Absorption of Sulfur Dioxide by Tetraglyme–Sodium Salt Ionic Liquid
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Ionic Liquids
2.2. Absorption and Desorption of SO2
3. Results and Discussion
3.1. Properties of Ionic Liquids
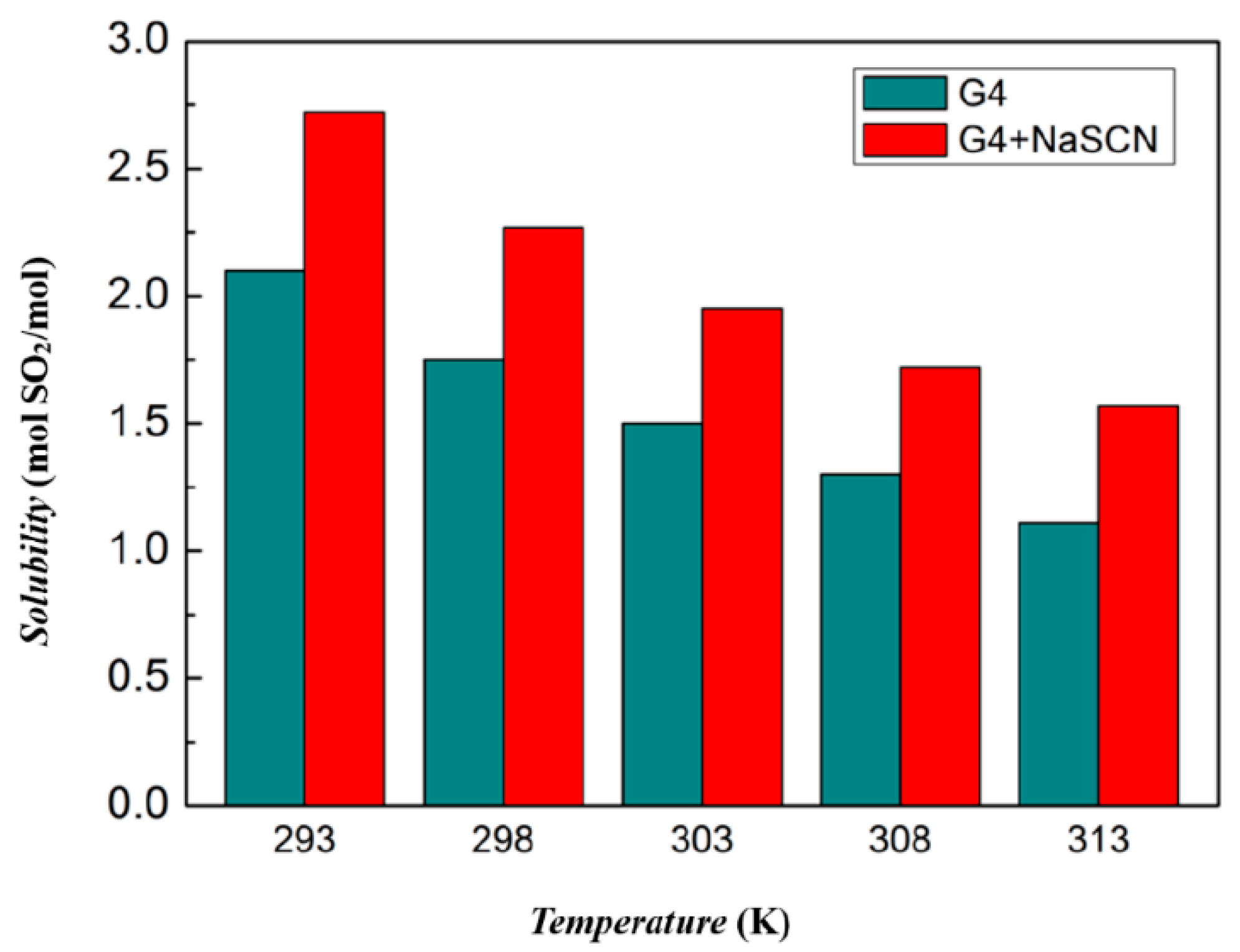
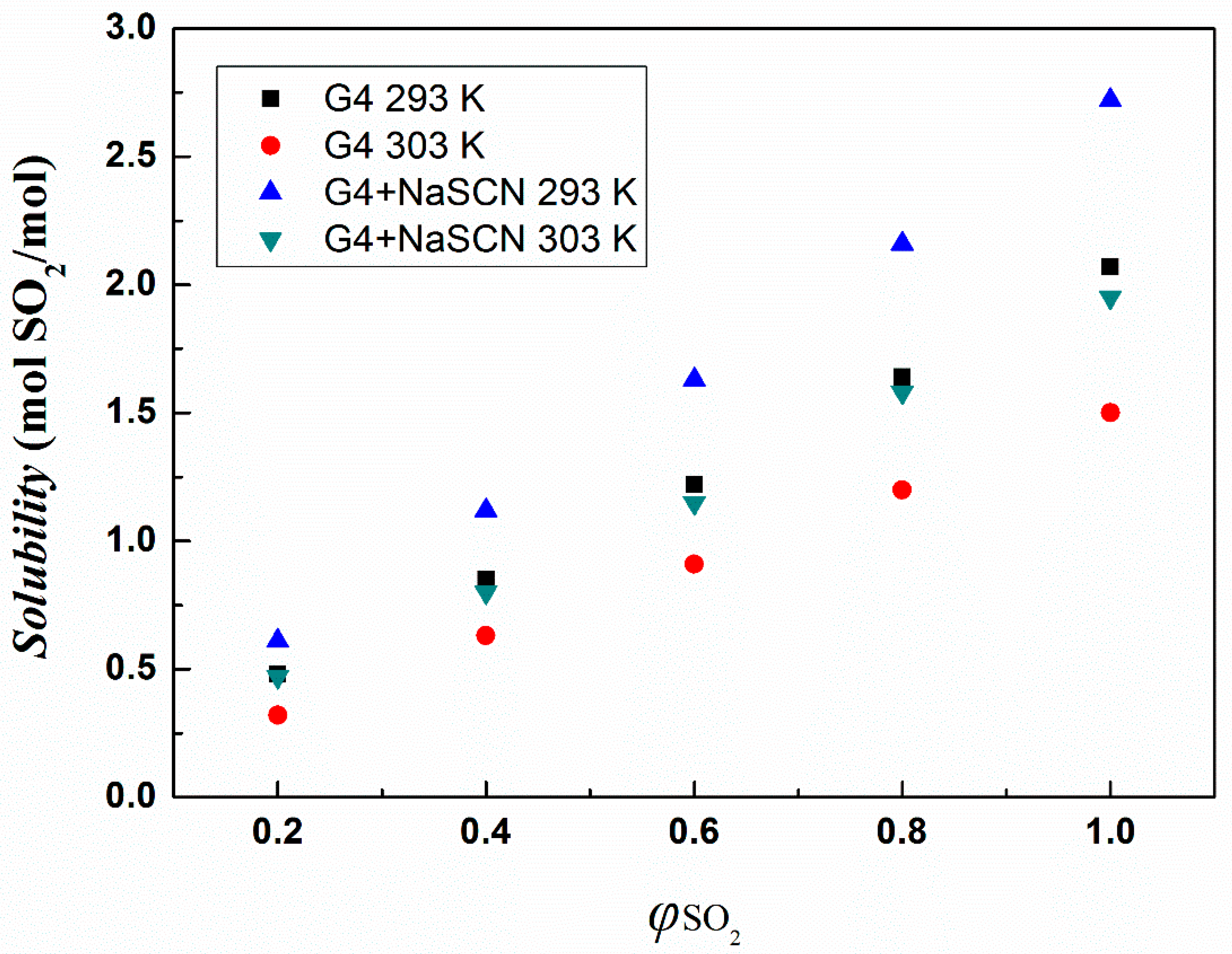
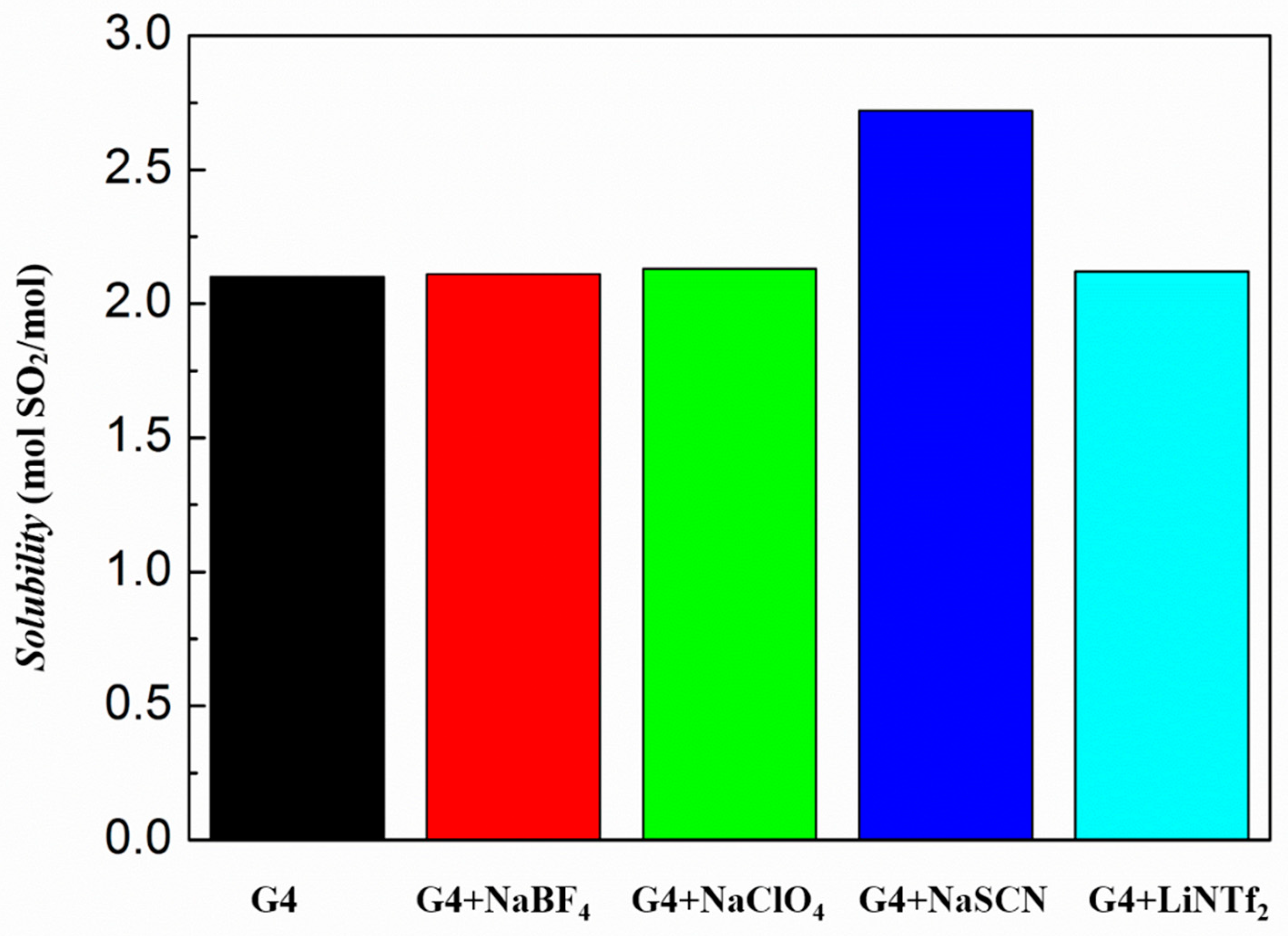
3.2. Absorption Capacity of Ionic Liquids
3.3. Regeneration
3.4. Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spengler, J.D.; Ferris, B.G., Jr.; Dockery, D.W.; Speizer, F.E. Sulfur dioxide and nitrogen dioxide levels inside and outside homes and the implications on health effects research. Environ. Sci. Technol. 1979, 13, 1276–1280. [Google Scholar] [CrossRef]
- Xue, J.; Yuan, Z.; Griffith, S.M.; Yu, X.; Lau, A.K.; Yu, J.Z. Sulfate Formation Enhanced by a Cocktail of High NOx, SO2, Particulate Matter, and Droplet pH during Haze-Fog Events in Megacities in China: An Observation-Based Modeling Investigation. Environ. Sci. Technol. 2016, 50, 7325–7334. [Google Scholar] [CrossRef] [PubMed]
- Renedo, M.J.; Fernandez, J. Preparation, Characterization, and Calcium Utilization of Fly Ash/Ca(OH)2 Sorbents for Dry Desulfurization at Low Temperature. Ind. Eng. Chem. Res. 2002, 41, 2412–2417. [Google Scholar] [CrossRef]
- Ma, X.; Kaneko, T.; Tashimo, T.; Yoshida, T.; Kato, K. Use of limestone for SO2 removal from flue gas in the semidry FGD process with a powder-particle spouted bed. Chem. Eng. Sci. 2000, 55, 4643–4652. [Google Scholar] [CrossRef]
- Hansen, B.B.; Kiil, S.; Johnsson, J.E.; Sønder, K.B. Foaming in Wet Flue Gas Desulfurization Plants: The Influence of Particles, Electrolytes, and Buffers. Ind. Eng. Chem. Res. 2008, 47, 3239–3246. [Google Scholar] [CrossRef]
- Van Dam, M.H.H.; Lamine, A.S.; Roizard, D.; Lochon, P.; Roizard, C. Selective sulfur dioxide removal using organic solvents. Ind. Eng. Chem. Res. 1997, 36, 4628–4637. [Google Scholar] [CrossRef]
- de Kermadec, R.; Lapicque, F.; Roizard, D.; Roizard, C. Characterization of the SO2−N-Formylmorpholine Complex: Application to a Regenerative Process for Waste Gas Scrubbing. Ind. Eng. Chem. Res. 2002, 41, 153–163. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhang, P.Y.; Chen, G.H.; Han, F.; Wei, X.H. Gas−Liquid Equilibrium Data for the Mixture Gas of Sulfur Dioxide/Nitrogen with Ethylene Glycol at Temperatures from (298.15 to 313.15) K under Low Pressures. J. Chem. Eng. Data 2008, 53, 1479–1485. [Google Scholar] [CrossRef]
- Sun, S.; Niu, Y.; Sun, Z.; Xu, Q.; Wei, X. Solubility properties and spectral characterization of sulfur dioxide in ethylene glycol derivatives. RSC Adv. 2015, 5, 8706–8712. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.; Gilea, M.A.; Lopes, J.N.; Rebelo, L.P.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Haumann, M.; Riisager, A. Hydroformylation in room temperature ionic liquids (RTILs): Catalyst and process developments. Chem. Rev. 2008, 108, 1474–1497. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Dao, R.; Zhang, W.; Lv, X.; Li, H.; Wang, C. Designing an anion-functionalized fluorescent ionic liquid as an efficient and reversible turn-off sensor for detecting SO2. Chem. Commun. 2017, 53, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Yokozeki, A.; Shiflett, M.B. Hydrogen purification using room-temperature ionic liquids. Appl. Energy 2007, 84, 351–361. [Google Scholar] [CrossRef]
- Jalili, A.H.; Rahmati-Rostami, M.; Ghotbi, C.; Hosseini-Jenab, M.; Ahmadi, A.N. Solubility of H2S in Ionic Liquids [bmim][PF6], [bmim][BF4], and [bmim][Tf2N]. J. Chem. Eng. Data 2009, 54, 1844–1849. [Google Scholar] [CrossRef]
- Revelli, A.L.; Mutelet, F.; Jaubert, J.N. Reducing of nitrous oxide emissions using ionic liquids. J. Phys. Chem. B 2010, 114, 8199–8206. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, J.; Gomes, M.F.C.; Husson, P.; Majer, V. Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric. J. Chem. Thermodyn. 2006, 38, 490–502. [Google Scholar] [CrossRef]
- Wu, W.; Han, B.; Gao, H.; Liu, Z.; Jiang, T.; Huang, J. Desulfurization of Flue Gas: SO2 Absorption by an Ionic Liquid. Angew. Chem. Int. Ed. 2004, 43, 2415–2417. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X. SO2 Capture by Guanidinium-Based Ionic Liquids: A Theoretical Study. J. Phys. Chem. B 2011, 115, 3466–3477. [Google Scholar] [CrossRef]
- Shang, Y.; Li, H.; Zhang, S.; Xu, H.; Wang, Z.; Zhang, L.; Zhang, J. Guanidinium-based ionic liquids for sulfur dioxide sorption. Chem. Eng. J. 2011, 175, 324–329. [Google Scholar] [CrossRef]
- Huang, J.; Riisager, A.; Wasserscheid, P.; Fehrmann, R. Reversible physical absorption of SO2 by ionic liquids. Chem. Commun. 2006, 38, 4027–4029. [Google Scholar] [CrossRef] [PubMed]
- Shiflett, M.B.; Yokozeki, A. Separation of Carbon Dioxide and Sulfur Dioxide Using Room-Temperature Ionic Liquid [bmim][MeSO4]. Energy Fuels 2009, 24, 1001–1008. [Google Scholar] [CrossRef]
- Tian, S.D.; Hou, Y.C.; Wu, W.Z.; Ren, S.H.; Zhang, C. Absorption of SO2 by thermal-stable functional ionic liquids with lactate anion. RSC Adv. 2013, 3, 3572–3577. [Google Scholar] [CrossRef]
- Ren, S.H.; Hou, Y.C.; Wu, W.Z.; Liu, Q.Y.; Xiao, Y.F.; Chen, X.T. Properties of Ionic Liquids Absorbing SO2 and the Mechanism of the Absorption. J. Phys. Chem. B 2010, 114, 2175. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, G.N.; Dai, Y.; Wu, Y.T.; Hu, X.B.; Zhang, Z.B. Dicarboxylic acid salts as task-specific ionic liquids for reversible absorption of SO2 with a low enthalpy change. RSC Adv. 2013, 3, 16264–16269. [Google Scholar] [CrossRef]
- Zeng, S.J.; Gao, H.S.; Zhang, X.C.; Dong, H.F.; Zhang, X.P.; Zhang, S.J. Efficient and reversible capture of SO2 by pyridinium-based ionic liquids. Chem. Eng. J. 2014, 251, 248–256. [Google Scholar] [CrossRef]
- Huang, K.; Chen, Y.L.; Zhang, X.M.; Xia, S.; Wu, Y.T.; Hu, X.B. SO2 absorption in acid salt ionic liquids/sulfolane binary mixtures: Experimental study and thermodynamic analysis. Chem. Eng. J. 2014, 237, 478–486. [Google Scholar] [CrossRef]
- Qu, G.F.; Zhang, J.; Li, J.Y.; Ning, P. SO2 Absorption/Desorption Characteristics of Two Novel Phosphate Ionic Liquids. Sep. Sci. Technol. 2013, 48, 2876–2879. [Google Scholar] [CrossRef]
- Yang, Z.Z.; He, L.N.; Zhao, Y.N.; Yu, B. Highly Efficient SO2 Absorption and Its Subsequent Utilization by Weak Base/Polyethylene Glycol Binary System. Environ. Sci. Technol. 2013, 47, 1598–1605. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhang, Z.J.; Sun, Y.L.; Jiang, B.; Li, X.G.; Ge, X.H.; Wang, J.T. Ether-Functionalized Ionic Liquids with Low Viscosity for Efficient SO2 Capture. Ind. Eng. Chem. Res. 2013, 52, 16335–16340. [Google Scholar] [CrossRef]
- Yokozeki, A.; Shiflett, M.B. Separation of Carbon Dioxide and Sulfur Dioxide Gases Using Room-Temperature Ionic Liquid [hmim][Tf2N]. Energy Fuels 2009, 23, 4701–4708. [Google Scholar] [CrossRef]
- Wang, C.; Cui, G.; Luo, X.; Xu, Y.; Li, H.; Dai, S. Highly Efficient and Reversible SO2 Capture by Tunable Azole-Based Ionic Liquids through Multiple-Site Chemical Absorption. J. Am. Chem. Soc. 2011, 133, 11916–11919. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.K.; Zheng, J.J.; Luo, X.Y.; Lin, W.J.; Ding, F.; Li, H.R.; Wang, C.M. Tuning Anion-Functionalized Ionic Liquids for Improved SO2 Capture. Angew. Chem. Int. Ed. 2013, 52, 10620–10624. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Zheng, J.J.; Cui, G.K.; Luo, X.Y.; Guo, Y.; Li, H.R. Highly efficient SO2 capture through tuning the interaction between anion-functionalized ionic liquids and SO2. Chem. Commun. 2013, 49, 1166–1168. [Google Scholar] [CrossRef]
- Sun, S.; Niu, Y.; Xu, Q.; Sun, Z.; Wei, X. Highly efficient sulfur dioxide capture by glyme–lithium salt ionic liquids. RSC Adv. 2015, 5, 46564–46567. [Google Scholar] [CrossRef]
- Reuter, K.; Dankert, F.; Donsbach, C.; von Hänisch, C. Structural Study of Mismatched Disila-Crown Ether Complexes. Inorganics 2017, 5, 11. [Google Scholar] [CrossRef]
- Bernazzani, L.; Borsacchi, S.; Catalano, D.; Gianni, P.; Mollica, V.; Vitelli, M.; Asaro, F.; Feruglio, L. On the interaction of sodium dodecyl sulfate with oligomers of poly (ethylene glycol) in aqueous solution. J. Phys. Chem. B 2004, 108, 8960–8969. [Google Scholar] [CrossRef]
- Guchhait, B.; Gazi, H.a.; Kashyap, H.K.; Biswas, R. Fluorescence Spectroscopic Studies of (Acetamide + Sodium/Potassium Thiocyanates) Molten Mixtures: Composition and Temperature Dependence. J. Phys. Chem. B 2010, 114, 5066–5081. [Google Scholar] [CrossRef]
- Mandai, T.; Nozawa, R.; Tsuzuki, S.; Yoshida, K.; Ueno, K.; Dokko, K.; Watanabe, M. Phase diagrams and solvate structures of binary mixtures of glymes and Na salts. J. Phys. Chem. B 2013, 117, 15072–15085. [Google Scholar] [CrossRef]
- Terada, S.; Mandai, T.; Nozawa, R.; Yoshida, K.; Ueno, K.; Tsuzuki, S.; Dokko, K.; Watanabe, M. Physicochemical properties of pentaglyme–sodium bis (trifluoromethanesulfonyl) amide solvate ionic liquid. Phys. Chem. Chem. Phys. 2014, 16, 11737–11746. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds including tetraglyme, NaSCN and NaBF4 are available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Jiang, W.; Xiao, J.; Wei, X. Absorption of Sulfur Dioxide by Tetraglyme–Sodium Salt Ionic Liquid. Molecules 2019, 24, 436. https://doi.org/10.3390/molecules24030436
Xu Q, Jiang W, Xiao J, Wei X. Absorption of Sulfur Dioxide by Tetraglyme–Sodium Salt Ionic Liquid. Molecules. 2019; 24(3):436. https://doi.org/10.3390/molecules24030436
Chicago/Turabian StyleXu, Qiang, Wei Jiang, Jianbai Xiao, and Xionghui Wei. 2019. "Absorption of Sulfur Dioxide by Tetraglyme–Sodium Salt Ionic Liquid" Molecules 24, no. 3: 436. https://doi.org/10.3390/molecules24030436
APA StyleXu, Q., Jiang, W., Xiao, J., & Wei, X. (2019). Absorption of Sulfur Dioxide by Tetraglyme–Sodium Salt Ionic Liquid. Molecules, 24(3), 436. https://doi.org/10.3390/molecules24030436





