triazolinediones under mild and heterogeneous conditions. Indian J. Chem. Sect. B: Org. Chem. Incl. Med. Chem. 2000, 39, 308–310. [Google Scholar]
- Hu, Q.-Y.; Allan, M.; Adamo, R.; Quinn, D.; Zhai, H.; Wu, G.; Clark, K.; Zhou, J.; Ortiz, S.; Wang, B.; et al. Synthesis of a well-defined glycoconjugate vaccine by a tyrosine-selective conjugation strategy. Chem. Sci. 2013, 4, 3827–3832. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955, 61, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Zolfigol, M.A.; Nasr-Isfahanib, H.; Mallakpourc, S.; Safaieea, M. Oxidation of Urazoles with 1,3-Dihalo-5,5-dimethylhydantoin, both in Solution and under Solvent-Free Conditions. Synlett 2005, 5, 0761–0764. [Google Scholar] [CrossRef]
- Cunha, C.B.; Klumpers, D.D.; Li, W.A.; Koshy, S.T.; Weaver, J.C.; Chaudhuri, O.; Granja, P.L.; Mooney, D.J. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 2014, 35, 8927–8936. [Google Scholar] [CrossRef]
- Ryall, J.P.; Dines, T.J.; Chowdhry, B.Z.; Leharne, S.A.; Withnall, R. Vibrational spectra and structures of the anions of urazole and 4-methylurazole: DFT calculations of the normal modes and the influence of hydrogen bonding. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 918–925. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2018; p. 286. [Google Scholar]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Raspanti, M.; Caravà, E.; Sgambato, A.; Natalello, A.; Russo, L.; Cipolla, L. The collaggrecan: Synthesis and visualization of an artificial proteoglycan. Int. J. Biol. Macromol. 2016, 86, 65–70. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guizzardi, R.; Vaghi, L.; Marelli, M.; Natalello, A.; Andreosso, I.; Papagni, A.; Cipolla, L. Gelatin-Based Hydrogels through Homobifunctional Triazolinediones Targeting Tyrosine Residues. Molecules 2019, 24, 589. https://doi.org/10.3390/molecules24030589
Guizzardi R, Vaghi L, Marelli M, Natalello A, Andreosso I, Papagni A, Cipolla L. Gelatin-Based Hydrogels through Homobifunctional Triazolinediones Targeting Tyrosine Residues. Molecules. 2019; 24(3):589. https://doi.org/10.3390/molecules24030589
Chicago/Turabian StyleGuizzardi, Roberto, Luca Vaghi, Marcello Marelli, Antonino Natalello, Ivan Andreosso, Antonio Papagni, and Laura Cipolla. 2019. "Gelatin-Based Hydrogels through Homobifunctional Triazolinediones Targeting Tyrosine Residues" Molecules 24, no. 3: 589. https://doi.org/10.3390/molecules24030589
APA StyleGuizzardi, R., Vaghi, L., Marelli, M., Natalello, A., Andreosso, I., Papagni, A., & Cipolla, L. (2019). Gelatin-Based Hydrogels through Homobifunctional Triazolinediones Targeting Tyrosine Residues. Molecules, 24(3), 589. https://doi.org/10.3390/molecules24030589