1. Introduction
In 2016 nearly 58% of the organic fraction of municipal solid waste (OFMSW) in the EU was sent directly to landfill or incineration (estimated using the Eurostat database accessed 28/11/2018: recycling of bio-waste (cei_wm030), generation of waste by waste category (ten00108) and population on 1 January (tps00001)), resulting in undesirable environmental effects, little to no value recovery, and hence a loss of resources. However, recycling in the EU is now increasing [
1], and hence separately collected organic waste is becoming more available for resource recovery or waste valorisation, i.e., the process of converting waste into energy, chemicals or materials. Technologies for bio-waste valorisation can be categorised as thermal or thermochemical such as hydrothermal liquefaction, pyrolysis and gasification, physicochemical like extraction and transesterification or biological conversion processes [
2,
3]. Biomass gasification has been proposed to homogenise various substrates to syngas and further process this for chemical production [
4]. Reviews are available regarding technologies for waste to energy [
5,
6], or waste to chemicals and materials [
7,
8,
9,
10]. The choice of treatment method will depend on several factors such as type and availability of organic waste streams, e.g., the waste’s organic strength measured by chemical oxygen demand (COD) [
11], relative content of biopolymers (i.e., cellulose, hemicellulose or lignin) [
12], or biomass type (woody biomass, types of agricultural residues, household organic waste and sewage sludge) [
13,
14]. Development of a circular economy where waste is used as resource for renewable energy and chemicals will require the integration of different types of conversion processes to deal with the complexity of bio-waste and maximize resource recovery [
15].
Established bio-waste valorisation technologies are composting and anaerobic digestion (AD), which each produce fertilizer and methane-rich biogas as end-products. However, the final products have relatively low economic value. For instance only € 2 worth of compost is obtained per tonne food waste [
16]. AD generates a slightly more valuable product: assuming the OFMSW typically contains 306.4 g
COD kg
−1 of anaerobic biodegradable content [
17] and that biogas conversion yields € 0.25 worth of biogas per kg of COD [
18], then a tonne of food waste will produce about € 76 worth of biogas. However, the intermediate fermentation compounds produced during AD have a higher market value.
Fermentation to accumulate the intermediate carboxylates is known as the carboxylate platform. Producing carboxylates through fermentation forms a sustainable alternative to their current production from fossil fuels or extraction in small amounts from natural oils [
19]. Compared to AD, the carboxylate platform shows lower conversion yields, yet the higher product value and broader applications can result in a higher economic value [
20]. In the last decade, particular interest has grown in medium chain carboxylic acids (MCCAs). They are defined as carboxylic acids with an aliphatic straight carbon chain of 6 to 12 carbon atoms, e.g.,
n-caproic acid has a straight chain of 6 carbon atoms (C6). MCCAs are more hydrophobic compared to shorter chain carboxylates, which makes them a more interesting fermentation product as it facilitates recovery from the fermentation broth [
21]. In terms of potential value, C6 has a market size of 25,000 tonne per year, with an unrefined value of
$1000, and refined value of
$2000 to
$3000 per tonne [
22,
23]. Overall, MCCAs have a wide range of applications: they can be applied as growth-promoting antibiotic replacements in animal feed [
24,
25], or be converted via various bio-, thermo-, or electro-chemical processes into bulk fuels or solvents [
14,
26,
27,
28]. The production of MCCAs as higher value products from organic waste can incentivise for improved recycling while simultaneously replacing current unsustainable production processes.
MCCAs are produced by certain bacteria in a strongly reduced anaerobic environment, via a metabolic pathway that has been recently reviewed by Spirito et al. [
29]. The bacteria gain energy by combining the oxidation of an electron donor, i.e., lactic acid or ethanol, to acetyl-CoA with the reductive elongation of acetyl-CoA with acetic acid (C2), propionic acid (C3), butyric acid (C4), pentanoic acid (C5), or caproic acid (C6) generating a carboxylic acid with 2 additional carbons at each step (
Figure 1). The reduction step is required to provide sufficient Gibbs free energy (ΔG) to generate ATP in the initial oxidation step, restore the NAD
+/NADH balance in the cell, and contribute to further energy generation via electron-transport phosphorylation. Ethanol and lactic acid have similar thermodynamic capacity to act as electron donors [
30]. This chain elongation pathway is called the reverse β-oxidation pathway, since it is seen as the reversed biochemical degradation or β-oxidation of fatty acids.
Instead of using pure or engineered cultures, a consortium of microorganisms has more potential to deal with complex and variable feedstock such as organic waste. Mixed microbial cultures (MMC), also referred to as microbiomes, are communities of microorganisms within a well-defined environment of specific physicochemical properties [
31]. Microbiomes are employed in biotechnology, for example in anaerobic digestion (AD), and in bioremediation by cultivating communities within contaminated soils [
32,
33]. The term “microbiome” is used to describe the mixed microbial communities related to the human and animal gut, mouth or skin, or plant rhizospheres.
The first report of MMC that produced MCCAs dates back to the mid-19th Century, where Béchamp attributed the production of approx. 6 g
COD L
−1 C6 from ethanol, meat extract and chalk in a fermentation reactor to microbial activity [
34]. A few decades later in the early 20th Century, an oily, immiscible layer comprising 5.3 g
COD L
−1 C4 and 6.4 g
COD L
−1 C6 was produced in a 30-day fermentation with impure cultures from a nutrient medium containing 24 g
COD L
−1 ethanol [
35]. Further microscopic study of the fermentation sludge revealed a consortia of microorganisms comprising methanogenic archaea and spore-forming bacteria [
35]. By contrast with pure cultures, MMC do not require sterilisation, can degrade a complex feedstock, show a resilience to operational upsets [
36], and allow continuous, long-term operation [
37]. These advantages provide a strong argument for utilising microbiomes.
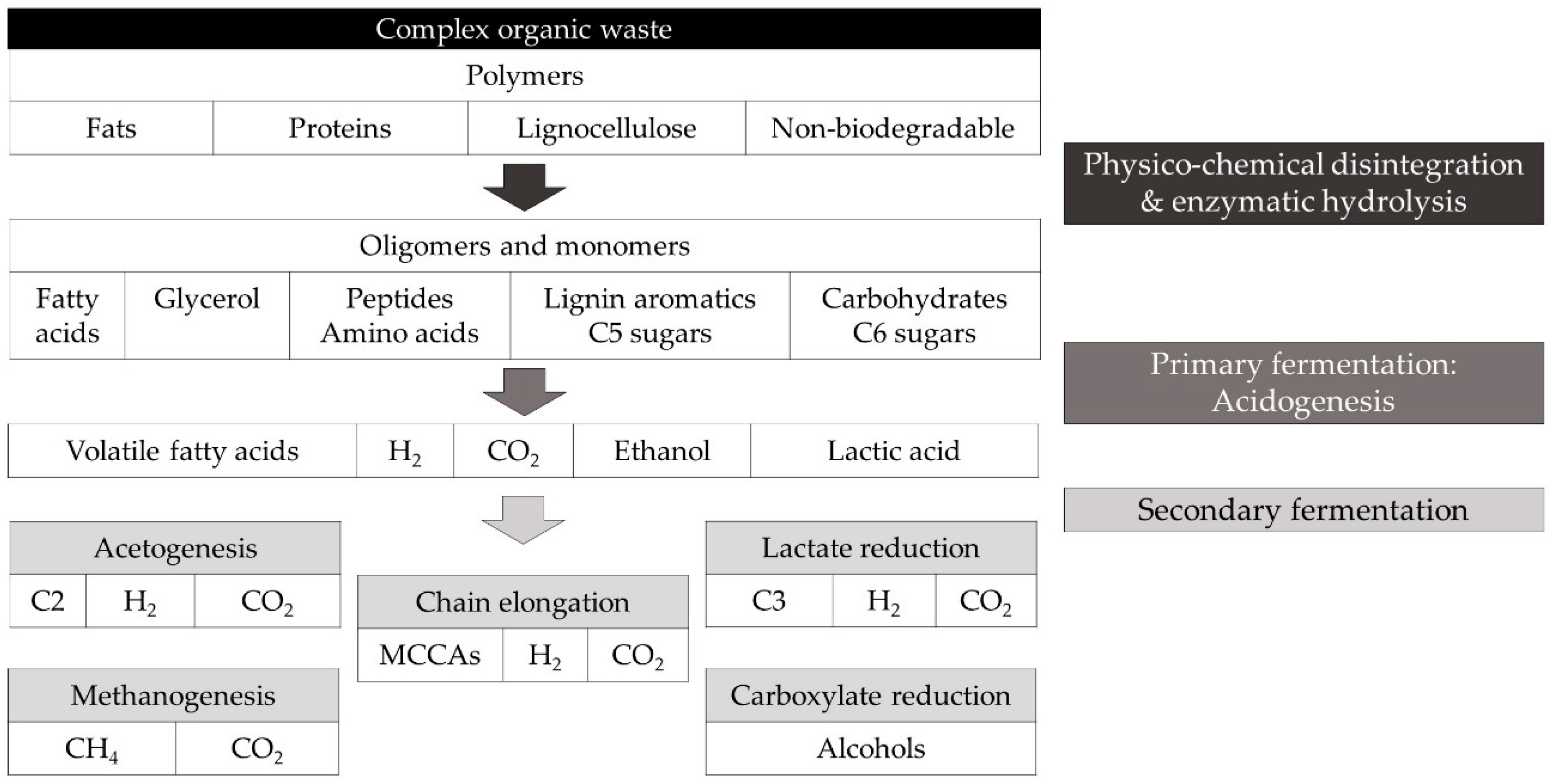
In MMC, conversion of organic substrates occurs following a cascade of steps catalysed by different microorganisms that form synergistic and competitive interactions, resulting in a complex microbial ecosystem with a versatile metabolic capacity [
38]. The different microbial groups can convert organic molecules into substrates available for chain elongating bacteria. In general, biodegradable organics are hydrolysed and fermented to intermediate compounds that acidify the medium, i.e., acidogenesis, including hydrogen gas (H
2), lactic acid, ethanol, formic acid (C1) and volatile fatty acids (VFAs), i.e., straight short chain carboxylic acids with 2 to 4 carbon atoms. The accumulated intermediates can undergo several secondary bioconversion steps, including chain elongation to produce MCCAs (
Figure 2) [
26]. For instance, co-culture of the chain elongating bacteria
Clostridium kluyveri with specific cellulolytic species or a rumen microbiome showed chain elongation potential from a cellulose substrate and ethanol [
39,
40]. The supporting community can even be designed or selected to allow chain elongation from a specific compound, such as glycerol or syngas (CO) [
41,
42,
43], or allow the use of alternative electron donors such as, for instance, the cathode in a bio-electrochemical system [
44,
45].
While it is generally believed that specific operational conditions allow development of a MMC for a functional and stable process [
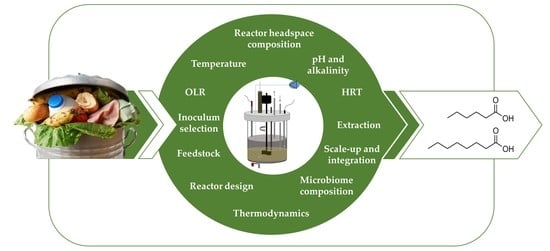
46], the broad metabolic capacity also gives rise to a set of various competitive reactions and by-products, especially when utilising a complex feedstock. Manipulating the environmental conditions, by regulating operation, allows some control to be exerted on the product spectrum, as it affects the thermodynamics of conversion processes, and therefore the microbiome composition that catalyses these conversions. However, current knowledge of control over the product outcome to improve MCCA yields in MMC fermentation is limited since experiments that use complex feedstock for MCCAs production have only emerged in the past few years.
While the operational conditions that select for other MMC fermentation products such as volatile fatty acids (VFAs) [
47] and hydrogen (H
2) [
48] have been reviewed, the operational conditions or process set-up that allow MCC to be steered towards MCCA formation have to be further evaluated. A recent review is available regarding the use of bio-electrochemical systems for MCCA production as a complementary technology to AD [
49]. Certain other reviews include a section on MCCAs as potential MMC fermentation products, either in the context of operational control applied in AD [
50], or the contexts of a biorefinery [
51], wastewater treatment [
11] or food waste treatment [
21,
52,
53,
54]. However, a focussed analysis of the literature to identify and connect key operational parameters to target MCCA production from MMC fermentation of complex feedstocks is lacking. Therefore, this work aims to analyse the current literature, and hence complement existing reviews. For this, studies were included that specifically target chain elongation, but the scope was extended to include other MMC-based studies that have noted MCCA as by-products from, for instance, VFA or H
2 production. Concentrations and production rates are converted to a COD-basis to allow comparison between studies using different reporting concentrations (
Appendix A). The review evaluates the key operational parameters for MCCA production from complex substrates using MMC, with the objective of stimulating and accelerating research to produce sustainable, bio-based fuels and chemicals from organic waste. In addition, a database was generated from the experimental data available in the literature regarding MCCA production using MMC fermentation [
55].
2. Chain Elongation Behaviour of Pure Cultures Can Be Extended for MMC
Chain elongation via ethanol is the most studied pathway to date. The mechanism has been elucidated by studying
Clostridium kluyveri, a gram-positive, spore-forming bacteria from the phylum of Firmicutes whose whole genome has been published [
56]. For each molecule of ethanol oxidized to C2, resulting in substrate-level ATP-generation and production of H
2, five molecules of ethanol enter the reverse β-oxidation pathway as acetyl-CoA and elongate five molecules of C2 to C4. Subsequently, C4 can be elongated to C6 via ethanol-derived acetyl-CoA addition (
Table 1, Equations (1) and (2)) [
29]. In reality, the pathway of
C. kluyveri has a more flexible stoichiometry influenced by substrate concentrations, ratio of ethanol to acetate, and the partial pressure of H
2 (
Table 1, Equations (3)–(6)) [
57,
58,
59]. It also has a broader substrate range including propanol as an electron donor, or propionate (C3), succinate, malonate, 3-butenoate, 4-hydroxybutyrate and crotonate as electron acceptors [
39,
60,
61]. Pure culture fermentations of
C. kluyveri fed with ethanol and C2 mixtures have been reported to produce C6 up to 10.2 g
COD L
−1 d
−1 in continuous culture [
62] and to reach concentrations up to 30.7 g
COD L
−1 after 72 h of batch culture [
60].
Chain elongation via lactic acid has been reported for other bacteria in the phylum of Firmicutes, such as
Megasphaera elsdenii [
63] and a
Ruminococcaceae bacterium CPB6 [
64]. Other wild-type bacteria are known to perform chain elongation and produce C6 (and C8) using more “exotic” chain elongating substrates such as simple sugars, polyols, methanol, amino acids and H
2 and CO
2 gas mixtures as reviewed by Angenent et al. [
58]. In addition, pathways have been engineered to produce C6. For instance, to improve yields the genes from
Megasphaera sp. were expressed in
Escherichia coli, and approx. 1.17 g
COD L
−1 d
−1 C6 was obtained after 36 h of batch fermentation [
65]. To develop a more thermo-tolerant and acid-resistant biocatalyst, biosynthetic pathways have been constructed in the yeast
Kluyveromyces marxianus [
66]. Single-strains or engineered cultures have their place when the product is of high enough value and require a certain purity. Overall, the production of MCCAs, and other medium chain chemicals, using pure, engineered cultures has been recently reviewed by other authors, e.g., Sarria et al. [
67] and Su et al. [
68].
However, when it comes to breaking down a complex feedstock such as organic waste, the focus of this review, pure cultures have limited metabolic capacity, reducing their potential for an effective treatment and requiring more expensive processing such as media sterilisation [
71]. This can be circumvented by using MMC instead of pure cultures. Chain elongation in MMC happens in a similar manner than with pure cultures. For instance, microbiomes grown in ethanol-rich conditions show similar characteristics to pure culture fermentation, such as higher specificity towards longer chain carboxylates at higher ethanol/acetate ratios [
72,
73] and elongation towards a mixture of even- and uneven MCCA in the presence of propanol or C3 [
74,
75]. It should be noted that MMC are unable to use either 4-carbon alcohols or 5-carbon carboxylates as initial substrate sources for chain elongation at similar concentrations than for example ethanol or acetate [
75]. This may be due to longer chain substrates having higher toxicity and possible inhibition of the microbiome. In addition, microbiomes are capable of adapting to substrate fluctuations: MMC obtained from ethanol-based chain elongation reactors acclimatised to produce C6 when fed with methanol or lactic acid as an alternate electron donor [
76,
77].
3. Thermodynamic Models and MMC Composition Determine Competitive Processes
Successful production of MCCAs requires elimination of competing reactions that could consume the substrate or product. Some example reactions include methanogenesis, sulphate reduction, lactate reduction to propionate (C3), excessive oxidation of ethanol, and reduction or oxidation of carboxylic acids, as described in
Table 2. Since anaerobic ecosystems are energy-limited with ΔG of conversion processes being close to 0 kJ mol
−1 (
Table 1 and
Table 2) [
78], the thermodynamic favourability of bioconversion processes can shift by small changes in substrate or product concentrations, pH and temperature, partial pressure of gases in reactor headspace, or substrate availability [
50,
79,
80]. The resulting thermodynamic constraints select for the viable bioconversion reactions and, hence, the composition of microbiome that has the most efficient catabolic system [
81,
82]. Therefore, strategies to inhibit competitive reactions can be classified as; (i) the inhibition of a specific, competitive trophic group, or (ii) the engineering of the fermentation environment to reduce the potential competitive reactions. For instance, methanogenesis, the ability to produce CH
4, is limited to certain archaea. Since CH
4 has the lowest free energy content per electron upon oxidation to CO
2 under anaerobic conditions, and automatically leaves the reactor as a gas, it will be produced by methanogens in MMC to optimally use the energy available [
71]. To ensure that C2 or H
2 are not lost to CH
4 and CO
2 (
Table 2, Equations (10) and (11)), specific methanogenic inhibitors can be added to promote chain elongation. For instance, in batch fermentation of a synthetic substrate containing ethanol and C2, the addition of 2-bromoethylsulfonate (BES) tripled C6 production to 19 g
COD L
−1 [
83]. Alternatively, to avoid the cost of such chemicals, specific operational conditions such as pH or hydraulic retention time (HRT) can be selected to inhibit methanogens as discussed subsequently. Another unwanted trophic group are the sulphate reducing bacteria. A sulphur-rich feedstock will result in sulphate reduction, as this is more thermodynamically favourable than C6 production (
Table 2, Equation (12)), generating sulphide, which is both toxic for most bacteria and corrosive to fermentation equipment [
84].
Thermodynamic models are useful tools to improve understanding of the chain elongation pathway in MMC and to determine which operational parameters allow to regulate the product spectrum. Research has developed kinetic and thermodynamic models based on pure culture chain elongation using
C. kluyveri [
69,
85] or metabolic energy-based models to predict MMC fermentation of simple substrates such as glucose [
86]. Such models can help understanding the occurrence of chain elongation at different ethanol concentrations [
58], or at varying H
2:CO
2 ratios [
30]. There is a lack of models that evaluate the thermodynamics of the lactic acid-based chain elongation route. At standard conditions lactate reduction (
Table 2, Equations (13) and (14)) releases more energy than chain elongation via lactic acid (
Table 1, Equation (9)). Experimentally, Kucek et al. [
77] found increasing lactate loading rate with a synthetic feedstock initially improved chain elongation in MMC fermentation, yet increasing influent lactic acid from 9.1 to 16.2 g
COD L
−1 d
−1 led to a collapse of C6 productivity to 3.0 g
COD L
−1 d
−1 while C3 production increased to 5.5 g
COD L
−1 d
−1. This was attributed to the competitive acrylate pathway being stimulated ahead of chain elongation at elevated lactic acid concentrations [
77,
87]. In contrast, another study operating with an excess of lactic acid did not report C3 production; the addition of three spikes in a fed batch-style adding a total about 26.7 g
COD L
−1 lactic acid to the synthetic medium resulted in C6 accumulation of up to 51.7 g
COD L
−1 [
88]. The development of thermodynamic models focusing on lactic acid-based chain elongation might shed more light on these competitive pathways.
Modelling thermodynamics only goes so far, and the composition of the microbiome must be considered as this can influence the microbiome’s metabolic capacity. The results from microbial community composition analysis using 16s rRNA gene sequencing of the mentioned studies cited above indicate they had a different microbiome structure. The fermentation where the acrylate pathway took over, had a wider variety of prokaryotic families and was dominated by
Acinetobacter spp. (approx. 60% relative abundance) and the operational taxonomic units belonging to
Ruminococcaceae were less than 10% [
77]. On the contrary, the study with minimal C3 production was dominated by a Clostridium cluster IV group (79.1%, belonging to
Ruminococcaceae) [
88].
Studying the microbiome composition improves the understanding of the MMC fermentation mechanisms. For instance, when following the microbial community dynamics of maize silage fermentation in a leach bed reactor (LBR), Sträuber et al. [
89] found
Lactobacillus and
Acetobacter strains dominated during the first days of operation, with lactic and acetic acid as concurrent products. However,
Clostridium species became dominant on Days 3 and 4 resulting in a pH increase and C4 and C6 production, and in turn these were overgrown during Days 5 to 7 by other phylotypes capable of using more complex polysaccharides by different metabolism [
89]. Further investigation of microbial interactions and synergies will allow better design of MCCA production processes from complex feedstocks, for example operating in sequential batch mode to allow the different trophic groups to first accumulate ethanol, lactic acid, H
2 or VFAs for subsequent chain elongation. Only 20 studies on MCCA production could be found, so far, that include an analysis of the microbial community. This usually involves DNA extraction and sequencing of 16s rRNA amplicon and comparison to sequence databases [
23,
70,
73,
75,
77,
83,
88,
90,
91,
92,
93,
94,
95,
96,
97,
98,
99], sometimes in addition to other community analysis such as flow cytometry [
100], analysis of terminal restriction fragment length polymorphisms (T-RFLP) [
101] or microscopic evaluation [
102].
Recently, Scarborough et al. [
103] combined metagenomic, metatranscriptomic and thermodynamic analysis of samples from a reactor microbiome fermenting a lignocellulosic-based feedstock in continuous stirred-tank reactor (CSTR) mode, which allowed, for instance, affiliation of
Lactobacillus and members of the
Coriobacteriaceae family to hydrolysis and primary fermentation, and organisms related to
Lachnospiraceae and
Eubacteriaceae to MCCA production. In addition, the recent advancements in metagenomic and metatranscriptomic analysis led to the proposition that other MCCA-producing pathways occur in a microbiome, such as the fatty acid biosynthesis pathway, alongside the reverse β-oxidation pathway [
104].
Research is necessary to expand the thermodynamic models to include both the MMC composition, which indicates the potential bio-reactions in a system, and the composition of complex feedstocks. The development of these models will complement the understanding obtained from experimental studies, and will help in determining the operational parameters which select for MCCA production over competitive reactions. In addition, culture-independent analysis and increased application of “omics” approaches on MMC fermentation studies will be essential to enhance our understanding of the underlying mechanisms that include competitive and synergistic processes and the importance of the MMC composition.
4. Bio-Waste Composition and Its Effect on Chain Elongation
A feedstock suitable for chain elongation should provide the necessary substrates, i.e., VFAs and electron donors such as ethanol or lactic acid. Chain elongation substrates can either be directly present in the feedstock, indirectly produced from primary fermentation in vivo, or supplemented. The highest MCCA production rates obtained in MMC fermentation used a synthetic feedstock, hence a readily bio-available substrate. In up-flow reactors (URs) with biomass retention, 115.2 g
COD L
−1 d
−1 for C6 [
105] and 19.4 g
COD L
−1 d
−1 for C8 [
96] were obtained from ethanol and C2 mixtures. These rates are more than 10 times higher than that achieved so far using complex, un-supplemented feedstocks (
Table 3). If electron donors, such as ethanol or lactic acid, are supplemented, selectivity of secondary fermentation is enhanced towards chain elongation. Ethanol-supplemented organic waste streams have reached production rates that lie somewhat in between synthetic and complex feedstocks. The maximum reported is 60.7 g
COD L
−1 d
−1 C6 and 2.13 g
COD L
−1 d
−1 C8 for pre-fermented OFMSW supplemented with 97.4 g
COD L
−1 d
−1 ethanol [
106]. Supplementation of 21.3 g
COD L
−1 lactic acid to pre-treated grass in batch fermentation with an adapted inoculum resulted in a total C6 concentration of 24.1 g
COD L
−1 after 1 day [
95].
When applying a supplementation strategy, certain experiments show that excessive concentrations of ethanol and lactic acid should be avoided. An upper limit for ethanol-based chain elongation is reported at 97.4 g
COD L
−1 after which it exerts an inhibitory effect [
107]. Grootscholten et al. [
108] spiked a LBR processing OFMSW at four intervals with 11.2 g
COD L
−1 ethanol during batch fermentation. This increased MCCA concentration from 4.0 to 6.0 g
COD L
−1 for C6 and 0.0 to 1.2 g
COD L
−1 for C8 compared to a non-supplemented control experiment, yet the total amount of carboxylic acids produced was lower [
108]. This was attributed to ethanol inhibition of hydrolytic and acidogenic bacteria [
108]. To avoid ethanol inhibition limiting primary fermentation, Grootscholten et al. [
106] suggested the use of a two-stage system where hydrolysis and primary fermentation of OFMSW occurs in the first batch phase, and in the second the pre-fermented OFMSW is supplemented with ethanol to select for chain elongation in a CSTR. The disadvantages of two-stage systems are the increased operational complexity and additional capital and operational costs.
A life-cycle assessment on C6 production from ethanol-supplemented food waste fermentation in a lab- and pilot-scale system revealed that the largest environmental effects (acidification and eutrophication potential) result from addition of caustic soda to control pH and ethanol as electron donor [
109]. Thus, supplementation of electron donors to stimulate chain elongation should be minimized or avoided. Instead of supplementing the feedstock, several studies have split the overall fermentation into two stages. Firstly, specific operational conditions are selected to accumulate ethanol or lactic acid. Then in a second phase, the leftover organics in the effluent are fermented towards VFAs and elongated with the electron donor under chain elongating conditions. Ethanol-rich substrates fed into chain elongation reactors have been obtained from yeast-based fermentations, such as for the production of bio-ethanol which generates an ethanol-rich beer and a residue after distillation called stillage [
27,
37,
93], or residues from the production of wine [
97], or effluent from syngas fermentation [
110]. Lactate-rich substrates can be obtained via MMC fermentations selective for lactic acid, such as effluent from thermophilic acid whey fermentation [
94], and pre-fermented grass (i.e., grass silage) [
95] or maize silage [
27,
89]. Whey is rich in lactose, simple sugars such as fructose that are easily fermented to produce lactic acid [
111]. Food waste contains lactic acid bacteria that are easily enriched in MMC fermentation, to produce lactic acid up to concentrations of 21.3 to 48 g
COD L
−1 [
112,
113,
114]. Maximum production using ethanol- and lactic acid-rich streams in MMC fermentation without additional supplementation have been reached using diluted beer (7.52 g
COD L
−1 d
−1 C6) [
46] and acidified whey from the quark industry (5.12 g
COD L
−1 d
−1 C6) [
85]. The disadvantages of two-stage systems for accumulation of electron donors are the increased operational complexity and additional capital and operational costs.
An alternative to supplemented or pre-fermented organic feedstock, would be to operate a single-phase process where the substrate itself is converted to lactic acid and/or ethanol in parallel with the chain elongation reactions. Recently, C6 has been produced in a one-stage LBR from food waste up to concentrations of 21.8 g
COD L
−1 and a rate of 3.12 g
COD L
−1 d
−1 [
91], comparable to a supplemented and/or two-stage system. In this study, batch tests inoculated with the LBR leachate showed that lactic acid was consumed in favour of ethanol as the electron donor [
91]. Other studies aimed at VFA or H
2 production have produced C6 in a single-stage approach from food waste, without supplementation, and at similar production rates. In studies aimed at H
2 production using glucose-rich synthetic wastewater [
115] or simulated food waste [
116] as feedstock, C6 was produced at rates higher than experiments which targeted MCCA production (
Table 3). Therefore, food waste and similar complex substrates might be a promising feedstock.
The use of complex feedstock comes with a set of challenges. Firstly, the composition of the feedstock will influence certain competitive processes and the intermediates that result from hydrolysis and acidogenesis, and therefore affects the potential for chain elongation. For example, VFA yields in MMC fermentation have shown to be less for lipid-rich waste compared to carbohydrate- or protein-rich wastes, due to difficulties in hydrolysis [
117]. In addition, the type of carbohydrates [
118] or proteins [
119] in the substrate also influences the metabolic pathways. Secondly, it is important to recognise that seasonal and geographical variation in organic waste streams impacts composition and availability of feedstock. This can often become a challenge when designing a suitable treatment or valorisation (bio-)process that is robust and flexible, especially when relying on pure microbial cultures. For instance, OFMSW may contain small quantities of bio-waste from various origins, such as discarded oils, fruits, animal-derived products and more lignocellulose-rich leaves and stems from vegetables, resulting in an overall feedstock comprising both easily biodegradable carbohydrates and proteins as more recalcitrant matter [
120]. It was found that a LBR fed with OFMSW which was richer in green waste had lower production of carboxylic acids compared to OFMSW containing more food waste [
121]. Lastly, not every type of substrate will allow chain elongation to occur. In batch experiments aimed at VFA production, seven types of “food waste” feedstocks were tested and only four produced C6. These were cheese whey, sugarcane molasses, the organic fraction of municipal solid waste, and winery effluent [
122]. Similarly, in sequential batch reactors (SBRs), the fermentation of cheese whey resulted in 0.12 g
COD L
−1 d
−1 C6, while in similar operating conditions the fermentation of sewage sludge produced more total carboxylic acids but very little C6 [
123]. Therefore, the right type of complex feedstock should be selected for MCCA production to be successful—this is not well understood and requires further investigation.
6. The Push towards Chain Elongation by Organic Overloading
For AD applications, organic overloading is defined as the COD loading rate exceeding the degradation capacity of the anaerobic microbiome, leading to accumulation of VFAs and hence a decrease of pH which inhibits methanogens [
152]. For VFA and MCCA production, organic overloading is deliberately employed to promote carboxylic acid accumulation and limit competitive processes. Elevated carboxylic acid concentrations inhibit methanogens, as found, for instance, for C4 where only ~2.4 g
COD L
−1 at pH 6 was shown to inhibit 90% of methanogens in a thermophilic batch fermentation [
130].
The organic load at start-up can be represented by the food-to-microorganisms ratio (F/M). This is defined as the amount of feedstock introduced, expressed as COD or volatile solids (VS), relative to the amount of biomass, estimated as VS or volatile suspended solids (VSS) in the inoculum [
9,
153]. In anaerobic consortia fermenting food waste, organic overloading and VFA accumulation usually occur at start-up with a F/M ratio > 1 gCOD
fed gVS
inoculum−1 and carboxylates accumulate at an optimal F/M ratio of 5 gCOD
fed gVS
inoculum−1 [
9]. As with the acidogenic fermentation of synthetic soft drink wastewater, a F/M ratio of 4.0 gCOD
fed gVSS
inoculum−1 was found to be optimum for C6 production compared to F/M ratios of either 1.6 and 6.4 gCOD
fed gVSS
inoculum−1 [
148].
A positive relationship is found for MCCA production and higher organic loading rates (OLRs) using synthetic or supplemented substrates and pure cultures or MMC [
96]. However, for complex feedstock, whilst the same mechanism would be expected, the relationship between OLR and MCCA production is less straightforward (
Figure 3). When using a synthetic medium or supplementation of electron donors the OLR, expressed in terms of total COD, is directly related to the amount of bio-available substrate. On the contrary, when using complex feedstock, the OLR does not necessarily indicate the presence of chain elongation substrates or anaerobic biodegradable content, which can be converted to chain elongation compounds. Namely, fractions can be present in complex feedstocks that can be chemically oxidised, and thus contribute to the total COD, but are not easily biologically degraded. For instance, bio-waste collected from municipalities had a higher anaerobic biodegradability (90.8 ± 3.7%) compared to food waste collected from a vegetarian restaurant (66.9 ± 6.4%) even though their total COD values were within a similar range, i.e., 337 ± 14 g
COD kg
WW−1 and 303 ± 18 g
COD kg
WW−1 respectively [
17]. MMC fermentation experiments for MCCA products using complex feedstocks have only recently been conducted, and little is known regarding the need for easily biodegradable feedstock in order to exert sufficient OLR to accumulate chain elongation substrates for C6 production. In AD, high-COD substrates such as food waste or food processing by-products result in problematic increased retention times and low throughput, to prevent organic overloading and hence such feedstocks show greater potential for production of MCCAs rather than biogas.
7. Effect of Inoculum on Achieving MCCA
Selection of the appropriate inoculum in biotechnological applications is critical to ensure catalysis of the required bio-conversion process. Cultures used for inoculating reactor experiments with complex substrates for carboxylate production contain a large variety of microorganisms (high richness) and include anaerobic sludges from AD [
147] or wastewater treatment [
133], marine sediments [
107,
129], rumen samples [
30,
40], mixtures of cultures [
155] or microbiomes enriched in chain elongators obtained from lab-scale reactors [
83,
91,
96]. For production of VFAs, H
2 or other MMC fermentation products, the inoculum can be physico-chemically pretreated in order to suppress methanogenesis, e.g., heat shock and/or acid/alkali conditioning [
156,
157]. However, no studies could be found on the effect of inoculum pretreatment on MCCA production specifically. Studies on MMC fermentation towards H
2 or VFAs have shown that the microbial composition was altered and the MMC fermentation product profile shifted depending on inoculum pretreatment method applied [
7,
47,
158,
159]. For chain elongation in particular, Cavalcante et al. [
69] has discussed thermal pretreatment as a potential selective pressure since various chain elongating bacteria have been allocated to the genus
Clostridium, that due to its spore-forming abilities could be selected for by heat shock. The limited knowledge regarding pretreatment of inoculum to select for chain elongation would be worthwhile to further investigate.
Separate inoculation is not always required. Some experiments showed C6 production from organic waste by simply using the endogenous MMC present in the feedstock [
108,
135]. In addition, regardless of the initial inoculum source (i.e., sludge from full-scale AD plant or lab-scale fermentation reactors) microbiomes grown on synthetic substrates with ethanol as the main electron donor are enriched by species closely related to
C. kluyveri due to adaptation over time [
70,
101].
As shown in
Figure 4, for batch experiments, the most commonly used inoculum types are AD sludge or enriched microbiomes, i.e., previously enriched and adapted to chain elongation conditions in the lab. Yields seem to be similar for both types of inoculum, perhaps due to high microbial richness. However, the total MCCA concentration accumulated with an AD inoculum had a 5 times lower upper range than when an adapted microbiome is used, indicating that tolerance towards toxic MCCA concentrations can be developed (
Figure 4).
This tolerance to higher C6 concentrations from acclimatised microbiomes has been recently reported using batch inhibition assays with synthetic media and differently cultured sludges [
160]. The same adaptability hypothesis was used to explain productions of longer MCCA such as C8 [
92]. However, batch experiments inoculated with an enriched microbiome are often operated at a higher organic load that could also enhance MCCA accumulation, and these parameters are inter-connected. From the analysed studies it also appears that bio-augmentation does not improve considerably the MCCA yields, but it does impact on the product concentration with more MCCAs produced. It has been shown that bio-augmentation with
C. kluyveri improves yields and even results in chain elongation up to decanoic acid (C10) [
90]. From the data currently available, inoculum selection could contribute to MCCA specificity, yet further investigations are needed to evaluate the importance of inoculum selection.
9. Reactor Design and the Relation to Retention and Organic Overload
The type of feedstock will influence the choice of fermentation reactor [
8]. In the case of a complex, solid-rich feedstock, such as OFMSW or unprocessed food waste, hydrolysis is rate limiting and stirring or pumping is impractical. Therefore, longer retention times are required to allow hydrolysis and solubilisation, and leach bed reactors (LBRs) are an effective option, as they generate a leachate rich in carboxylic acids. For instance, Yesil et al. [
172] obtained ± 30 g
COD of carboxylic acids per kg of solid waste of which approx. 10% was C6, in batch LBR. Nzeteu et al. [
91] used a LBR set-up that allowed semi-continuous operation and obtained a maximum C6 production rate (3.12 g
COD L
−1 d
−1) from food waste by replacing 75% of the reactor content with fresh feedstock and diluting the leachate with water by 1/15 every 7 days. However, this approach does not easily allow operation with homogenous pH or temperature stability. Another study has shown that operation stability for the production of H
2 by MMC fermentation of food waste is enhanced by mixing and agitation [
116], however, this has not yet been determined for chain elongation.
To exert better process control, complex feedstock can be mechanically pre-treated, i.e., crushing, chopping or blending, to obtain a mixture that can be pumped and stirred, and therefore allows the use of (semi-)continuous stirred tank reactors (sCSTRs). This has been done in some studies focussing on carboxylic acid production from food waste [
125,
126]. In addition, the feed stream can be diluted with water, or blended with a liquid waste stream or recycled liquor from sludge dewatering to modify the composition. For a more fluid stream such as synthetic feedstock, e.g., ethanol and acetate mixtures, or more easily degradable substrates such as potato-processing or brewery wastewater or food waste leachate, less hydrolysis is required and the chain elongation itself becomes rate-limiting. To allow higher flow rates, whilst maintaining high biomass to counter the rate-liming effect of chain elongation, reactor configurations such as membrane bioreactors [
169], CSTRs with in situ settlers [
128] and up-flow reactors (UR) with mechanisms for biomass retention, e.g., filter, sludge blankets or packed beds, have been used (
Table 3). It has been suggested that biomass retention and cell density are important parameters which are often unreported for MMC fermentation [
95]. Indeed, studies using reactors with biomass retention have shown the highest MCCAs production rates so far (
Figure 3). Such reactors are well established for other processes, and are worthy of investigation.
In continuous systems, the inoculum acclimation and biomass retention, also expressed as sludge retention time (SRT), favour specific microbial populations. For instance, C4 producers have often been shown to have a longer doubling time than lactic acid producers, and hence wash out more easily from continuous systems [
47]. In stirred tank reactors without a settling phase, the SRT is equal to the hydraulic retention time (HRT) and inversely proportional to the OLR. Therefore, in order to operate at a sufficient biomass retention, the OLR can only increase with an increase of substrate COD composition. A suboptimal residence time results in lactic acid or VFA accumulation without chain elongation, and a reduction in hydrolysis [
97,
128]. In fermentation of salad and vegetable waste in a sCSTR, a retention time of 10 days left a fraction of the substrate unutilized and no C6 was produced; whilst retention time of 20 and 30 days increased C6 production [
126]. Similarly, a step-wise decrease of HRT from 20 to 12 to 8 days in cheese whey fermentation, generated C6 concentrations which decreased from 2.24 g
COD L
−1 to 1.45 g
COD L
−1 to 0.41 g
COD L
−1. Analysis of the microbiome composition revealed certain microbial groups were removed by lowering the HRT, via washout, with a dominant presence of lactic acid-producing
Lactobacillus sp. at HRT of 8 days [
123]. Thus, a minimum HRT is required to sustain chain elongation when working with reactors without biomass retention. However, if the HRT is too high competing processes such as methane production are more favoured [
36,
126]. Methanogens are relatively slow growers, thus reducing HRT has been suggested as a tactic to wash them out, and increase MCCA production rates [
72,
106]. Therefore, a compromise to reduce methanogens and enhance chain elongation must be found.
Studies on chain elongation have varied HRT from less than 1 day to over 2 weeks resulting in various MCCA production yields (
Figure 5). A lower HRT can generate similar yields by altering reactor design to include biomass retention and hence decoupling the HRT from the SRT. Using an UR with biomass retention, chain elongation was performed using a HRT of only 4 h, resulting in a maximum MCCA production rate of 57.4 g L
−1 d
−1 using a synthetic ethanol and C2 feed supplemented with methanogenic inhibitors, yeast extract and CO
2 at neutral pH [
105]. In addition, increasing HRT reduces product toxicity by eliminating accumulation, e.g., by gradually reducing HRT from 20 to 2.5 days C6 production from cheese whey rate increased to ~5 g
COD L
−1 d
−1 [
100].
It is important to note that other operational factors come in to play alongside HRT. For instance, increasing HRT from 8 to 12 days and operating at 35 °C increased C6 concentrations in food waste fermentation from approx. 1.64 g
COD L
−1 to 6.55 g
COD L
−1, and even more C6 (10.26 g
COD L
−1) was obtained at a HRT of 8 days by operating at 45 °C [
125]. Therefore, the optimal HRT to stimulate chain elongation in complex feedstock fermentations will vary according to the type of system used, as it strongly depends on the reactor configuration, hydrolysis rate, sludge retention time, and other operational parameters such as pH and temperature.
10. Overcoming Product Toxicity by In Situ Extraction, Biofilm Formation or Acclimation
Low MCCA concentrations in the fermentation broth result in poor product recovery and high cost of down-stream processing. MCCA concentration can be limited in MMC fermentation for three reasons: (i) the substrate is poor in electron donors or in easily biodegradable organics, and hence prevents in situ substrate accumulation to drive chain elongation; (ii) product accumulation lowers the thermodynamic favourability of chain elongation; and (iii) the antimicrobial properties of MCCAs in their protonated state can result in product toxicity. To evaluate which cause is limiting MCCA production, Weimer et al. [
40] measured residual substrate and product concentrations and calculated ΔG for C6 production. Incomplete substrate consumption in their MMC fermentation study enriched with
C. kluyveri and ethanol-supplemented lignocellulosic feedstock still gave a negative ΔG, thus suggesting product toxicity and/or limited incubation time had prevented further MCCA production [
40]. Toxicity limits can be offset by employing in situ extraction, or biofilm formation and/or acclimation of the MMC.
In situ extraction methods have the advantage of continuously removing carboxylic acids from the fermentation broth, thereby alleviating product toxicity and thermodynamic constraints [
130]. Various in situ extraction systems for carboxylic acids have been proposed for pure culture and MMC fermentation (
Table 6). Electrochemical extraction has been applied to recover C2 to C6 from stillage fermentation, and has shown to simultaneously control pH and stimulate chain elongation by OH
− and H
2 production at the cathode [
23]. However, for MMC fermentation of a complex substrate, a MCCA-selective extraction method is preferred to maintain low MCCA concentrations, whilst VFAs remain in the fermentation broth as substrates for chain elongation. Pertraction has been used in various MMC studies as an in situ extraction method selective for MCCA. This is an in-line liquid-liquid, membrane-assisted extraction method driven by a pH-gradient, and is usually performed with mineral oil containing a phase transfer catalyst, e.g., TOPO, and an alkaline recovery phase [
37,
77,
93,
96,
130]. Pertraction can be combined with membrane electrolysis to drive further separation and obtain a MCCA-rich oil [
174]. Whilst the majority of in situ extraction studies report enhanced production rates and chain elongation, two studies did not report a significant improvement [
62,
155]. The advantages of implementing in situ extraction systems must outweigh the increased complexity and cost in process operation.
Recently, different strategies to overcome product toxicity have been suggested. Allowing biofilms and microscale aggregates to develop improves interactions within a microbiome and tolerance to toxic compounds [
175]. Addition of 2 g L
−1 biochar to a UR fed with synthetic ethanol and C2 significantly improved MCCA production and reduced by-product formation [
102]. In this case, microscopic observations revealed the community structure and the spatial distribution of microorganisms changed to dense microbial aggregates around the biochar; it was postulated this improved cell-cell interactions and energy efficiency via stabilising relationships between trophic partners, and increased tolerance to product toxicity [
102]. Formation of microbial aggregates has been noted for bioreactors without providing a specific means of biofilm formation; granules were formed in a C3 and ethanol fed CSTR producing C7 as chain elongation product [
173]. Therefore, reactor configuration and feedstock that allow microbial aggregates are expected to improve chain elongation, yet research on this is limited. In addition, recent research found the MCCA concentration in the fermentation broth influenced the microbial community structure. It has been suggested that elevated C6 and C8 concentrations lead to a more acclimatized and resistant MMC [
92,
169]. A microbiome adapted to operating at elevated C6 concentrations had a 10 times higher productivity in an environment with elevated C6 (33 g
COD L
−1) [
160]. Further development of a resistant, highly productive MMC would allow accumulation of MCCA resulting in less complex extraction methods with reduced economic burden due to downstream processing.
11. MMC Fermentation Scale up and Integration within a Bio-Refinery Context
Biological MCCA production is mostly in the experimental phase, but some scale-up has also been studied. For example, Hegner et al. [
101] showed MCCA-producing MMC can be scaled up from 0.11 L serum bottles to 2.2 L bioreactors by maintaining similar reactor operation and without loss of performance or a change in microbial composition. Pilot scale projects are now being started. For instance, the MixAlco
TM process has been operated in four parallel 3.78 m
3 scale fed-batch fermenters processing chicken manure, urea and shredded paper to produce a mixture of carboxylate salts from processed fermentation effluent (containing approx. 6.8 g
COD L
−1 C6) in an 11-month time period as precursors for jet fuel and gasoline [
179]. The first start-ups and university spin-offs using MMC fermentation for production of MCCA and other bio-based standard chemicals as starting to appear, such as ChainCraft B.V. in the Netherlands [
180].
In order to increase the potential of food waste as a feedstock for production of renewable chemicals, MCCA-producing MMC fermentation can be integrated within a bio-refinery. The term bio-refinery, defined as “the sustainable processing of biomass into a spectrum of marketable products and energy”, is inspired by traditional oil refineries where biomass replaces fossil fuels as feedstock for coproducing chemicals and power through various conversion technologies [
181]. Process integration allows production of various compounds such as fuels, chemicals, solvents, biomaterials, food and feed ingredients, fibres and heat and power, thus increasing resilience and robustness against market price fluctuations while minimizing waste [
182]. Using bio-waste as the renewable biomass feedstock, known as the 3rd generation bio-refinery concept, not only allows replacement of fossil fuel sources with a renewable alternative, but also stabilises waste streams with maximal use of resource, thus contributing to a circular economy [
15]. Integration into a bio-refinery concept could include mechanical pre-treatment of waste streams to obtain pumpable mixtures, or pre-fermentation steps to obtain streams rich in lactic acid or ethanol. The combination of physical and biological processes for organic waste valorisation including MCCA will favour a myriad of product and energy goods that are market competitive (
Table 7). Agler et al. gave an overview of bio-, thermo-, or electro-chemical post-processes, to convert carboxylic acids from fermentation into carbonyls, esters, alcohols or alkanes applicable as bulk fuels, solvents [
26].
12. Conclusions
MCCAs, such as caproic and caprylic acid, are compounds of interest due to their broad range of potential applications. In contrast to chemical or single-culture biotechnological processes, using the consorted action of MMC allows to produce MCCA from complex organic feedstocks, such as food waste, in open, non-sterile systems via the natural process of chain elongation. However, the yields, concentrations and selectivity of this process must be improved in order to increase its viability. Therefore, we have summarised the current knowledge on the underlying mechanism of chain elongation by MMC, discussed the current state of the art on the use of complex organic feedstock and reviewed key operational parameters, and their interactions.
Some of the key findings lie with the fact that with complex substrates and microbial cultures, there must be a greater emphasis on managing competing reactions and positively selecting for chain elongation microbiomes. Since the microbial diversity of MMC ecosystems has been shown to be distinct from pure cultures and clean substrates, existing thermodynamic and kinetic models should be expanded to include complex feedstock and mixed cultures. Advances in microbial culture analysis, such as improved implementation of various “-omics” methods on complex samples, will boost current understanding of MMC fermentation.
Most common complex feedstocks trialled so far include residues from the bio-ethanol and dairy industries, different types of cellulosic wastes, syngas fermentation effluent and different types of organic food waste. These type of feedstocks have resulted in maximum production rates up to 8.02 gCOD L−1 d−1. Supplementation of complex waste-derived feedstock with chain elongation substrates such as ethanol increased production rates, with maxima reported up to 62.8 gCOD L−1 d−1. However, the negative environmental effects from chemical addition have also been reported. The use of synthetic substrates allowed production rates up to 115.2 gCOD L−1 d−1.
Through an extensive review of the literature, including studies targeting MCCAs or reporting MCCAs as by-products, various key operational parameters were identified and discussed to highlight the research gaps. Mesophilic temperatures are so far a preferred choice for chain elongation, yet there is little justification for this. The preferred operational pH seems to lie in a slight acidic range from pH 5 to 7, in order to limit the activity of methanogens. The relationship between organic loading rate (OLR) and MCCA production rates showed a positive correlation to some extent, however this is complicated by the degree of biodegradability of the feedstock. Linked to the organic load is the substrate-inoculum ratio (F/M) at the start-up of the process which favours the accumulation of intermediate compounds instead of methane production when F/M > 5. In addition, whilst increased OLR tends to improve chain elongation, this must be coupled with sufficiently long residence times and biomass retention. OLR and retention times will have to be optimized depending on whether the reactor design has included mechanisms for biomass retention, and the biodegradability of the feedstock.
The literature study revealed very little information is available on some specific operational parameters that have been studied for other MMC applications. For example, in similar MMC fermentation processes a minimal alkalinity was beneficial to stabilise the process and reduce the need for pH controlling agents. However, the buffer capacity required to stimulate chain elongation has not been thoroughly investigated. The partial pressures of CO2 and H2 in the reactor headspace have been identified to influence chain elongation, however production of these gases during fermentation, and their accumulation in reactor headspace is rarely considered. In order to circumvent the antimicrobial limitations imposed by MCCAs on the microbiome, in situ extraction is often proposed, but the alternative strategies which promote the development of biofilm or granule formation, and MMC adaptation, are worthy of further research. Finally, the development of down-stream processing methods, and integration within a bio-refinery context, are crucial issues to transform MCCA production from organic waste streams into a competitive waste valorisation technology that will contribute to the development of a circular economy.