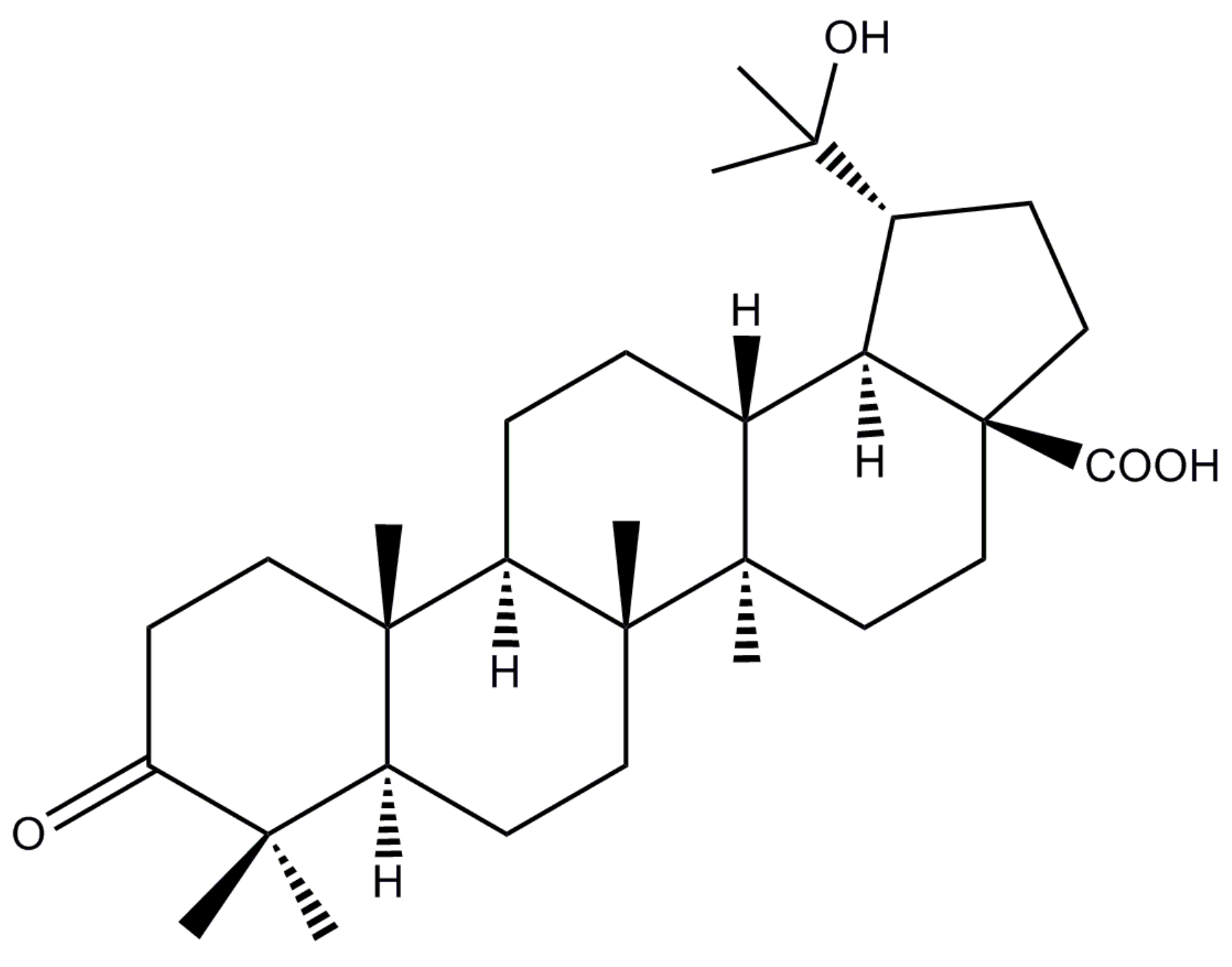
20-Hydroxy-3-Oxolupan-28-Oic Acid Attenuates Inflammatory Responses by Regulating PI3K–Akt and MAPKs Signaling Pathways in LPS-Stimulated RAW264.7 Macrophages
Abstract
1. Introduction
2. Results
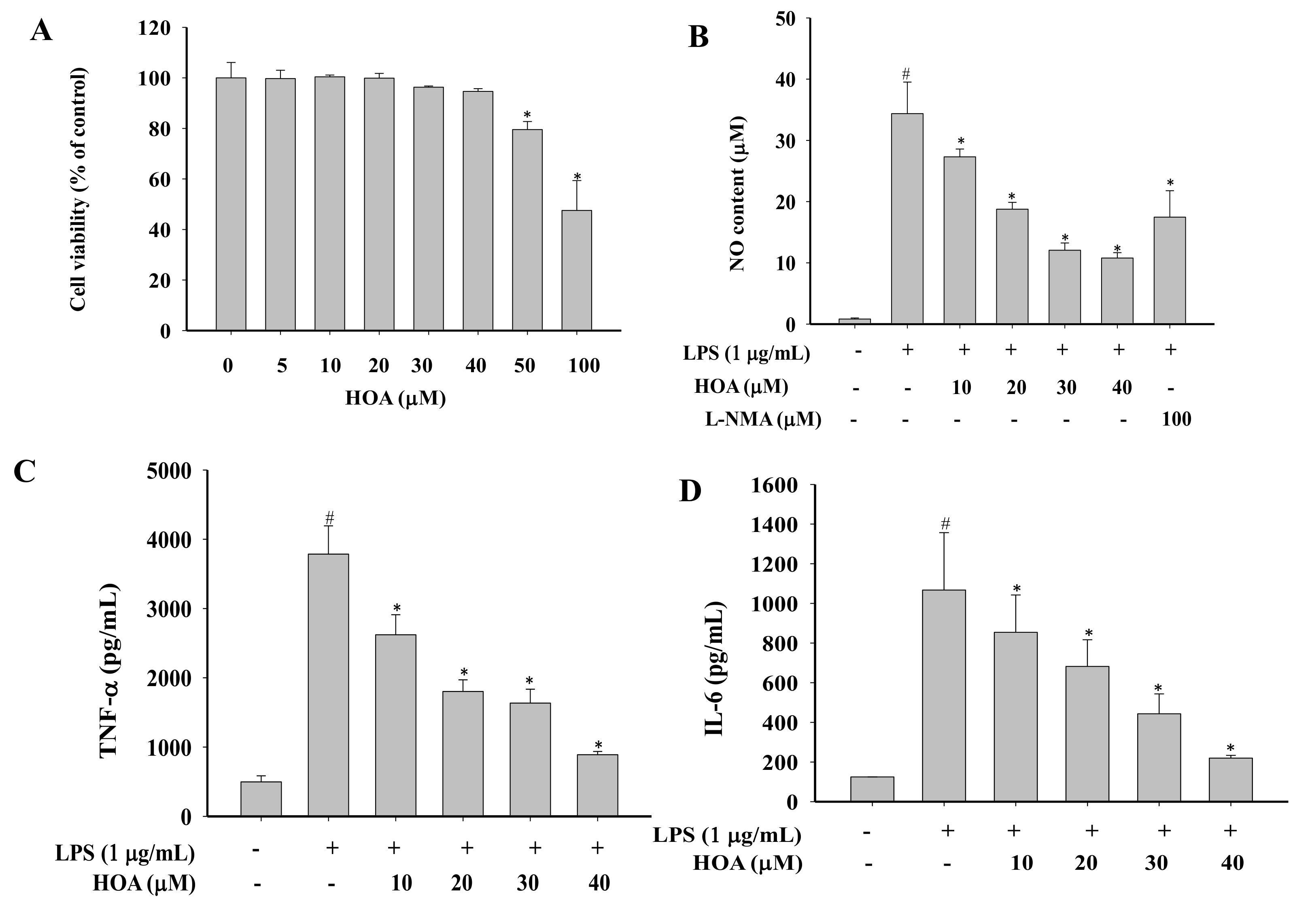
2.1. Effects of HOA on the Viability of RAW264.7 Cells
2.2. Effect of HOA on NO Production and Pro-Inflammatory Cytokine Production in LPS-Stimulated RAW264.7 Cells
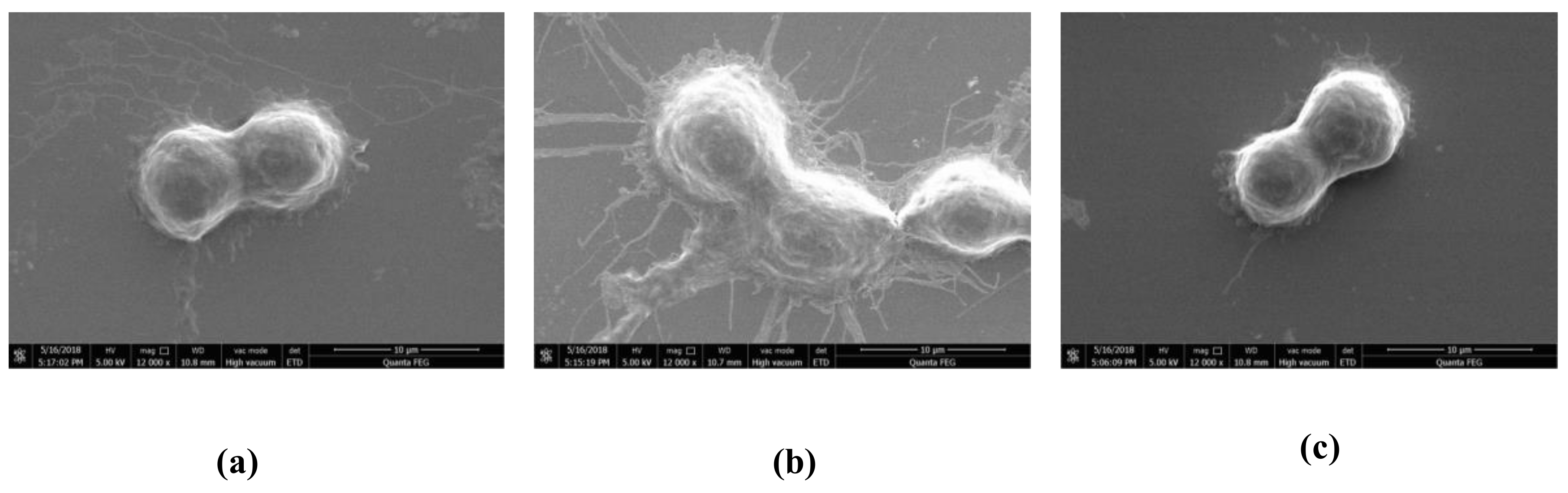
2.3. Effect of HOA on Morphology of LPS-Stimulated RAW264.7 Cells
2.4. Effect of HOA on Expression of Pro-Inflammatory Cytokines in LPS-Stimulated RAW264.7 Cells
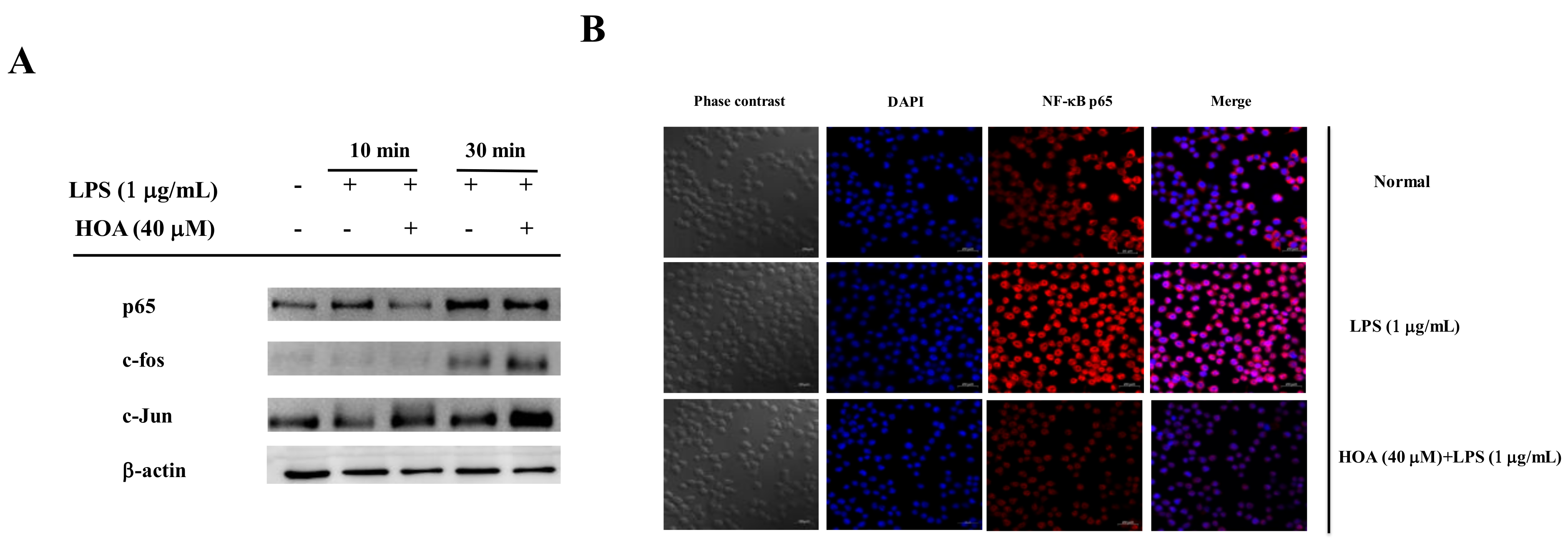
2.5. Effects of HOA on the Regulation of Transcription Factors and Its Upstream Signalling Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Cell Cytotoxicity Assay
4.3. Determination of NO, TNF-α, and IL-6 Content
4.4. Scanning Electron Microscopy (SEM)
4.5. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-qPCR) Analysis
4.6. Western Blot Analysis
4.7. Immunofluorescence and Confocal Microscopy
4.8. Transfections and Luciferase Assay
4.9. Data Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Branzk, N.; Gronke, K.; Diefenbach, A. Innate lymphoid cells, mediators of tissue homeostasis, adaptation and disease tolerance. Immunol. Rev. 2018, 286, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Belkai, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signalling pathway: Pivotal roles in inflammation. Bba-Mol. Basis Dis. 2016, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Lavieri, R.; Rubartelli, A.; Carta, S. Redox stress unbalances the inflammatory cytokine network: Role in autoinflammatory patients and healthy subjects. J. Leukoc. Biol. 2016, 99, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C. Inflammatory response of macrophages in infection. HBPD Int. 2014, 13, 138–152. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2017, 223, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signalling pathways. Cell. Signal. 2013, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mu, Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 175, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, L.; Qiang, Q.; Ji, L.; Wang, X.; Luo, H.; Wu, H.; Jiang, H.; Wang, G.; Shen, T. The dichloromethane fraction from Mahonia bealei (Fort.) Carr. leaves exerts an anti-inflammatory effect both in vitro and in vivo. J. Ethnopharmacol. 2016, 188, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, W.; Zhang, Y.; Yang, B.; Fu, Z.; Li, X.; Tian, J. Proteomics analysis of Mahonia bealei leaves with induction of alkaloids via combinatorial peptide ligand libraries. J. Proteom. 2014, 110, 59–71. [Google Scholar] [CrossRef]
- Hu, W.; Yu, L.; Wang, M.H. Antioxidant and antiproliferative properties of water extract from Mahonia bealei (Fort.) Carr. Leaves. Food Chem. Toxicol. 2011, 49, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Dong, Y.; Sheng, G.; Dong, X.; Sun, X.; Fu, J. Isolation and structure determination of anti-influenza component from Mahonia bealei. J. Ethnopharmacol. 2006, 108, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Li, H.; He, X.; Zhang, R.Q.; Sun, Y.H.; Zhang, C.F.; Wang, C.Z.; Yuan, C.S. Alkaloids from Mahonia bealei posses anti-H+/K+-ATPase and anti-gastrin effects on pyloric ligation-induced gastric ulcer in rats. Phytomedicine 2014, 21, 1356–1363. [Google Scholar] [CrossRef]
- Kohoutková, M.; Korimová, A.; Brázda, V.; Kohoutek, J. Early inflammatory profiling of schwannoma cells induced by lipopolysaccharide. Histochem. Cell Biol. 2017, 148, 607–615. [Google Scholar] [CrossRef]
- Kai, K.; Zhou, X.; Wong, H.L.; Ng, C.F.; Fu, W.M.; Leung, P.C.; Peng, G.; Ko, C.H. In vivo and in vitro anti-inflammatory effects of Zao-Jiao-Ci (the spine of Gleditsia sinensis Lam.) aqueous extract and its mechanisms of action. J. Ethnopharmacol. 2016, 192, 192–200. [Google Scholar]
- Liu, L.; Chen, L.; Jiang, C.; Xie, Y.; Cheng, Z. Berberine inhibits the LPS-induced proliferation and inflammatory response of stromal cells of adenomyosis tissues mediated by the LPS/TLR4 signalling pathway. Exp. Ther. Med. 2017, 14, 6125. [Google Scholar] [CrossRef]
- Cuadrado, I.; Amesty, A.; Cedrón, J.C.; Oberti, J.C.; Estévez-Braun, A.; Hortelano, S.; Heras, B. Semisynthesis and Inhibitory Effects of Solidagenone Derivatives on TLR-Mediated Inflammatory Responses. Molecules 2018, 23, 3197. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.J.; Jung, U.; Eom, H.S.; Shin, H.J.; Park, H.R. Inhibition of Lipopolysaccharide-Induced Proinflammatory Responses by Buddleja officinalis Extract in BV-2 Microglial Cells via Negative Regulation of NF-κB and ERK1/2 Signalling. Molecules 2013, 18, 9195–9206. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9’-fucoxanthinone via inhibition of MAPKs and NF-κB signalling pathway in LPS-stimulated RAW264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Min, L.; Wei, J.; Gou, H.; Bao, Z.; Wang, Z.; Huang, Y.; An, B. Heliangin inhibited lipopolysaccharide-induced inflammation through signalling NF-κB pathway on LPS-induced RAW264.7 cells. Biomed. Pharmacother. 2017, 88, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Alves, C.Q.; Brandão, H.N.; David, J.P.; Silva, R.L.; Franchin, M.; Cunha, T.M.; Martins, F.T.; Oliveirra, C.M. Poligalen, a new coumarin from Polygala boliviensis, reduces the release of TNF and IL-6 independent of NF-κB downregulation. Fitoterapia 2016, 113, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhao, Y.; Guo, X.; Liu, J. Chiisanoside, a triterpenoid saponin, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis. RSC Adv. 2017, 7, 41640–41650. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, H.; Li, Z.H.; Wang, G.K.; Feng, T.; Liu, J.K. Anti-inflammatory lupane triterpenoids from Menyanthes trifoliata. J. Asian Nat. Prod. Res. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mabhida, S.E.; Dludla, P.V.; Johnson, R.N.; Ndlovu, M.L.; Johan, O.; Andy, R.M.; Rebamang, A. Protective effect of triterpenes against diabetes-induced β-cell damage: An overview of in vitro and in vivo studies. Pharmacol. Res. 2018, 137, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Zhang, Z.; Karin, M.; Beyaert, R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017, 542, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic Res. 2018, 52, 507–543. [Google Scholar] [CrossRef]
- Kipanyula, M.J.; Etet, P.F.S.; Vecchio, L.; Farahna, M.; Nukenine, E.; Kamdje, A.H.N. Signalling pathways bridging microbial-triggered inflammation and cancer. Cell. Signal. 2013, 25, 403–416. [Google Scholar] [CrossRef]
- Takano, K.; Ishida, N.; Kawabe, K.; Moriyama, M.; Hibino, S.; Choshi, T.; Hori, O.; Nakamura, Y. A dibenzoylmethane derivative inhibits lipopolysaccharide-induced NO production in mouse microglial cell line BV-2. Neurochem. Int. 2018, 119, 126–131. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Stadler, M.; Chen, H.P.; Huang, Y.; Gan, X.Q.; Feng, T.; Liu, J.K. Lanostane triterpenoids from Tricholoma pardinum with NO production inhibitory and cytotoxic activities. Phytochemistry 2018, 152, 105–112. [Google Scholar] [CrossRef]
- Cao, T.; Tran, M.H.; Kim, J.A.; Tran, P.T.; Lee, J.H.; WOO, M.H.; Lee, H.K.; Min, B.S. Inhibitory effects of compounds from Styrax obassia on NO production. Bioorg. Med. Chem. Lett. 2015, 25, 5087–5091. [Google Scholar] [CrossRef]
- Valilou, S.F.; Keshavarz-Fathi, M.; Silvestris, N.; Argentiero, A.; Rezaei, N. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev. 2018, 39, 46–61. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, C.; Chen, J.; Zhao, G. Different levels of pro- and anti-inflammatory cytokines in patients with unipolar and bipolar depression. J. Affect. Disord. 2018, 237, 65–72. [Google Scholar] [CrossRef]
- Naseem, S.; Hussain, T.; Manzoor, S. Interleukin-6: A promising cytokine to support liver regeneration and adaptive immunity in liver pathologies. Cytokine Growth Factor Rev. 2018, 39, 39–45. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signalling pathway by poly Anti-inflammatory potential of hentriacontane in LPS stimulated RAW264.7 cells and mice model phenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Chong, C.; Ai, N.; Ke, M.; Tan, Y.; Huang, Z.; Li, Y.; Lu, J.H.; Ge, W.; Su, H. Roles of Nitric Oxide Synthase Isoforms in Neurogenesis. Mol. Neurobiol. 2018, 55, 2645–2652. [Google Scholar] [CrossRef]
- Genovese, S.; Taddeo, V.A.; Fiorito, S.; Epifano, F.; Marrelli, M.; Conforti, F. Inhibition of nitric oxide production by natural oxyprenylated coumarins and alkaloids in RAW264.7 cells. Phytochem. Lett. 2017, 20, 181–185. [Google Scholar] [CrossRef]
- Khajuria, V.; Gupta, S.; Sharma, N.; Kumar, A.; Lone, N.A.; Khullar, M.; Dutt, P.; Sharma, P.R.; Bhagat, A.; Ahmed, Z. Anti-inflammatory potential of hentriacontane in LPS stimulated RAW264.7 cells and mice model. Biomed. Pharmacother. 2017, 92, 175–186. [Google Scholar] [CrossRef]
- Han, H.; Shin, J.; Lee, S.; Park, J.C.; Lee, K.T. Cirsimarin, a flavone glucoside from the aerial part of Cirsium japonicum var. ussuriense (Regel) Kitam. ex Ohwi, suppresses the JAK/STAT and IRF-3 signalling pathway in LPS-stimulated RAW264.7 macrophages. Chem. Biol. Interact. 2018, 293, 38–47. [Google Scholar] [CrossRef]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Lee, S.; Lee, W.S.; Shin, J.; Jang, D.S.; Lee, K.T. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW264.7 macrophages. Int. Immunopharmacol. 2017, 49, 21–29. [Google Scholar] [CrossRef]
- Chen, C.; Peng, W.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Jung, D.H.; Park, H.J.; Byun, H.E.; Park, Y.M.; Kim, T.W.; Kim, B.O.; Um, S.H.; Pyo, S. Diosgenin inhibits macrophage-derived inflammatory mediators through downregulation of CK2, JNK, NF-κB and AP-1 activation. Int. Immunopharmacol. 2010, 10, 1047–1054. [Google Scholar] [CrossRef]
- Kwon, D.J.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-κB and AP-1 activation in RAW264.7 macrophages. Food Chem. Toxicol. 2013, 58, 479–486. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Shin, J.M.; Bae, Y.S.; Cho, H.J.; Park, K.K.; Choe, J.Y.; Han, S.M.; Moon, S.K.; Kim, W.J.; Choi, Y.H.; et al. Melittin has a chondroprotective effect by inhibiting MMP-1 and MMP-8 expressions via blocking NF-κB and AP-1 signalling pathway in chondrocytes. Int. Immunopharmacol. 2015, 25, 400–405. [Google Scholar] [CrossRef]
- Paul, A.; Edwards, J.; Pepper, C.; Mackay, S. Inhibitory-κB Kinase (IKK) α and Nuclear Factor-κB (NF-κB)-Inducing Kinase (NIK) as anti-cancer drug targets. Cells 2018, 7, 176. [Google Scholar] [CrossRef]
- Courtois, G.; Fauvarque, M.O. The many roles of ubiquitin in NF-κB signaling. Biomedicines 2018, 6, 43. [Google Scholar] [CrossRef]
- Durham, W.J.; Li, Y.P.; Gerken, E.; Eric, G.; Farid, M.; Arbogast, S.; Wolfe, R.R.; Reid, M.B. Fatiguing exercise reduces DNA binding activity of NF-kappaB in skeletal muscle nuclei. J. Appl. Physiol. 2004, 97, 1740–1745. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Aksoylar, H.I.; Horng, T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin. Immunol. 2015, 27, 286–296. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, M.Y.; Cho, J.Y. Syk and Src-targeted anti-inflammatory activity of aripiprazole, an atypical antipsychotic. Biochem. Pharmacol. 2018, 148, 1–12. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.C.; Choi, J.S.; Byun, D.S.; Utstuki, T.; Ingram, D.; Kim, H.R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, J.H.; Park, H.I.; Sung, J.M.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Clin. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef]
- Ku, K.Y.; Huang, Y.L.; Huang, Y.J.; Chiou, W.F. Miyabenol A Inhibits LPS-Induced NO Production via IKK/IκB Inactivation in RAW 264.7 Macrophages: Possible Involvement of the p38 and PI3K Pathways. J. Agric. Food Chem. 2008, 56, 8911–8918. [Google Scholar] [CrossRef]
- EL-Gamal, A. Cytotoxic lupane secolupane and oleanane type triterpenes from Viburnum awabuki. Nat. Prod. Res. 2008, 22, 191–197. [Google Scholar] [CrossRef]
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Zhao, X.; Si, C.L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-d-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signalling pathways in RAW264.7 macrophages and protects mice against endotoxin shock. Food Funct. 2016, 7, 1002–1013. [Google Scholar] [CrossRef]
- Bai, Y.; Jiang, Y.; Liu, T.; Li, F.; Zhang, J.; Luo, Y.; Zhang, L.; Yan, G.; Feng, Z.; Li, X.; et al. Xinjiang herbal tea exerts immunomodulatory activity via TLR2/4-mediated MAPK signalling pathways in RAW264.7 cells and prevents cyclophosphamide-induced immunosuppression in mice. J. Ethnopharmacol. 2019, 228, 179–187. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Gene Name | Primer Squence (5′-3′) |
|---|---|
| iNOS | F: CATTGATCTCCGTGACAGCC |
| R: CATGCTACTGGAGGTGGGTG | |
| IL-6 | F: TGGGACTGATGCTGGTGACAAC |
| R: AGCCTCCGACTTGTGAAGTGGT | |
| TNF-α | F: TGCCTATGTCTCAGCCTCTTC |
| R: GAGGCCATTTGGGAACTTCT | |
| GAPDH | F: CACTCACGGCAAATTCAACGGCACA |
| R: GACTCCACGACATACTCAGCAC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Li, F.; Luo, Y.; Zhang, L.; Lu, S.; Xing, R.; Yan, B.; Zhang, H.; Hu, W. 20-Hydroxy-3-Oxolupan-28-Oic Acid Attenuates Inflammatory Responses by Regulating PI3K–Akt and MAPKs Signaling Pathways in LPS-Stimulated RAW264.7 Macrophages. Molecules 2019, 24, 386. https://doi.org/10.3390/molecules24030386
Cao Y, Li F, Luo Y, Zhang L, Lu S, Xing R, Yan B, Zhang H, Hu W. 20-Hydroxy-3-Oxolupan-28-Oic Acid Attenuates Inflammatory Responses by Regulating PI3K–Akt and MAPKs Signaling Pathways in LPS-Stimulated RAW264.7 Macrophages. Molecules. 2019; 24(3):386. https://doi.org/10.3390/molecules24030386
Chicago/Turabian StyleCao, Yufeng, Fu Li, Yanyan Luo, Liang Zhang, Shuya Lu, Rui Xing, Bingjun Yan, Hongyin Zhang, and Weicheng Hu. 2019. "20-Hydroxy-3-Oxolupan-28-Oic Acid Attenuates Inflammatory Responses by Regulating PI3K–Akt and MAPKs Signaling Pathways in LPS-Stimulated RAW264.7 Macrophages" Molecules 24, no. 3: 386. https://doi.org/10.3390/molecules24030386
APA StyleCao, Y., Li, F., Luo, Y., Zhang, L., Lu, S., Xing, R., Yan, B., Zhang, H., & Hu, W. (2019). 20-Hydroxy-3-Oxolupan-28-Oic Acid Attenuates Inflammatory Responses by Regulating PI3K–Akt and MAPKs Signaling Pathways in LPS-Stimulated RAW264.7 Macrophages. Molecules, 24(3), 386. https://doi.org/10.3390/molecules24030386







