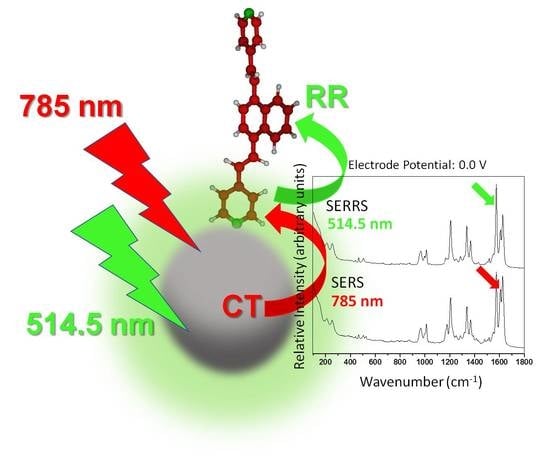
Intramolecular and Metal-to-Molecule Charge Transfer Electronic Resonances in the Surface-Enhanced Raman Scattering of 1,4-Bis((E)-2-(pyridin-4-yl)vinyl)naphthalene
Abstract
:1. Introduction
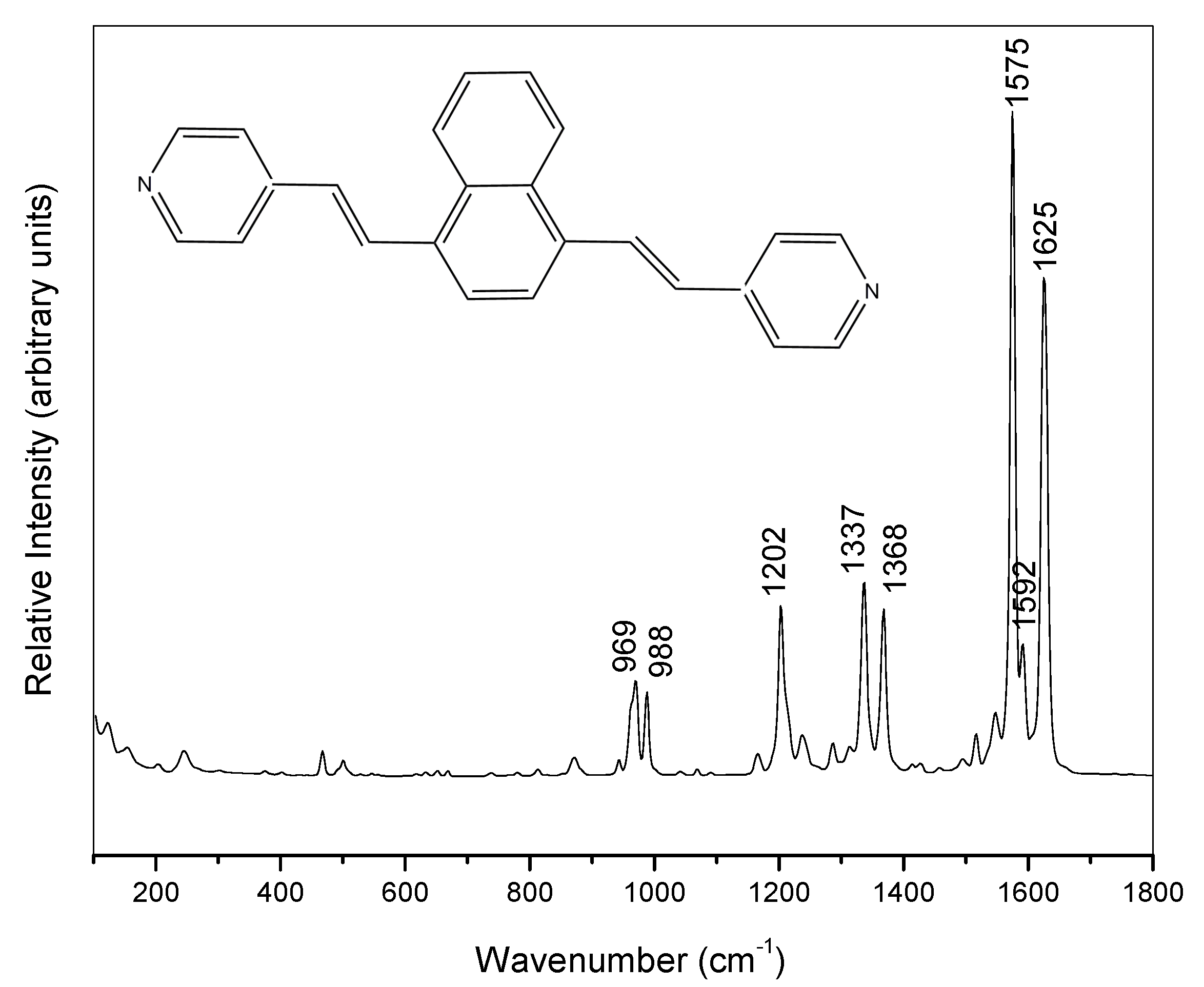
2. Results and Discussion
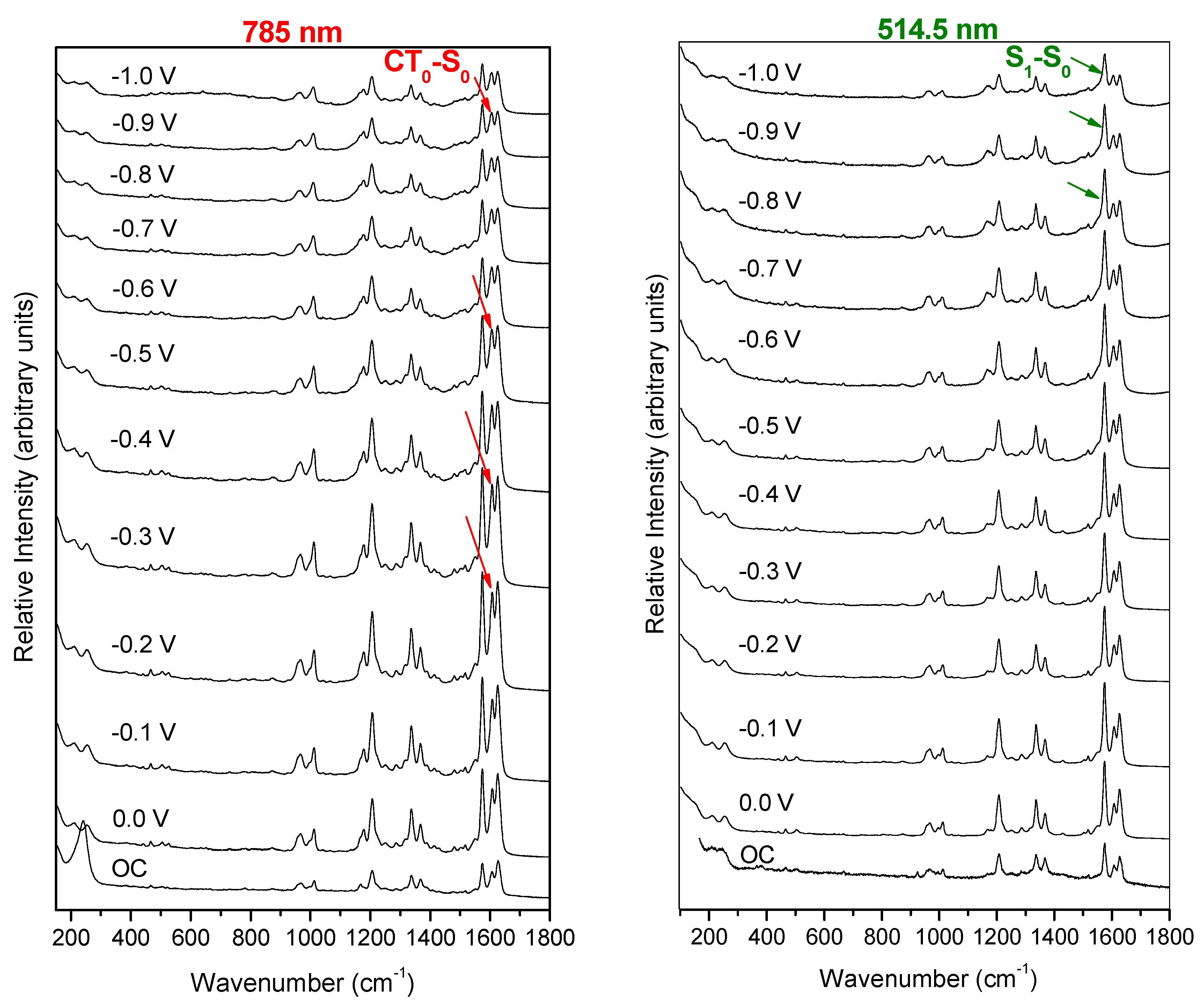
2.1. Electrochemical SERS Spectra
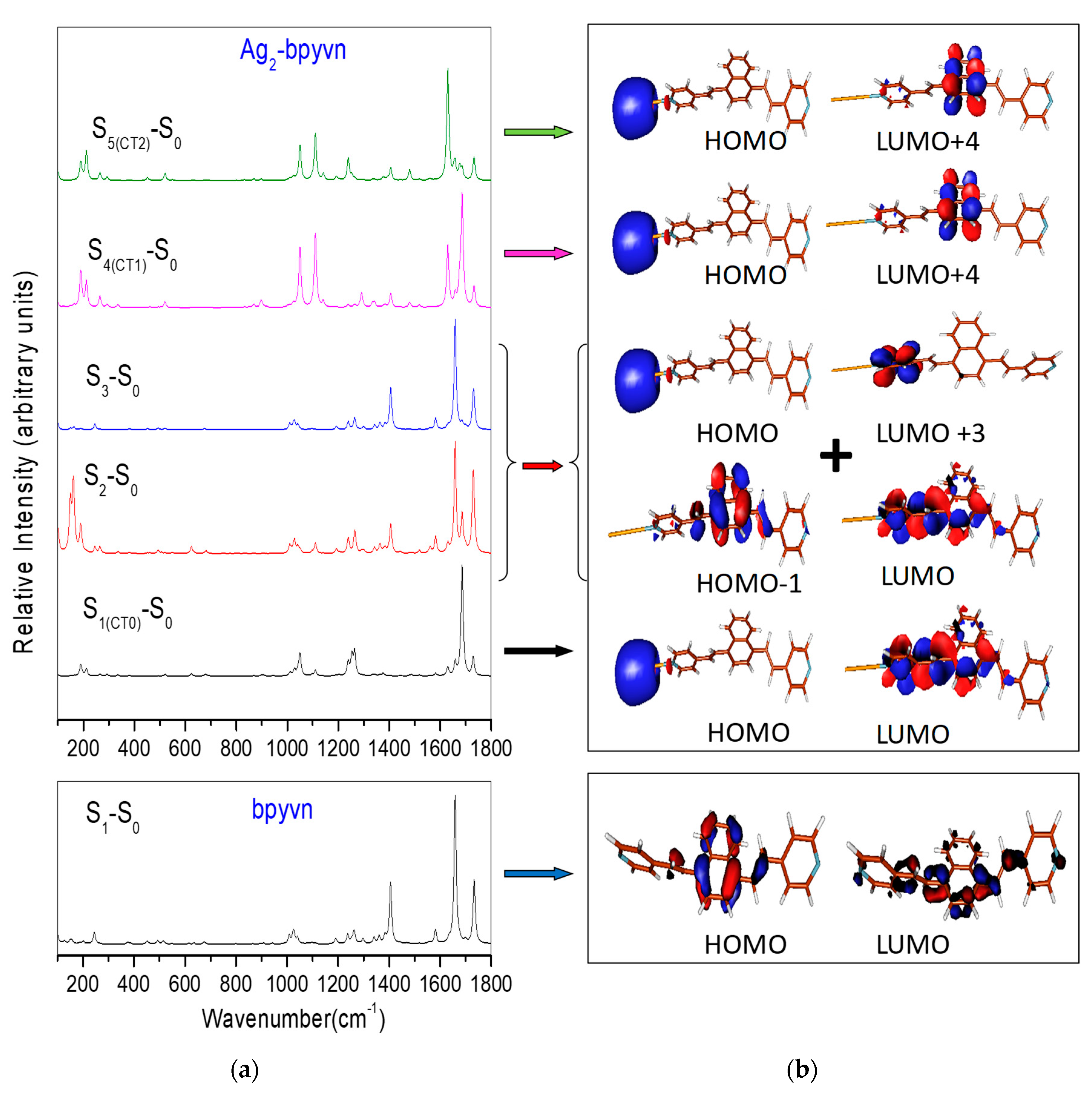
2.2. Electronic Structure of bpyvn and Ag2-bpyvn Complex
2.3. Theoretical Resonance Raman Spectra
3. Conclusions
4. Materials and Methods
4.1. Experimental Section
4.1.1. Synthesis of 1,4-bis((E)-2-(pyridin-4-yl)vinyl)naphthalene, (bpyvn)
4.1.2. Electrochemical SERS Records
4.2. Computational Details
4.2.1. DFT Calculations
4.2.2. Computational Resonance Raman Spectra
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Le Ru, E.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects; Elsevier: Amsterdam, The Netherland, 2008. [Google Scholar]
- Furini, L.N.; Constantino, C.J.L.; Sanchez-Cortes, S.; Otero, J.C.; Lopez-Tocon, I. Adsorption of carbendazim pesticide on plasmonic nanoparticles studied by surface-enhanced Raman scattering. J. Colloid Interface Sci. 2016, 465, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Furini, L.N.; Sanchez-Cortes, S.; Lopez-Tocon, I.; Otero, J.C.; Aroca, R.F.; Constantino, C.J.L. Detection and quantitative analysis of carbendazim herbicide on Ag nanoparticles via surface-enhanced Raman scattering. J. Raman Spectrosc. 2015, 46, 1095–1101. [Google Scholar] [CrossRef]
- Lopez-Tocon, I.; Otero, J.C.; Arenas, J.F.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Trace Detection of Triphenylene by Surface Enhanced Raman Spectroscopy Using Functionalized Silver Nanoparticles with Bis-Acridinium Lucigenine. Langmuir 2010, 26, 6977–6981. [Google Scholar] [CrossRef]
- López-Tocón, I.; Valdivia, S.; Soto, J.; Otero, J.C.; Muniz-Miranda, F.; Menziani, M.C.; Muniz-Miranda, M. A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles. Nanomaterials 2019, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Soto, J.; Imbarack, E.; Lopez-Tocon, I.; Sanchez-Cortes, S.; Otero, J.C.; Leyton, P. Application of surface-enhanced resonance Raman scattering (SERS) to the study of organic functional materials: Electronic structure and charge transfer properties of 9,10-bis((E)-2-(pyridin-4-yl)vinyl)anthracene. RSC Adv. 2019, 9, 14511–14519. [Google Scholar] [CrossRef] [Green Version]
- Arenas, J.F.; Tocon, I.L.; Otero, J.C.; Marcos, J.I. The charge transfer mechanism in the SERS of 2-methylpyrazine on silver electrode. Vibr. Spectrosc. 1999, 19, 213–221. [Google Scholar] [CrossRef]
- Arenas, J.F.; Otero, J.C.; Centeno, S.P.; Tocon, I.L.; Soto, J. Evidences for the contribution of a resonant charge transfer process to the surface-enhanced Raman scattering of 2,6-dimethylpyridine. Surf. Sci. 2002, 511, 163–170. [Google Scholar] [CrossRef]
- Arenas, J.F.; Centeno, S.P.; Lopez-Tocon, I.; Otero, J.C. Surface-enhanced Raman scattering of 2,3-dimethylpyrazine adsorbed on silver electrode: Selective enhancement explained through the charge transfer mechanism. Vibr. Spectrosc. 2004, 35, 39–44. [Google Scholar] [CrossRef]
- Arenas, J.F.; Soto, J.; Pelaez, D.; Fernandez, D.J.; Otero, J.C. Understanding complex surface-enhanced Raman scattering, using quantum chemical calculations. Int. J. Quantum Chem. 2005, 104, 681–694. [Google Scholar] [CrossRef]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic structure methods for studying surface-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 1061–1073. [Google Scholar] [CrossRef]
- Barnes, B.; García Vidal, F.; Aizpurua, J. Strong coupling of molecules to cavities. ACS Photonics 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Chulhai, D.V.; Cheng, X.; Jenssen, L. Simulating Ensemble-Averaged Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2016, 120, 20833–20842. [Google Scholar] [CrossRef]
- Roman-Perez, J.; Lopez-Tocon, I.; Castro, J.L.; Arenas, J.F.; Soto, J.; Otero, J.C. The electronic structure of metal-molecule hybrids in charged interfaces: Surface-enhanced Raman selection rules derived from plasmon-like resonances. Phys. Chem. Chem. Phys. 2015, 17, 2326–2329. [Google Scholar] [CrossRef]
- Roman-Perez, J.; Centeno, S.P.; Lopez-Ramirez, M.R.; Arenas, J.F.; Soto, J.; Lopez-Tocon, I.; Otero, J.C. On the dual character of charged metal-molecule hybrids and the opposite behaviour of the forward and reverse CT processes. Phys. Chem. Chem. Phys. 2014, 16, 22958–22961. [Google Scholar] [CrossRef]
- Aranda, D.; Valdivia, S.; Soto, J.; Lopez-Tocon, I.; Avila, F.J.; Otero, J.C. Theoretical Approaches for Modeling the Effect of the Electrode Potential in the SERS Vibrational Wavenumbers of Pyridine Adsorbed on a Charged Silver Surface. Front. Chem. 2019, 7, 423. [Google Scholar] [CrossRef] [Green Version]
- Aranda, D.; Valdivia, S.; Avila, F.J.; Soto, J.; Otero, J.C.; Lopez-Tocon, I.; Soto, J.; Otero, J.C.; Lopez-Tocon, I. Charge transfer at the nanoscale and the role of the out-of-plane vibrations in the selection rules of surface-enhanced Raman scattering. Phys. Chem. Chem. Phys. 2018, 20, 29430–29439. [Google Scholar] [CrossRef]
- Sardo, M.; Ruano, C.; Castro, J.L.; Lopez-Tocon, I.; Soto, J.; Ribeiro-Claro, P.; Otero, J.C. Surface-enhanced Raman scattering of 5-fluorouracil adsorbed on silver nanostructures. Phys. Chem. Chem. Phys. 2009, 11, 7437–7443. [Google Scholar] [CrossRef]
- Avila, F.; Ruano, C.; Lopez-Tocon, I.; Arenas, J.F.; Soto, J.; Otero, J.C. How the electrode potential controls the selection rules of the charge transfer mechanism of SERS. Chem. Commun. 2011, 47, 4213–4215. [Google Scholar] [CrossRef]
- Li, W.X.; Li, H.X.; Li, H.Y.; Chem, M.M.; Shi, Y.X.; Lang, J.P. 1,4-Bis(2-(pyridin-4-yl)vinyl)naphthalene and Its Zinc(II) Coordination Polymers: Synthesis, Structural Characterization, and Selective Luminescent Sensing of Mercury(II) Ion. Cryst. Growth Des. 2017, 17, 3948–3959. [Google Scholar] [CrossRef]
- Annan, N.K.; Cook, P.R.; Mulfins, S.T.; Lowe, G. Evidence for cross-linking DNA by bis-intercalators with rigid and extended linkers is provided by knotting and catenation. Nucleic Acids Res. 1992, 20, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Aranda, D.; Lopez-Tocon, I.; Soto, J.; Otero, J.C.; Avila, F. An approach to the electronic structure of molecular junctions with metal clusters of atomic thickness. Phys. Chem. Chem. Phys. 2016, 18, 27179–27184. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Guo, X. Molecule–electrode interfaces in molecular electronic devices. Chem. Soc. Rev. 2013, 42, 5642–5660. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput.-Aided Mol. Design 2000, 14, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Mrozek, I.; Grabhorn, H.; Akemann, W. Surface-enhanced Raman Scattering. J. Phys. Condens. Matter 1992, 4, 1143–1212. [Google Scholar] [CrossRef]
- Creighton, J.A. Spectroscopy of Surfaces; Clark, R.H.J., Hester, R.E., Eds.; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Jeanmaire, D.L.; van Duyne, D.L. Surface Raman Spectroelectrochemistry. Part, I. heterocyclic, aromatic and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Arenas, J.F.; Lopez-Tocon, I.; Castro, S.P.; Lopez-Ramirez, M.R.; Otero, J.C. Resonant charge transfer on the nanoscale: Studying doublet states of adsorbates by surface-enhanced Raman scattering. J. Raman Spectrosc. 2005, 36, 515–521. [Google Scholar] [CrossRef]
- Arenas, J.F.; Lopez-Tocon, I.; Centeno, S.P.; Soto, J.; Otero, J.C. How a resonant charge transfer mechanism determines the relative intensities in the SERS spectra of 4-methylpyridine. Vibr. Spectrosc. 2002, 29, 147–154. [Google Scholar] [CrossRef]
- Centeno, S.P.; Lopez-Tocon, I.; Roman-Perez, J.; Arenas, J.F.; Soto, J.; Otero, J.C. Franck-Condon Dominates the Surface-Enhanced Raman Scattering of 3-Methylpyridine: Propensity Rules of the Charge-Transfer Mechanism under Reduced Symmetry. J. Phys. Chem. C 2012, 116, 23639–23645. [Google Scholar] [CrossRef]
- Soto, J.; Fernandez, D.J.; Centeno, S.P.; Tocon, I.L.; Otero, J.C. Surface orientation of pyrazine adsorbed on silver from the surface-enhanced Raman scattering recorded at different electrode potentials. Langmuir 2002, 18, 3100–3104. [Google Scholar] [CrossRef]
- Avila, F.; Arenas, J.F.; Pelaez, D.; Otero, J.C.; Bando, Y.; Golberg, D.; Huang, Q.; Soto, J. Role of Dimethyl Sulfoxide in the Hydrolytic Peeling of Boron Nitride Nanotubes. J. Phys. Chem. C 2009, 113, 15565–15568. [Google Scholar] [CrossRef]
- Soto, J.; Rosas, J.M.; Otero, J.C.; Rodriguez-Mirasol, J.; Cordero, T. Reaction Mechanisms of 2-Butanol Dehydration over a Phosphorus-Containing Activated Carbon Acid Catalyst. J. Phys. Chem. C 2018, 122, 16772–16778. [Google Scholar] [CrossRef]
- Pelaez, D.; Arenas, J.F.; Otero, J.C.; Soto, J. Dependence of N-nitrosodimethylamine photodecomposition on the irradiation wavelength: Excitation to the S-2 state as a doorway to the dimethylamine radical ground-state chemistry. J. Org. Chem. 2007, 72, 4741–4749. [Google Scholar] [CrossRef]
- Soto, J.; Otero, J.C.; Avila, F.J.; Pelaez, D. Conical intersections and intersystem crossings explain product formation in photochemical reactions of aryl azides. Phys. Chem. Chem. Phys. 2019, 21, 2389–2396. [Google Scholar] [CrossRef]
- Soto, J.; Pelaez, D.; Otero, J.C.; Avila, F.J.; Arenas, J.F. Photodissociation mechanism of methyl nitrate. A study with the multistate second-order multiconfigurational perturbation theory. Phys. Chem. Chem. Phys. 2009, 11, 2631–2639. [Google Scholar] [CrossRef]
- Pelaez, D.; Arenas, J.F.; Otero, J.C.; Avila, F.J.; Soto, J. Photochemistry of protonated nitrosamine: Chemical inertia of NH(2)NOH(+) versus reactivity of NH(3)NO(+). J. Phys. Chem. A 2008, 112, 8394–8402. [Google Scholar] [CrossRef]
- Soto, J.; Otero, J.C. Conservation of El-Sayed’s Rules in the Photolysis of Phenyl Azide: Two Independent Decomposition Doorways for Alternate Direct Formation of Triplet and Singlet Phenylnitrene. J. Phys. Chem. A 2019, 123, 9053–9060. [Google Scholar] [CrossRef]
- Albretch, A.C. On the theory of Raman intensities. J. Chem. Phys. 1961, 34, 1476–1484. [Google Scholar] [CrossRef]
- Long, D.A. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules; John Wiley & Sons, Ltd.: West Sussex England, UK, 2002. [Google Scholar]
- Johansson Seechurn, C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. Engl. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Knappke, C.E.; von Wangelin, A.J. 35 years of palladium-catalyzed cross-coupling with Grignard reagents: How far have we come? Chem Soc. Rev. 2011, 40, 4948–4962. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 16, Revision, A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Aranda, D.; Avila, F.J.; Lopez-Tocon, I.; Arenas, J.F.; Otero, J.C.; Soto, J. An MS-CASPT2 study of the photodecomposition of 4-methoxyphenyl azide: Role of internal conversion and intersystem crossing. Phys. Chem. Chem. Phys. 2018, 20, 7764–7771. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Ramirez, M.R.; Ruiz, D.A.; Ferrer, F.J.A.; Centeno, S.P.; Arenas, J.F.; Otero, J.C.; Soto, J. Analysis of the Potential Dependent Surface-Enhanced Raman Scattering of p-Aminothiophenol on the Basis of MS-CASPT2 Calculations. J. Phys. Chem. C 2016, 120, 19322–19328. [Google Scholar] [CrossRef]
- Avila, F.; Soto, J.; Arenas, J.F.; Rodriguez, J.A.; Pelaez, D.; Otero, J.C. Outstanding Role of Silver Nanoparticles in the Surface-Enhanced Resonance Raman Scattering of p-Benzosemiquinone. J. Phys. Chem. C 2009, 113, 105–108. [Google Scholar] [CrossRef]
- Arenas, J.F.; Centeno, S.P.; Marcos, J.I.; Otero, J.C.; Soto, J. A method to improve the agreement between calculated and observed vibrational frequencies after scaling of a quantum mechanical force field. J. Chem. Phys. 2000, 113, 8472–8477. [Google Scholar] [CrossRef]
- Aranda, D.; Román-Pérez, J.; López-Tocón, I.; Soto, J.; Avila, F.; Otero, J.C. Comment on ‘‘Elucidation of charge-transfer SERS selection rules by considering the excited stateproperties and the role of electrode potential’’ by M.; Mohammadpour, M.H.; Khodabandeh, L.; Visscherand, Z.; Jamshidi, Phys. Chem. Chem. Phys., 2017, 19, 7833. Phys. Chem. Chem. Phys. 2017, 19, 27888–27891. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound, 1,4-bis((E)-2-(pyridin-4-yl)vinyl)naphthalene (bpyvn), is available from the author (E.I.). |
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| bpyvn | E (eV) | 3.590 | 4.369 | 4.628 | 4.747 | 4.765 | 4.789 | 5.047 | 5.087 | 5.094 | 5.113 |
| f 1 | 1.113 | 0.000 | 0.102 | 0.017 | 0.017 | 0.160 | 0.194 | 0.015 | 0.023 | 0.031 | |
| S1(CT0) | S2 | S3 | S4(CT1) | S5(CT2) | S6 | S7 | S8 | S9 | S10(CT3) | ||
| Ag2-bpyvn | E (eV) | 2.611 | 3.237 | 3.535 | 3.808 | 3.824 | 4.049 | 4.160 | 4.348 | 4.505 | 4.604 |
| f 1 | 0.001 | 1.150 | 0.680 | 0.002 | 0.007 | 0.274 | 0.289 | 0.001 | 0.090 | 0.010 | |
| Ag2 charge 2 | 0.59 | −0.23 | −0.24 | 0.60 | 0.59 | −0.23 | −0.21 | −0.21 | −0.22 | 0.27 | |
| ∆q 3 | −0.80 | 0.02 | 0.03 | −0.81 | −0.80 | 0.02 | 0.00 | 0.00 | 0.01 | −0.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Tocón, I.; Imbarack, E.; Soto, J.; Sanchez-Cortes, S.; Leyton, P.; Otero, J.C. Intramolecular and Metal-to-Molecule Charge Transfer Electronic Resonances in the Surface-Enhanced Raman Scattering of 1,4-Bis((E)-2-(pyridin-4-yl)vinyl)naphthalene. Molecules 2019, 24, 4622. https://doi.org/10.3390/molecules24244622
López-Tocón I, Imbarack E, Soto J, Sanchez-Cortes S, Leyton P, Otero JC. Intramolecular and Metal-to-Molecule Charge Transfer Electronic Resonances in the Surface-Enhanced Raman Scattering of 1,4-Bis((E)-2-(pyridin-4-yl)vinyl)naphthalene. Molecules. 2019; 24(24):4622. https://doi.org/10.3390/molecules24244622
Chicago/Turabian StyleLópez-Tocón, Isabel, Elizabeth Imbarack, Juan Soto, Santiago Sanchez-Cortes, Patricio Leyton, and Juan Carlos Otero. 2019. "Intramolecular and Metal-to-Molecule Charge Transfer Electronic Resonances in the Surface-Enhanced Raman Scattering of 1,4-Bis((E)-2-(pyridin-4-yl)vinyl)naphthalene" Molecules 24, no. 24: 4622. https://doi.org/10.3390/molecules24244622