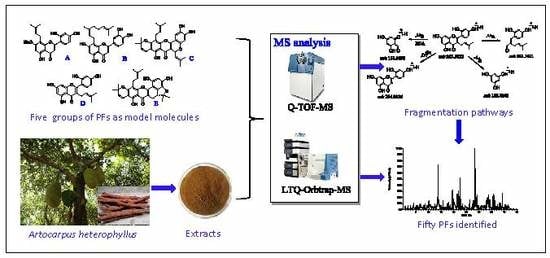
Characterization and Identification of Prenylated Flavonoids from Artocarpus heterophyllus Lam. Roots by Quadrupole Time-Of-Flight and Linear Trap Quadrupole Orbitrap Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
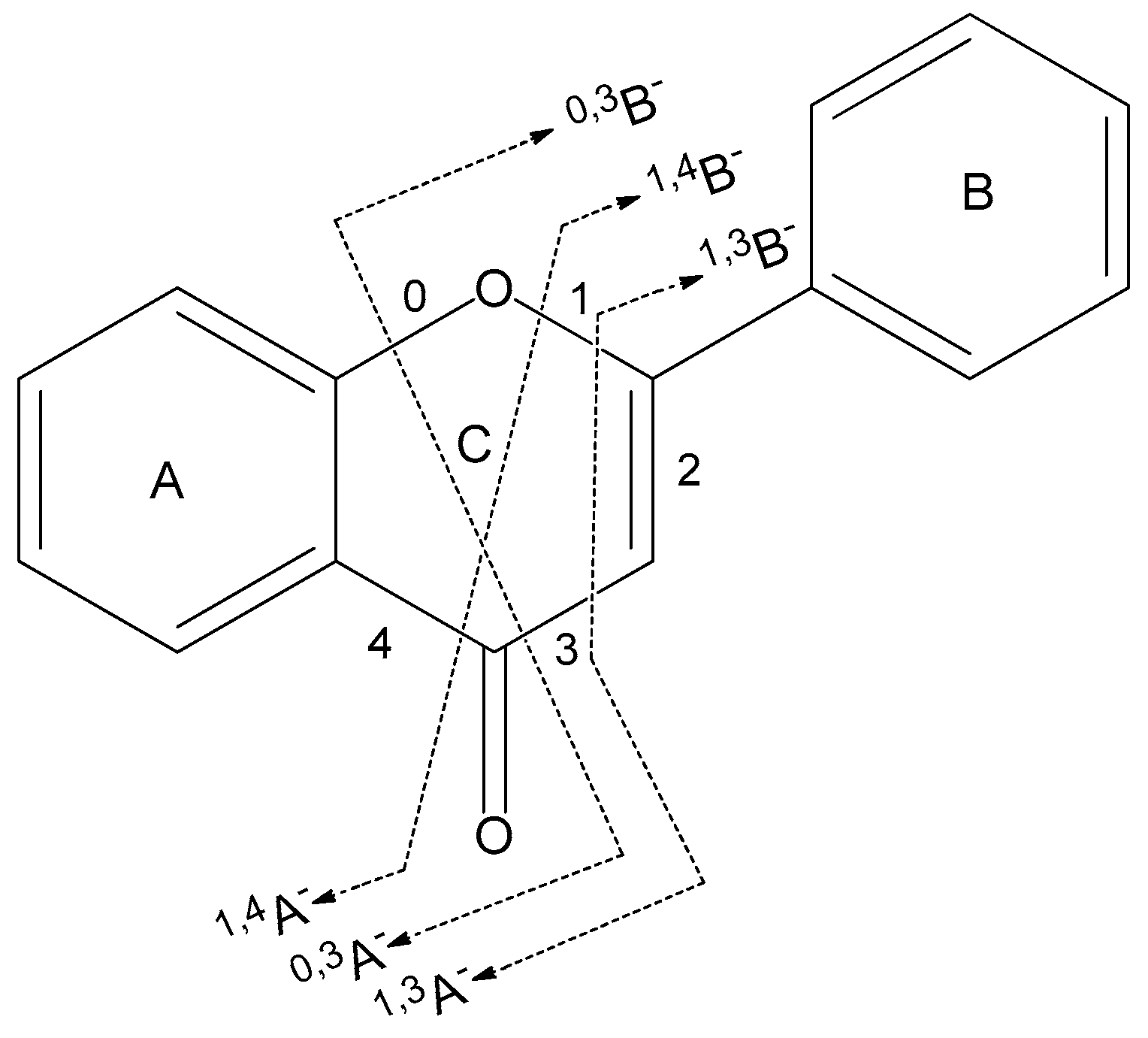
2.1. Fragment Naming Rules
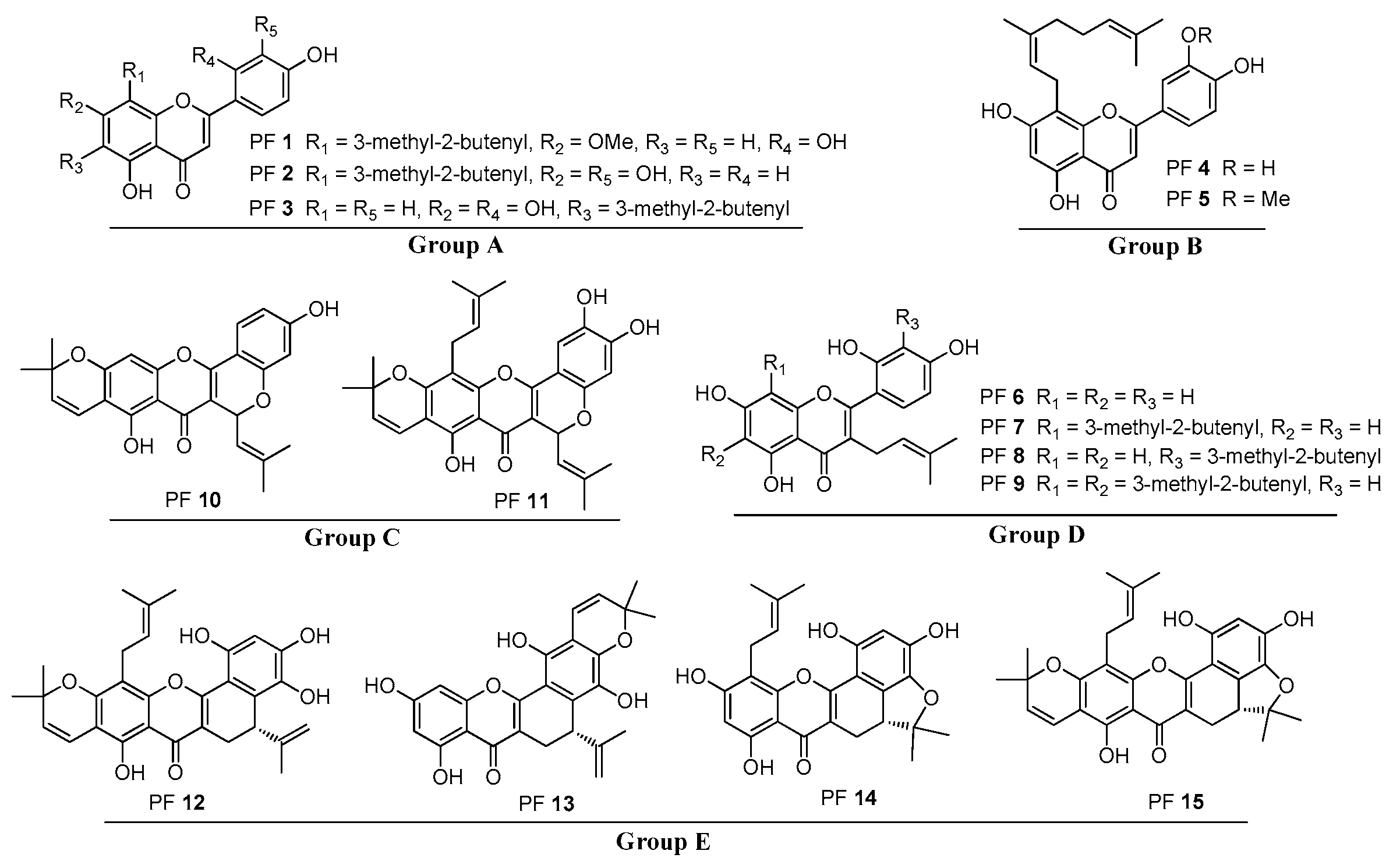
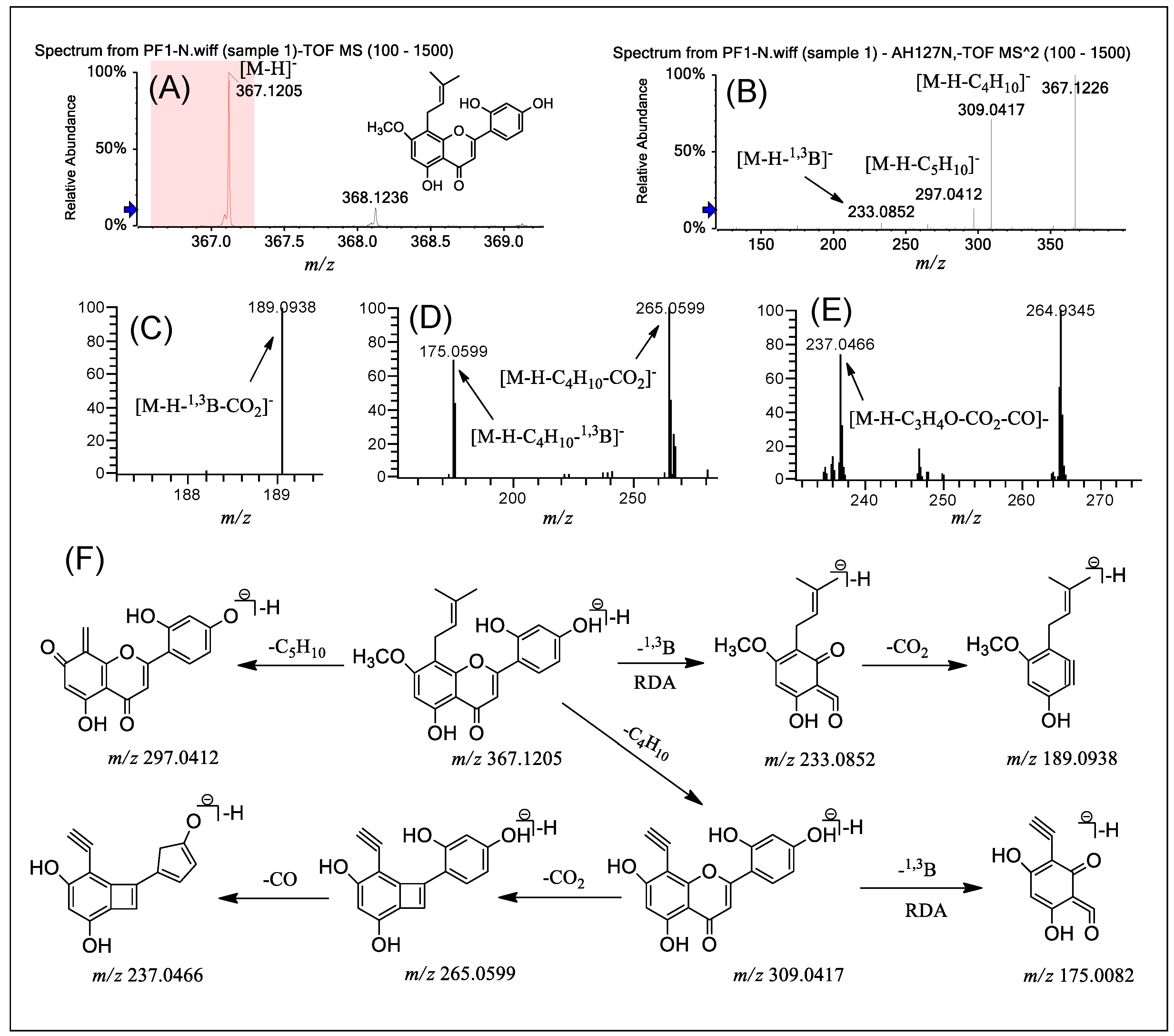
2.2. Fragmentation of PFs 1–3
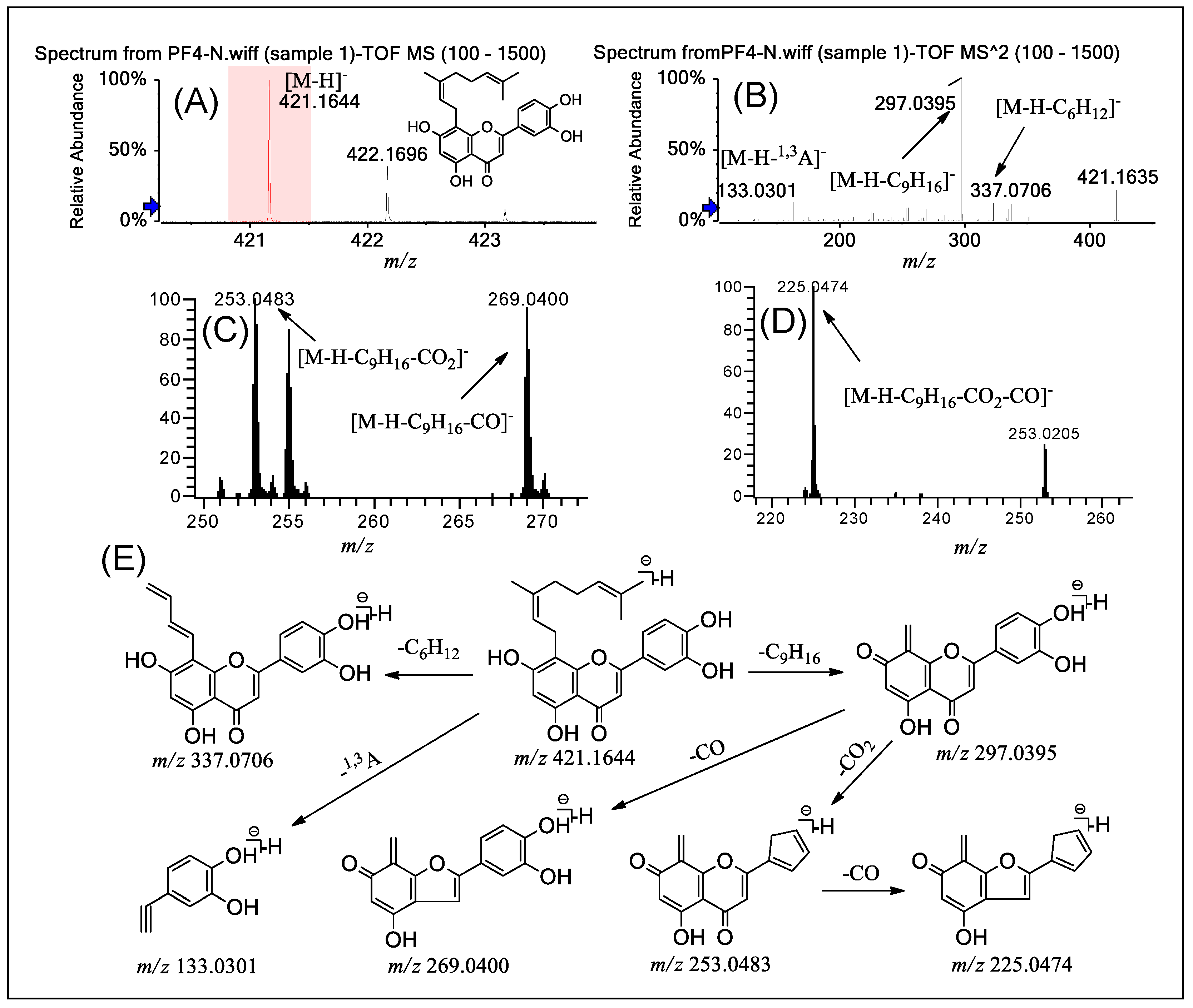
2.3. Fragmentation of PFs 4 and 5
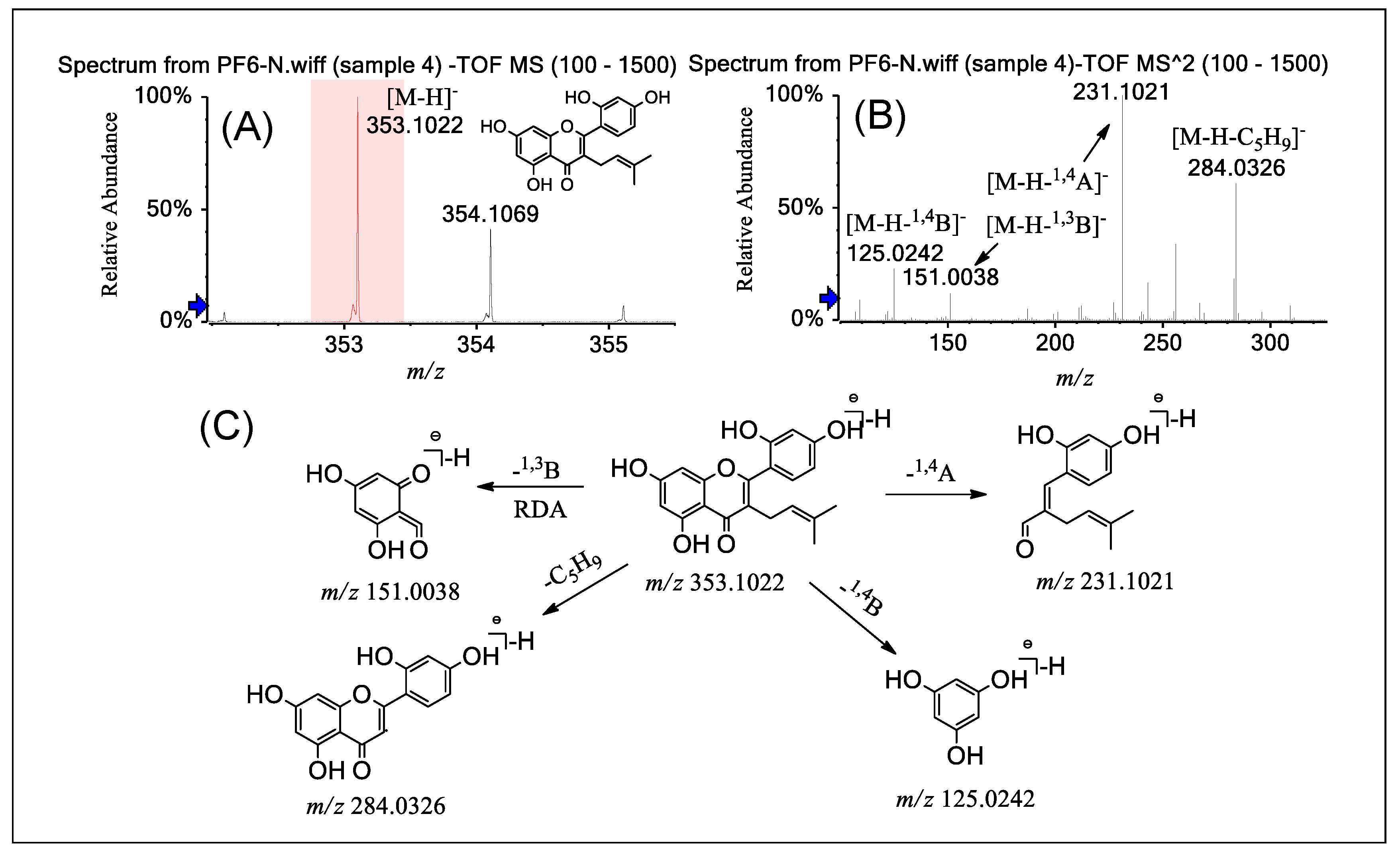
2.4. Fragmentation of PFs 6–9
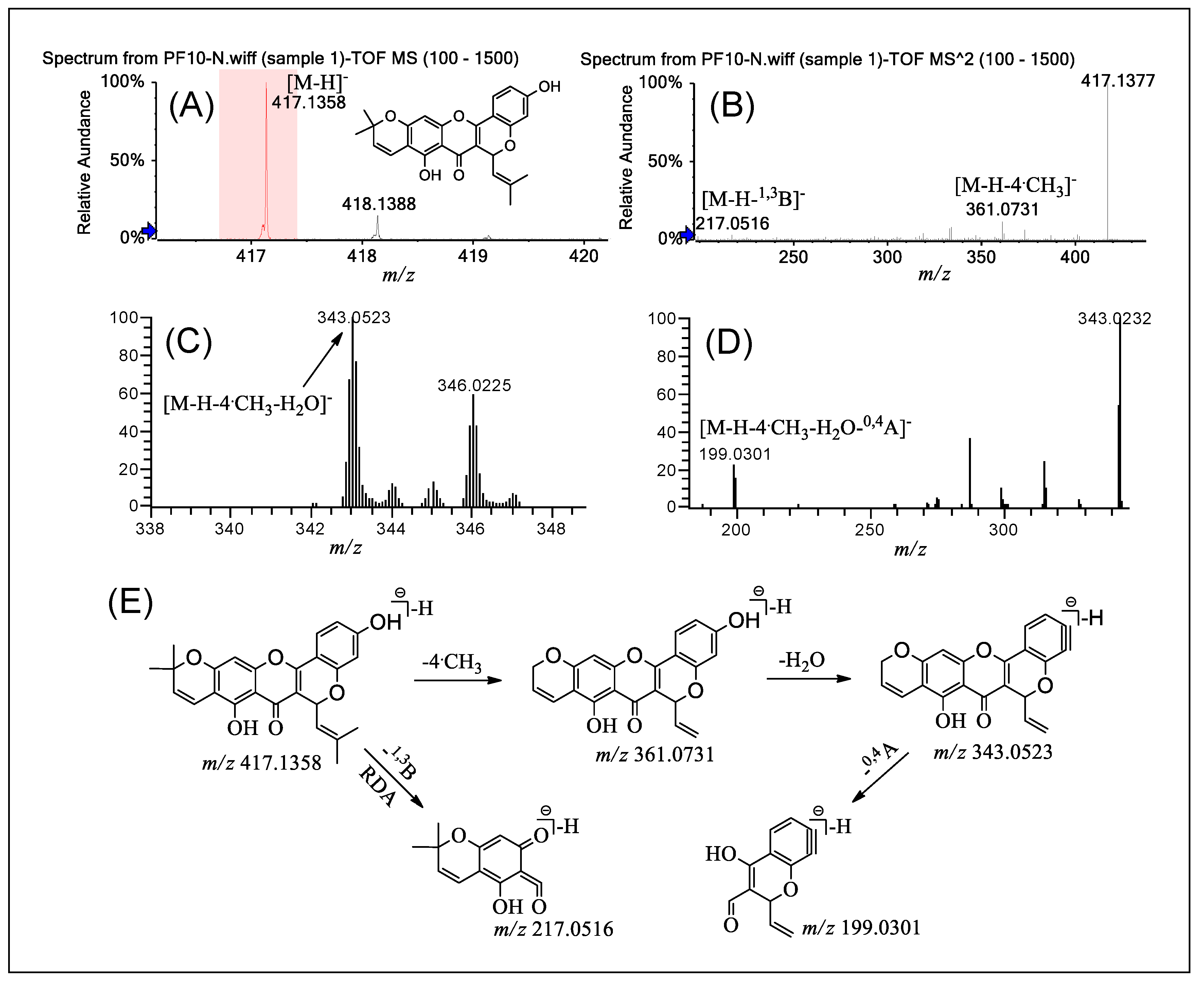
2.5. Fragmentation of PFs 10 and 11
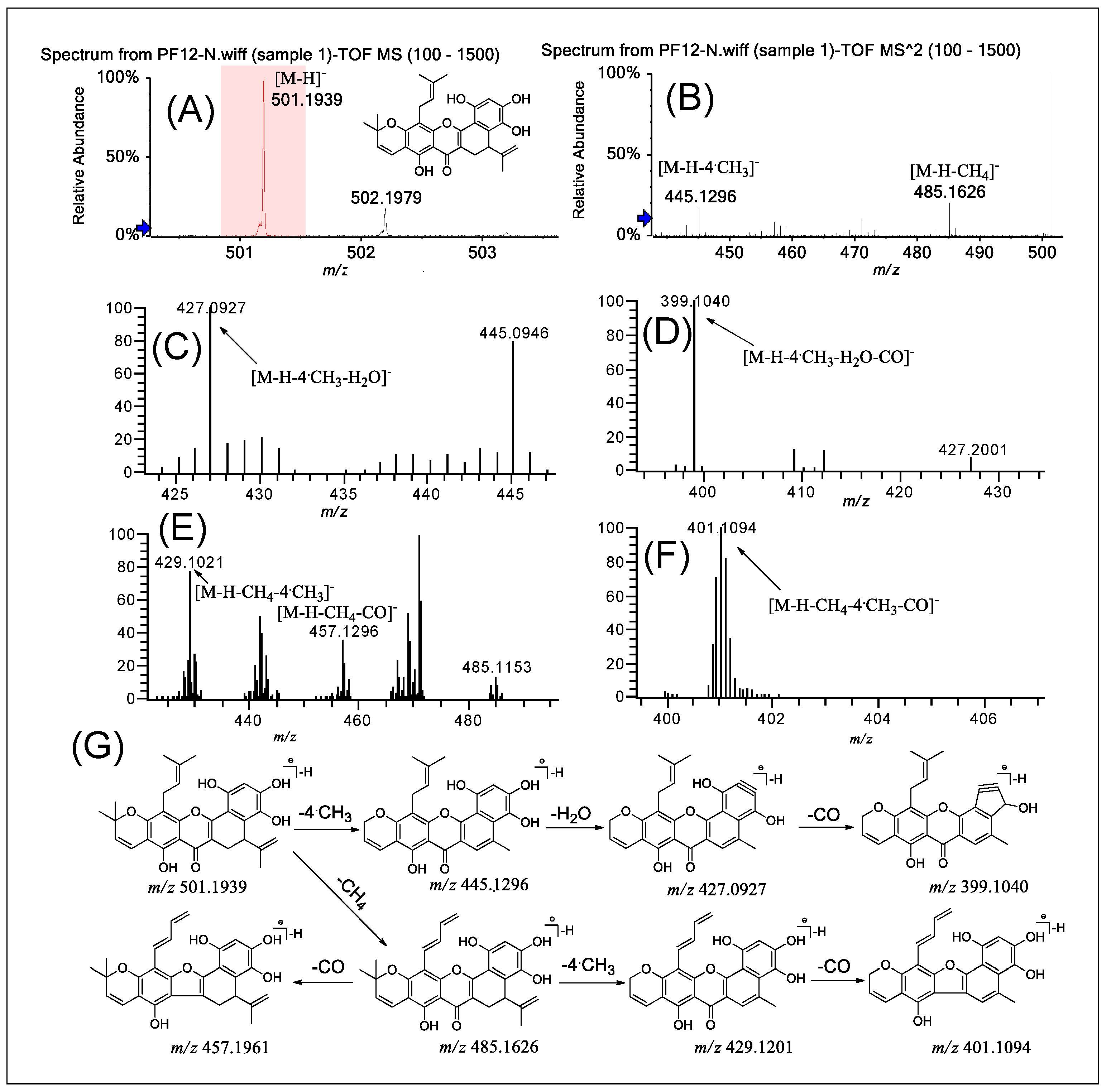
2.6. Fragmentation of PFs 12–15
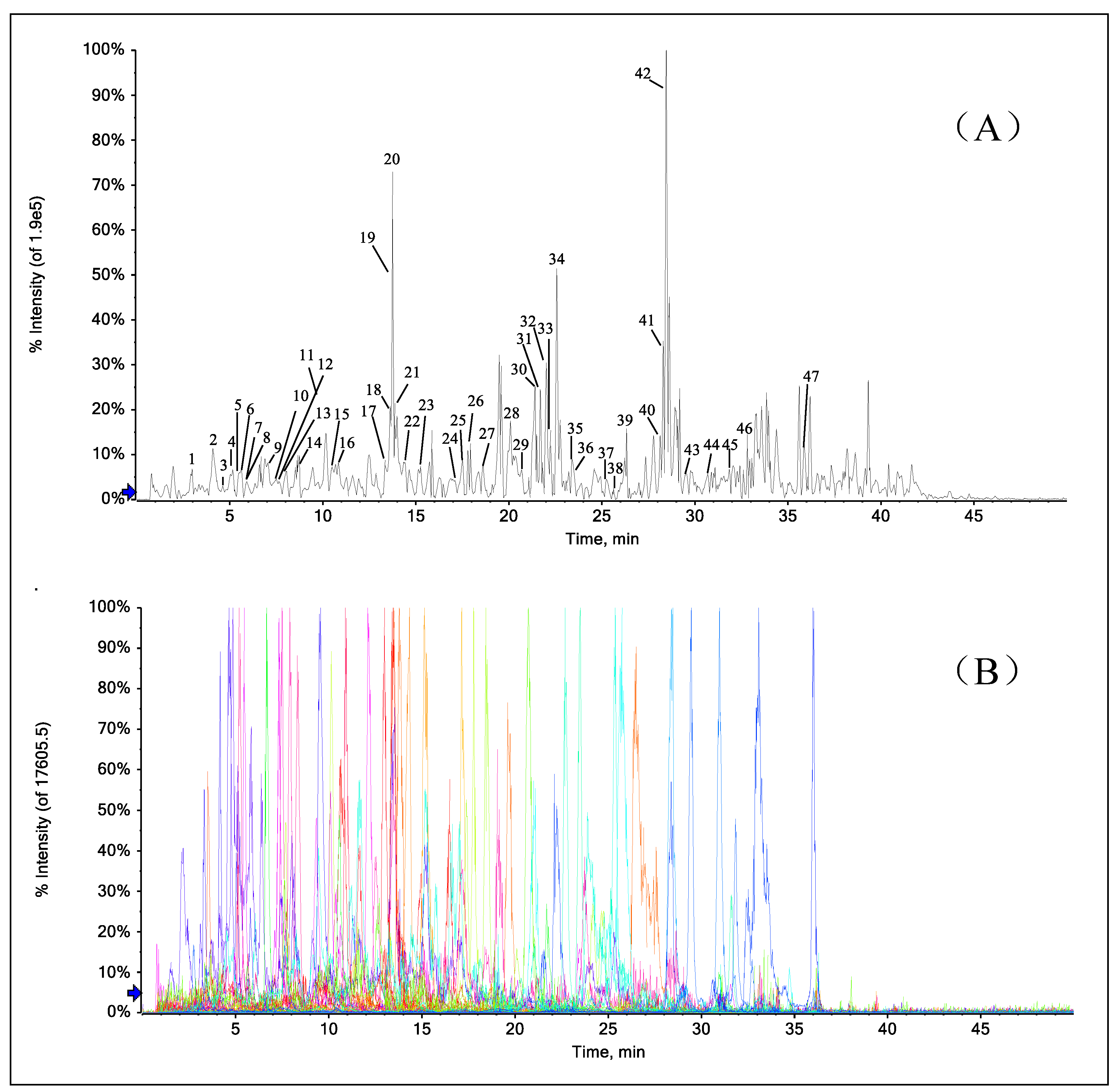
2.7. Rapid Identification of PFs in the Extract of A. heterophyllus Roots
3. Materials and Methods
3.1. Plant Material
3.2. Model Molecules and Reagents
3.3. Sample Preparation
3.4. Ultra-High Performance Liquid Chromatography (UPLC)-Q-TOF-MS/MS and UPLC-LTQ-Orbitrap-MSn Analysis of PFs
3.5. Data Preprocessing Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liou, S.S.; Shieh, W.L.; Chen, T.H.; Won, S.J.; Lin, C.N. γ-Pyrone compounds as potential anti-cancer drugs. J. Pharm. Pharmacol. 1993, 45, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wu, J.W.; Wang, M.; Yu, M.H.; Zhao, Q.S.; Wang, H.Y.; Hou, A.J. 2-Arylbenzofuran, flavonoid, and tyrosinase inhibitory constituents of Morus yunnanensis. J. Nat. Prod. 2012, 75, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Offen, P.; Taylor, P.B.; Votta, B.J.; Johnson, R.K. A new dimeric dihydrochalcone and a new prenylated flavone from the bud covers of Artocarpus altilis: Potent inhibitors of cathepsin k. J. Nat. Prod. 2002, 65, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.J.; Yuan, J.B.; Peng, J.B.; Ding, Y.Q.; Zhu, J.X.; Ren, G. Flavonoids from the roots of Artocarpus heterophyllus. Fitoterapia 2017, 117, 133–137. [Google Scholar] [CrossRef]
- Bourjot, M.; Apel, C.; Martin, M.T.; Grellier, P.; Nguyen, V.H.; Gueritte, F.; Litaudon, M. Antiplasmodial, antitrypanosomal, and cytotoxic activities of prenylated flavonoids isolated from the stem bark of Artocarpus styracifolius. Planta Med. 2010, 76, 1600–1604. [Google Scholar] [CrossRef]
- Hanáková, Z.; Hošek, J.; Kutil, Z.; Temml, V.; Landa, P.; Vaněk, T.; Schuster, D.; Dall’Acqua, S.; Cvačka, J.; Polanský, O.; et al. Anti-inflammatory activity of natural geranylated flavonoids: Cyclooxygenase and lipoxygenase inhibitory properties and proteomic analysis. J. Nat. Prod. 2017, 80, 999–1006. [Google Scholar] [CrossRef]
- Ren, G.; Xiang, H.Y.; Hu, Z.C.; Liu, R.H.; Yi, W.F.; Peng, J.B.; Yuan, J.B. Inhibitory effects of phenolic compounds from Artocarpus styracifolius on respiratory burst of rat neutrophils. Pharm. Biol. 2014, 52, 944–950. [Google Scholar] [CrossRef]
- Nomura, T.; Hano, Y.; Aida, M. Isoprenoid-substituted flavonoids from Artocarpus plants (Moraceae). Heterocycles 1998, 47, 1179–1205. [Google Scholar] [CrossRef]
- Ren, G.; Peng, J.B.; Yi, W.F.; Yuan, W.J. Advance on chemical constituents from Artocarpus plant and its bioactivities in recent five years. Chin. J. Exp. Tradit. Med. Formul. 2014, 20, 234–239. [Google Scholar]
- Zhang, X.S.; Wu, Z.Y. Zhongguo Zhiwu Zhi (Flora of China); Science Press: Beijing, China, 1998. [Google Scholar]
- Li, Y.; Mao, Q.; Feng, F.; Ye, C. Genetic diversity within a jackfruit (Artocarpus heterophyllus Lam.) germplasm collection in China using AFLP markers. Agric. Sci. China 2010, 9, 1263–1270. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, M.H.; Nguyen, H.X.; Bui, N.K.; Nguyen, M.T. Tyrosinase inhibitors from the wood of Artocarpus heterophyllus. J. Nat. Prod. 2012, 75, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Xu, Y.; Qin, C.; Zhang, S.; Gu, X.; Lin, Y.; Xie, G.; Wang, M.; Chen, J. Characterization of antiproliferative activity constituents from Artocarpus heterophyllus. J. Agric. Food Chem. 2014, 62, 5519–5527. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Zhang, Y.B.; Chen, S.Q.; Fu, Y. Characterization and identification of the chemical constituents in the root of Lindera reflexa Hemsl. using ultra-high performance liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2016, 126, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ye, J.; Liang, X.; Zhang, X.; SU, J.; Fu, P.; Lv, D.Y.; Zhang, W.D. Mass spectrometric profiling of valepotriates possessing various acyloxy groups from Valeriana jatamansi. J. Mass Spectrom. 2015, 50, 1294–1304. [Google Scholar] [CrossRef]
- Kang, L.P.; Zhao, Y.; Pang, X.; Yu, H.S.; Xiong, C.Q.; Zhang, J.; Gao, Y. Characterization and identification of steroidal saponins from the seeds of Trigonella foenum-graecum by ultra high-performance liquid chromatography and hybrid time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 257–267. [Google Scholar] [CrossRef]
- Zhai, X.X.; Zhong, G.Y.; Yao, P.C.; Lin, Q.H.; Yuan, W.J.; Ren, G. Phenolic constituents from root of Arocarpus heterophyllus. Chin. Tradit. Herb. Drugs 2016, 47, 3959–3964. [Google Scholar]
- Xiao, C.Y.; Li, W.Y.; Zhai, X.X.; Zhu, L.Z.; Zhou, Y.F.; Yao, P.C.; Shu, Q.X.; Ren, G. Prenylated flavonoids from the roots of Artocarpus heterophyllus. Acta Pharm. Sin. 2018, 53, 592–597. [Google Scholar]
- Lin, Q.H.; Yuan, J.B.; Ma, Z.L.; Liang, J.; Zhai, X.X.; Khan, I.A.; Ding, Y.Q.; Ren, G. Isoprenylated flavonoids from roots of Artocarpus styracfolius. Nat. Prod. Commun. 2016, 11, 1843–1846. [Google Scholar]
- Lin, Q.H.; Yao, P.C.; Xiao, C.Y.; Zhai, X.X.; Yuan, W.J.; Ren, G. Chemical constituents from the root of Artocarpus stracifolius. J. Chin. Med. Mater. 2017, 40, 839–844. [Google Scholar]
- Simons, R.; Vincken, J.P.; Bohin, M.C.; Kuijpers, T.F.M.; Verbruggen, M.A.; Gruppen, H. Identification of prenylated pterocarpans and other isoflavonoids in Rhizopus spp. elicited soya bean seedlings by electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 25, 55–65. [Google Scholar] [CrossRef]
- Yu, L.; Wei, F.; Liang, J.; Ren, G.; Liu, X.; Wang, C.Z.; Yuan, J.; Zeng, J.; Luo, Y.; Bi, Y.; et al. Target molecular-based neuroactivity screening and analysis of Panax ginseng by affinity ultrafiltration, UPLC-QTOF-MS and molecular docking. Am. J. Chin. Med. 2019, 47, 1345–1363. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, B.M.; Ngadjui, B.T.; Dongo, E.; Tamboue, H. Prenylated chalcones and flavones from the leaves of Dorstenia kameruniana. Phytochemistry 2010, 49, 1147–1150. [Google Scholar] [CrossRef]
- Nomura, T.; Aida, M.; Shinomiya, K.; Hano, Y. Artonins J, K, and L, three new isoprenylated flavones from the root bark of Artocarpus heterophyllus Lamk. Heterocycles 1993, 36, 575–580. [Google Scholar] [CrossRef]
- Ferrari, F.; Messana, I.; de Araujo, M. Three new flavone derivatives, brosimones G, H, and I, from Brosimopsis oblongifolia. Planta Med. 1989, 55, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Butler, M.S.; Buss, A.D. Flavonoids from Artocarpus Lanceifolius. Nat. Prod. Lett. 2003, 17, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Hakim, E.H.; Aimi, N.; Kitajima, M.; Takayama, H. Artoindonesianin P, a new prenylated flavone with cytotoxic activity from Artocarpus lanceifolius. Fitoterapia 2003, 73, 668–673. [Google Scholar] [CrossRef]
- Suhartati, T.; Achmad, S.A.; Aimi, N.; Hakim, E.H.; Kitajima, M.; Takayama, H.; Takeya, K. Artoindonesianin L, a new prenylated flavone with cytotoxic activity from Artocarpus rotunda. Fitoterapia 2001, 72, 912–918. [Google Scholar] [CrossRef]
- Jayasinghe, U.L.B.; Samarakoon, T.B.; Kumarihamy, B.M.M.; Hara, N.; Fujimoto, Y. Four new prenylated flavonoids and xanthones from the root bark of Artocarpus nobilis. Fitoterapia 2008, 79, 37–41. [Google Scholar] [CrossRef]
- Lin, C.N.; Shieh, W.L. Pyranoflavonoids from Artocarpus communis. Phytochemistry 1992, 31, 2922–2924. [Google Scholar]
- Syah, Y.M.; Achmad, S.A.; Ghisalberti, E.L.; Hakim, E.H.; Makmur, L.; Mujahidin, D. Artoindonesianins Q–T, four isoprenylated flavones from Artocarpus champeden Spreng. Phytochemistry 2002, 61, 949–953. [Google Scholar] [CrossRef]
- Nomura, T.; Aida, M.; Yamaguchi, N.; Hano, Y. Artonols A, B, C, D, and E, five new isoprenylated phenols from the bark of Artocarpus communis forst. Heterocycles 1997, 45, 163–175. [Google Scholar] [CrossRef]
- Hakim, E.H.; Fahriyati, A.; Kau, M.S.; Achmad, S.A.; Makmur, L.; Ghisalberti, E.L.; Nomura, T. Artoindonesianins A and B, two new prenylated flavones from the root of Artocarpus champeden. J. Nat. Prod. 1999, 62, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.N.; Cheng, Z.J.; Lin, C.N.; Teng, C.M. Scavenger and antioxidant properties of prenylflavones isolated from Artocarpus heterophyllus. Free Radic. Biol. Med. 1998, 25, 160–168. [Google Scholar] [CrossRef]
- Widyawaruyanti, A.; Kalauni, S.K.; Awale, S.; Nindatu, M.; Zaini, N.C.; Syafruddin, D.; Asih, P.B.; Tezuka, Y.; Kadota, S. New prenylated flavones from Artocarpus champeden, and their antimalarial activity in vitro. J. Nat. Med. 2007, 61, 410–413. [Google Scholar] [CrossRef]
- Wang, X.L.; Di, X.X.; Shen, T.; Wang, S.Q.; Wang, X.N. New phenolic compounds from the leaves of Artocarpus heterophyllus. Chin. Chem. Lett. 1998, 28, 37–40. [Google Scholar] [CrossRef]
- Ramli, F.; Rahmani, M.; Kassim, N.K.; Hashim, N.M.; Sukari, M.A.; Akim, A.M.; Go, R. New diprenylated dihyrochalcones from leaves of Artocarpus elasticus. Phytochem. Lett. 2013, 6, 582–585. [Google Scholar] [CrossRef]
- Aida, M.; Shinomiya, K.; Matsuzawa, K.; Hano, Y. Artonins Q, R, S, T, and U, five new isoprenylated phenols from the bark of Artocarpus heterophyllus Lamk. Heterocycles 1994, 39, 847–858. [Google Scholar]
- Arung, E.; Shimizu, K.; Kondo, R. Inhibitory effect of isoprenoid-substituted flavonoids Iisolated from Artocarpus heterophylluson Melanin biosynthesis. Planta Med. 2006, 72, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Wenkert, E.; Gottlieb, H.E. Carbon-13 nuclear magnetic resonance spectroscopy of flavonoid and isoflavonoid compounds. Phytochemistry 1977, 16, 1811–1816. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T.; Yamada, S.; Katayanagi, M. Studies on the constituents of the cultivated mulberry tree II. Photo-oxidative cyclization of morusin. Chem. Pharm. Bull. 1978, 26, 1431–1436. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.I.; Lu, C.M.; Huang, P.L.; Lin, C.N. Prenylflavonoids of Artocarpus heterophyllus. Phytochemistry 1995, 40, 1279–1282. [Google Scholar] [CrossRef]
- Kijjoa, A.; Cidade, H.M.; Pinto, M.M.M.; Gonzalez, M.J. Prenylflavonoids from Artocarpus elasticus. Phytochemistry 1996, 43, 691–694. [Google Scholar] [CrossRef]
- Nomura, T.; Hano, Y.; Inami, R. Components of the bark of Artocarpus rigida Bl. 1. structures of two new isoprenylated flavones, Artonins G and H. Heterocycles 1990, 31, 2173–2179. [Google Scholar] [CrossRef]
- Rao, A.R.; Varadan, M.; Venkataraman, K. Coloring matters of wood of Artocarus heterophyllus. 7. Isocycloheterophyllin, a new flavone. Indian J. Chem. 1973, 11, 298–299. [Google Scholar]
- Syah, Y.M.; Achmad, S.A.; Ghisalberti, E.L.; Hakim, E.H.; Makmur, L.; Mujahidin, D. Artoindonesianins G-I, three new isoprenylated flavones from Artocarpus lanceifolius. Fitoterapia 2001, 72, 765–773. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Meechai, I.; Puripattanavong, J.; Kummee, S. Antityrosinase and antimicrobial activities from Thai medicinal plants. Arch. Pharm. Res. 2013, 37, 473–483. [Google Scholar] [CrossRef]
- Nomura, T.; Hano, Y.; Aida, M.; Shiina, M.; Kawai, T.; Ohe, H.; Kagei, K. Artonins A and B, two new prenylflavonoes from the root bark of Artocarpus heterophyllus Lamk. Heterocycles 1989, 29, 1447–1453. [Google Scholar] [CrossRef]
- Fujimoto, T.; Hano, Y.; Nomura, T.; Uzawa, J. Components of root bark of Cudrania tricuspidata 2. structures of two new isoprenylated flavones, Cudraflavones A and B. Planta Med. 1984, 50, 161–163. [Google Scholar] [CrossRef]
- Septama, A.W.; Panichayupakaranant, P. Antibacterial assay-guided isolation of active compounds from Artocarpus heterophyllus heartwoods. Pharm. Biol. 2015, 53, 1–6. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (PFs 1–15) are available from the authors. |
| Compounds | MSn | Precursor Ions | Product Ions m/z (Relative Abundance, %) |
|---|---|---|---|
| PF 1 | MS/MS | 367 [M − H]− | 175 (3.4); 233 (3.9); 265 (4.2); 297 (18.9); 309 (77.7); 337 (1.6); 352 (1.7); 367 (100.0) |
| MS3 | 233 | 133 (20.4); 165 (61.7); 189 (23.7); 217 (100.0) | |
| 309 | 175 (61.7); 265 (100.0); 291 (26.7) | ||
| MS4 | 265 | 173 (100.0); 193 (36.4); 215 (62.1); 221 (75.1); 237 (70.1) | |
| PF 2 | MS/MS | 353 [M − H]− | 133 (6.8); 297 (15.5); 309 (15.5); 353 (100.0) |
| MS3 | 297 | 225 (20.1); 227 (21.4); 253 (97.5); 255 (83.4); 269 (100.0) | |
| 309 | 265 (18.8); 267 (19.9); 309 (100.0) | ||
| MS4 | 269 | 225 (53.4); 227 (35.2); 241 (75.7); 251 (100.0); 269 (39.3) | |
| 267 | 211 (10.4); 223 (17.9); 239 (44.7); 252 (20.5); 267 (100.0) | ||
| PF 3 | MS/MS | 353 [M − H]− | 133 (2.0); 219 (9.8);309 (3.1); 353 (100.0) |
| MS3 | 219 | 133 (80); 151 (39.4); 175 (100.0) | |
| MS4 | 175 | 65 (13.4); 133 (100.0) | |
| PF 4 | MS/MS | 421 [M − H]− | 133 (12.7); 161 (8.9); 255(9.4); 269 (8.7); 297 (100.0); 323(12.4); 337 (12.0); 421 (21.9) |
| MS3 | 297 | 163 (9.8); 225(22.8); 227 (22.1); 253(100.0); 255 (84.4); 269 (95.9) | |
| MS4 | 253 | 211 (10.7); 225 (100.0); 253 (22.9) | |
| PF 5 | MS/MS | 435 [M − H]− | 133 (6.7); 269 (8.); 297(84.5); 309 (30.1); 311 (13.5); 323(21.1); 351 (100.0); 420 (35.4); 435 (32.8) |
| MS3 | 297 | 163 (9.0); 225(20.9); 227 (21.0); 253(88.6); 255 (75.6); 269 (100.0) | |
| 351 | 307 (9.2); 309 (19.1); 333 (24.0); 351 (100.0) | ||
| MS4 | 253 | 211 (8.38); 225 (100.0); 253 (20.2) | |
| PF 6 | MS/MS | 353 [M − H]− | 109 (16.7); 125 (30.6); 151 (24.8); 231 (100.0); 243 (13.2); 283 (11.6); 284 (62.0); 353 (50.5) |
| MS3 | 231 | 145 (23.1); 147 (23.2); 149 (24.0); 151 (76.7); 163 (23.0); 187 (100.0); 189 (28.2) | |
| 284 | 256 (100.0) | ||
| MS4 | 256 | 122 (100.0); 212 (56.7); 227 (43.6); 228 (38.3) | |
| PF 7 | MS/MS | 421 [M − H]− | 193 (8.91); 219 (1.9); 227 (1.8); 283 (2.4); 297 (7.7); 299 (20.9); 309 (38.8); 311 (3.1); 421 (100.0) |
| MS3 | 193 | 125 (43.1); 149 (100.0); 151 (36.2) | |
| 309 | 175 (92.2); 265 (100.0) | ||
| MS4 | 149 | 107 (100.0) | |
| 265 | 173 (59.8); 175 (44.4); 215 (74.5); 221 (76.7); 223 (100.0); 237 (56.2) | ||
| PF 8 | MS/MS | 421 [M − H]− | 193 (7.1); 293 (7.7); 299 (27.7); 309 (47.1); 421 (100.0) |
| MS3 | 299 | 244 (90.0) | |
| 309 | 175 (10.2); 244 (100.0); 256 (19.7); 299 (78.7) | ||
| MS4 | 299 | 244 (100.0); 299 (58.0) | |
| 244 | 123 (100.0); 149 (35.3); 150 (69.0); 203 (53.3); 215 (35.8); 216 (77.4); 229 (99.11) | ||
| PF 9 | MS/MS | 489 [M − H]− | 261 (6.1); 351 (6.0); 365 (10.3); 367 (100.0); 377 (75.9); 489 (90.4) |
| MS3 | 367 | 257 (10.6); 269 (24.5); 312 (100.0); 367 (71.2) | |
| 377 | 269 (10.1); 312 (31.5); 367 (100.0) | ||
| MS4 | 312 | 257 (51.4); 269 (100.0) | |
| PF 10 | MS/MS | 417 [M − H]− | 199 (2.3); 217 (3.0); 333 (7.3); 347 (2.4); 361 (11.7); 373 (6.29); 387 (2.8); 401 (3.3) |
| MS3 | 361 | 319 (7.5); 334 (13.5); 343 (100.0); 346 (59.4) | |
| MS4 | 343 | 199 (22.1); 287 (36.2); 299 (10.0); 315 (24.2); 343 (100.0) | |
| PF 11 | MS/MS | 501 [M − H]− | 285 (3.8); 429 (3.7); 457 (43.22); 473 (3.3); 501 (100.0) |
| MS3 | 285 | 285 (5.2); 243 (22.0); 241 (100.0); 217 (8.5) | |
| 457 | 402 (20.1); 439 (100.0); 442 (10); 457 (18.7) | ||
| MS4 | 241 | 133 (67.5); 157 (32.2); 173 (77.2); 193 (26.6); 197 (100.0); 213 (32.5) | |
| 439 | 357 (58.70); 383 (21.73); 395 (53.51); 411 (100.0); 424 (20.26) | ||
| MS5 | 411 | 329 (35.9); 341 (87.8); 354 (73.8); 356 (87.3); 367 (54.4); 383 (76.9); 393 (58.3); 396 (100.0); 411 (22.5) | |
| PF 12 | MS/MS | 501 [M − H]− | 415 (8); 417 (5.0); 429 (7.3); 445 (14.8); 457 (4.2); 471 (13.0); 483 (4.4); 501 (100.0) |
| MS3 | 445 | 355 (25.3); 373 (38.8); 399 (39.0); 403 (26.7); 417 (100.0); 427 (94.2); 445 (74.6) | |
| 485 | 429 (100.0); 442 (60.3); 457 (29.9); 469 (46.5); 471 (85.7) | ||
| MS4 | 427 | 355 (53.9); 383 (10.5); 399 (100.0); 427 (34.5) | |
| 429 | 401 (100.0); 411 (31.5); 414 (20.5) | ||
| MS5 | 399 | 343 (52.5); 355 (100.0); 356 (41.6) | |
| 401 | 357 (88.5); 373 (100.0); 383 (26.6); 386 (70.6); 401 (19.3) | ||
| PF 13 | MS/MS | 433 [M − H]− | 373 (11.3); 389 (37.0); 390 (63.2); 405 (25.3); 417 (11.2); 433 (100.0) |
| MS3 | 389 | 361 (15.2); 372 (100.0); 373 (34.3); 375 (17.5); 389 (27.9) | |
| 405 | 335 (17.4); 350 (32.9); 361 (27.9); 360 (30.4); 372 (21.9); 389 (15.1); 405 (100.0) | ||
| MS4 | 372 | 326 (34.9); 327 (35.9); 343 (71.7); 371 (100.0) | |
| 350 | 309 (21.6); 335 (100.0); 349 (22.2) | ||
| MS5 | 343 | 299 (13.9); 343 (100.0) | |
| 335 | 276 (16.0); 291 (68.1); 308 (25.6); 317 (34.2); 335 (100.0) | ||
| MS6 | 299 | 263 (14.0); 276 (100.0); 291 (16.7) | |
| 291 | 263 (14.0); 276 (100.0); 291 (16.7) | ||
| PF 14 | MS/MS | 435 [M − H]− | 349 (34.2); 363 (5.0); 377 (20.7); 379 (12.4); 391 (12.4); 393 (6.3); 419 (6.3); 435 (100.0) |
| MS3 | 349 | 277 (21.4); 305 (15.0); 321 (32.9); 349 (100.0) | |
| 391 | 337 (29.5); 349 (100.0); 363 (23.9); 377 (17.8) | ||
| MS4 | 321 | 264 (100.0); 276 (40.7); 279 (62.5); 293 (90.0) | |
| 337 | 309 (100.0) | ||
| MS5 | 309 | 237 (21.1); 263 (32.6); 365 (23.3); 279 (27.0); 281 (70.3); 291 (100.0); 309 (31.4) | |
| PF 15 | MS/MS | 501 [M − H]− | 417 (5.0); 429 (5.5); 443 (5.8); 445 (12.2); 457 (7.3); 471 (7.7); 485 (14.8); 501 (100.0) |
| MS3 | 445 | 355 (27.4); 373 (41.3); 399 (38.9); 417 (99.6); 427 (100.0); 445 (80.8) | |
| 485 | 429 (100.0); 442 (60.8); 457 (34.2); 469 (47.9); 471 (88.0) | ||
| MS4 | 427 | 355 (26.4); 371 (56.0); 385 (27.2); 399 (100.0) | |
| 429 | 357 (26.3); 374 (19.6); 401 (100.0); 411 (29.9) | ||
| MS5 | 399 | 355 (100.0); 384 (18.3) | |
| 401 | 357 (91.0); 373 (100.0); 383 (32.7); 385 (48.8); 401 (21.8) |
| Peak No. | Compounds | RT (min) | Molecular Formula | [M − H]− (m/z) | Error (Δppm) | Product Ions (m/z) | Error (Δppm) | Fragment Ions Identified |
|---|---|---|---|---|---|---|---|---|
| 1 | 6-(3-Methylbutyl-2-enyl)apigenin [23] | 3.49 | C20H18O5 | 337.10903 | 2.6 | 219.0681 | 8.2 | [M – H − 1,3B]− |
| 281.0473 | 6.4 | [M − H − C4H8]− | ||||||
| 2 | Artonin K [24] | 4.14 | C21H18O7 | 381.09924 | 3.3 | 337.0738 | 5.9 | [M – H − C2H4O]− |
| 365.0687 | 5.5 | [M – H − CH4]− | ||||||
| 3 | Albanin A [25] | 4.66 | C20H18O6 | 353.10459 | 4.3 | 125.0246 | 4.8 | [M – H − 1,4B]− |
| 231.0668 | 2.2 | [M – H − 1,4A]− | ||||||
| 4 | 14-Hydroxyartonin E [26] | 4.87 | C25H24O8 | 451.14188 | 4.5 | 393.0999 | 4.8 | [M – H − C3H6O]− |
| 433.1289 | 0.9 | [M − H − H2O]− | ||||||
| 5 | Artoindonesianin P [27] | 5.22 | C22H20O7 | 395.11548 | 4.7 | 337.0736 | 5.3 | [M – H − C3H6O]− |
| 351.0515 | 1.4 | [M – H − 3·CH3]− | ||||||
| 6 | Artonin J [28] | 5.47 | C25H24O7 | 435.14663 | 3.9 | 379.0839 | 4.2 | [M – H − C4H8]− |
| 365.0681 | 3.8 | [M – H − C5H10]− | ||||||
| 7 | Artelastoxanthone [18] | 6.67 | C25H22O7 | 433.12925 | −0.1 | 405.1399 | −1.2 | [M – H − CO]− |
| 389.1017 | 3.6 | [M – H − CO2]− | ||||||
| 8 | Artobiloxanthone [29] | 6.68 | C25H22O7 | 433.13224 | 6.8 | 405.1364 | 4.9 | [M – H − CO]− |
| 403.0850 | 6.7 | [M – H − 2CH3]− | ||||||
| 9 | Artonin E [28] | 7.23 | C25H24O7 | 435.14663 | 3.9 | 379.0841 | 4.7 | [M – H − C4H8]− |
| 351.0887 | 3.7 | [M – H − C5H4O]− | ||||||
| 10 | Cyclocommunol [30] | 7.31 | C20H16O6 | 351.08818 | 2.2 | 199.0777 | 6.0 | [M – H − 1,3A]− |
| 295.0259 | 3.7 | [M – H − C4H8]− | ||||||
| 11 | Artoindonesianin Q [4] | 7.47 | C22H22O7 | 397.12891 | −0.9 | 137.0247 | −2.2 | [M – H − 1,4B]− |
| 313.0345 | 2.9 | [M – H − CH3 − C5H9]− | ||||||
| 311.0195 | 0.6 | [M – H − OCH3 − C4H7]− | ||||||
| 365.1021 | −2.2 | [M – H − OCH3]− | ||||||
| 12 | Artoindonesianin R [31] | 7.49 | C22H22O7 | 397.13136 | 5.1 | 313.0357 | 1.0 | [M – H − C6H12]− |
| 137.0242 | −1.5 | [M – H − 1,4B]− | ||||||
| 13 | Artonol E [32] | 7.91 | C26H24O7 | 447.14548 | 1.2 | 415.1155 | −7.7 | [M – H − OCH4]− |
| 391.0831 | 2.0 | [M – H − C4H8]− | ||||||
| 14 | Artoindonesianin T [31] | 8.31 | C22H20O7 | 395.11548 | 4.7 | 339.0518 | 2.4 | [M – H − C4H8]− |
| 365.0655 | −3.3 | [M – H − 2CH3]− | ||||||
| 15 | Cycloartobiloxanthone [27] | 10.48 | C25H22O7 | 433.13134 | 4.7 | 391.0824 | 0.3 | [M – H − C3H6]− |
| 415.1177 | −2.4 | [M – H − H2O]− | ||||||
| 16 | Artoindonesianin B [33] | 10.92 | C26H28O8 | 467.17322 | 4.4 | 261.0758 | −3.8 | [M – H − 1,4A]− |
| 451.1399 | 0.2 | [M – H − CH4]− | ||||||
| 17 | Artocarpetin A [34] | 13.07 | C21H20O6 | 367.11769 | −2.8 | 233.0829 | −4.3 | [M – H − 1,3B]− |
| 297.0410 | −1.7 | [M – H − C5H10]− | ||||||
| 309.0763 | 1.6 | [M – H − C3H4O]− | ||||||
| 18 | Heteroflavanone C [35] | 13.44 | C23H26O7 | 413.16078 | 0.5 | 247.0975 | −0.4 | [M – H − C9H10O3]− |
| 235.0982 | 2.6 | [M – H − 1,2B]− | ||||||
| 19 | Artocarpesin [36] | 13.48 | C20H18O5 | 337.10903 | 2.6 | 133.0294 | −0.8 | [M – H − 1,3A]− |
| 281.0477 | 7.8 | [M – H − C4H8]− | ||||||
| 20 | 6-(3-Methylbut-2-enyl) Apigenin [18] | 13.5 | C20H18O5 | 337.10809 | −0.2 | 281.0464 | −3.2 | [M – H − C4H8]− |
| 117.0349 | −2.6 | [M – H − 1,3A]− | ||||||
| 21 | Styracifolin D [4] | 13.88 | C30H32O8 | 519.20258 | 0.3 | 461.1583 | 5.0 | [M – H − C3H6O]− |
| 491.2075 | 0.0 | [M – H − CO]− | ||||||
| 501.1919 | 0.0 | [M – H − H2O]− | ||||||
| 22 | Cycloartocarpesin [37] | 14.28 | C20H16O5 | 335.09274 | 0.7 | 133.0301 | 4.5 | [M – H − 1,3A]− |
| 305.0462 | 2.3 | [M – H − 2CH3]− | ||||||
| 23 | Artonin U [38] | 15.17 | C21H20O5 | 351.12462 | 2.4 | 281.0469 | 5.0 | [M – H − C5H10]− |
| 24 | Norartocarpin [39] | 17.16 | C25H26O6 | 421.16567 | 0 | 227.0725 | 4.8 | [M – H − 1,4A]− |
| 309.0391 | −4.5 | [M – H − C8H16]− | ||||||
| 365.1009 | -6.0 | [M – H − C4H8]− | ||||||
| 25 | Mulberrochromene [40] | 17.50 | C25H24O6 | 419.15040 | 0.9 | 199.0781 | 8.0 | [M – H − 1,3A]− |
| 349.0726 | 2.3 | [M – H − C5H10]− | ||||||
| 26 | Dihydroisocycloartomunin [31] | 17.79 | C26H26O7 | 449.16080 | 0.5 | 191.0715 | 0.5 | [M – H − 1,4B]− |
| 365.0694 | 7.4 | [M – H − C6H12]− | ||||||
| 27 | Morusin [41] | 18.48 | C25H24O6 | 419.15040 | 0.9 | 191.0728 | 7.3 | [M – H − 1,4B]− |
| 217.0515 | 4.1 | [M – H − 1,3B]− | ||||||
| 227.0725 | 4.8 | [M – H − 1,4A]− | ||||||
| 28 | Heterophyllin [17] | 20.55 | C30H32O7 | 503.20842 | 2.1 | 217.0879 | 4.1 | [M – H − 1,3A]− |
| 243.0662 | −0.4 | [M – H − 1,4A]− | ||||||
| 259.1351 | 4.2 | [M – H − 1,4B]− | ||||||
| 285.1112 | 5.6 | [M – H − 1,3B]− | ||||||
| 29 | Artocarpetin B [42] | 20.72 | C22H22O6 | 381.13518 | 2.2 | 233.0806 | −5.6 | [M – H − 1,3B]− |
| 311.0549 | −3.9 | [M – H − C5H10]− | ||||||
| 30 | Artelastofuran [43] | 21.07 | C30H34O7 | 505.22363 | 0.9 | 277.1455 | 3.6 | [M – H − 1,4B]− |
| 285.1147 | 5.3 | [M – H − 0,3B]− | ||||||
| 31 | 5’-Hydroxycudraflavone A [17] | 21.81 | C25H22O7 | 433.12925 | −0.1 | 389.1372 | 5.7 | [M – H − CO2]− |
| 415.1192 | −1.2 | [M – H − H2O]− | ||||||
| 217.0516 | −4.6 | [M – H − 1,3A]− | ||||||
| 377.0663 | 1.1 | [M – H − C3H4O]− | ||||||
| 32 | Artonin F [44] | 22.04 | C30H30O7 | 501.19231 | 0.9 | 429.1343 | −0.2 | [M – H − C4H8O]− |
| 473.1945 | −5.3 | [M – H − CO]− | ||||||
| 33 | Isocycloheterophyllin [45] | 22.23 | C30H30O7 | 501.19290 | −2.0 | 445.1267 | −2.2 | [M – H − CH4]− |
| 485.1615 | −1.9 | [M – H − C3H4O]− | ||||||
| 443.1511 | −2.5 | [M – H − C3H6O]− | ||||||
| 34 | Artoindonesianin I [46] | 22.70 | C30H34O7 | 505.22363 | 0.9 | 285.1137 | 1.8 | [M – H − 0,3B]− |
| 447.1806 | −1.6 | [M – H − C3H6O]− | ||||||
| 35 | Artocarpin [47] | 23.48 | C26H28O6 | 435.18167 | 0.8 | 207.1039 | 5.8 | [M – H − 1,4B]− |
| 233.0818 | −0.4 | [M – H − 1,3B]− | ||||||
| 36 | Cannflavin C [20] | 23.59 | C26H28O6 | 435.18022 | −2.5 | 351.0878 | −1.1 | [M – H − C6H12]− |
| 323.0939 | −4.3 | [M – H − C6H7 − OCH3]− | ||||||
| 313.1452 | −2.2 | [M – H − C9H14]− | ||||||
| 37 | Artonin H [44] | 25.10 | C30H34O7 | 505.22363 | 0.9 | 191.0728 | 7.3 | [M – H − 1,4B]− |
| 217.0512 | 2.8 | [M – H − 1,3B]− | ||||||
| 38 | Artonin S [38] | 25.36 | C26H28O7 | 451.17621 | 0.0 | 393.1357 | 3.3 | [M – H − C3H6O]− |
| 421.1311 | 4.3 | [M – H − 2CH3]− | ||||||
| 39 | Artonin G [44] | 26.21 | C30H32O7 | 503.20862 | 2.2 | 447.1422 | −6.0 | [M – H − C4H8]− |
| 475.2103 | −4.8 | [M – H − CO]− | ||||||
| 40 | Artonin A [48] | 28.15 | C30H30O7 | 501.19231 | 0.9 | 457.1286 | −1.5 | [M – H − C3H8]− |
| 473.1950 | −4.2 | [M – H − CO]− | ||||||
| 41 | Artonin B [48] | 28.39 | C30H30O7 | 501.19230 | 0.8 | 485.1602 | 0.8 | [M – H − CH4]− |
| 445.1296 | −0.7 | [M – H − C3H4O]− | ||||||
| 42 | Cudraflavone A [49] | 28.41 | C25H22O6 | 417.13475 | 0.9 | 217.0515 | 4.1 | [M – H − 1,3B]− |
| 373.1072 | −2.4 | [M – H − C2H4O]− | ||||||
| 43 | Artoindonesianin G [46] | 29.45 | C30H32O6 | 487.21308 | 1.0 | 259.1351 | 4.2 | [M – H − 1,4B]− |
| 285.1116 | −5.6 | [M – H − 1,3B]− | ||||||
| 365.1762 | 1.1 | [M – H − C7H6O2]− | ||||||
| 44 | Cycloartocarpin [50] | 30.97 | C26H26O6 | 433.16608 | 1.0 | 199.0777 | 6.0 | [M – H − 1,3A]− |
| 363.0878 | 1.1 | [M – H − C5H10]− | ||||||
| 375.0878 | 1.1 | [M – H − C4H10]− | ||||||
| 45 | Artoindonesianin H [46] | 31.80 | C30H32O6 | 487.21308 | 1.0 | 227.0731 | 7.5 | [M – H − C9H18]− |
| 361.0731 | 3.6 | [M – H − 1,4A]− | ||||||
| 473.1966 | −0.8 | [M – H − CO]− | ||||||
| 46 | Cycloheterophyllin [17] | 33.05 | C30H30O7 | 501.19231 | 0.9 | 285.1145 | 4.6 | [M – H − 1,3B]− |
| 445.1281 | −2.7 | [M – H − C4H8]− | ||||||
| 473.1966 | −0.8 | [M – H − CO]− | ||||||
| 47 | Artelastochromene [43] | 36.01 | C30H30O6 | 485.19690 | −0.1 | 285.1145 | 4.6 | [M – H − 1,3B]− |
| 469.1659 | 0.4 | [M – H − CH4]− |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.-B.; Ren, G.; Li, W.-Y.; Zhong, G.-Y.; Zhang, M.; Yuan, J.-B.; Lu, T. Characterization and Identification of Prenylated Flavonoids from Artocarpus heterophyllus Lam. Roots by Quadrupole Time-Of-Flight and Linear Trap Quadrupole Orbitrap Mass Spectrometry. Molecules 2019, 24, 4591. https://doi.org/10.3390/molecules24244591
Ye J-B, Ren G, Li W-Y, Zhong G-Y, Zhang M, Yuan J-B, Lu T. Characterization and Identification of Prenylated Flavonoids from Artocarpus heterophyllus Lam. Roots by Quadrupole Time-Of-Flight and Linear Trap Quadrupole Orbitrap Mass Spectrometry. Molecules. 2019; 24(24):4591. https://doi.org/10.3390/molecules24244591
Chicago/Turabian StyleYe, Jin-Bao, Gang Ren, Wen-Yan Li, Guo-Yue Zhong, Min Zhang, Jin-Bin Yuan, and Ting Lu. 2019. "Characterization and Identification of Prenylated Flavonoids from Artocarpus heterophyllus Lam. Roots by Quadrupole Time-Of-Flight and Linear Trap Quadrupole Orbitrap Mass Spectrometry" Molecules 24, no. 24: 4591. https://doi.org/10.3390/molecules24244591
APA StyleYe, J.-B., Ren, G., Li, W.-Y., Zhong, G.-Y., Zhang, M., Yuan, J.-B., & Lu, T. (2019). Characterization and Identification of Prenylated Flavonoids from Artocarpus heterophyllus Lam. Roots by Quadrupole Time-Of-Flight and Linear Trap Quadrupole Orbitrap Mass Spectrometry. Molecules, 24(24), 4591. https://doi.org/10.3390/molecules24244591