A New Strategy for Effective Succinic Acid Production by Enterobacter sp. LU1 Using a Medium Based on Crude Glycerol and Whey Permeate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Culture Conditions
2.3. Batch and Fed-Batch Fermentations
2.4. Description of Experiments
2.4.1. Increase of Lactose Content in Fermentation Medium
2.4.2. Fermentation Medium Based on Crude Glycerol and Whey Permeate
2.4.3. Reduced Yeast Extract Content and Flask Based Inoculum
2.4.4. Reduced YE Content and Bioreactor Based Inoculum
2.4.5. Microaerobic Cultivation on Crude Glycerol and Whey Permeate
2.4.6. Microaerobic Cultivation with Glycerol as the Sole Carbon Source
2.5. Analytical Methods
2.6. Calculation of Fermentation Parameters
3. Results
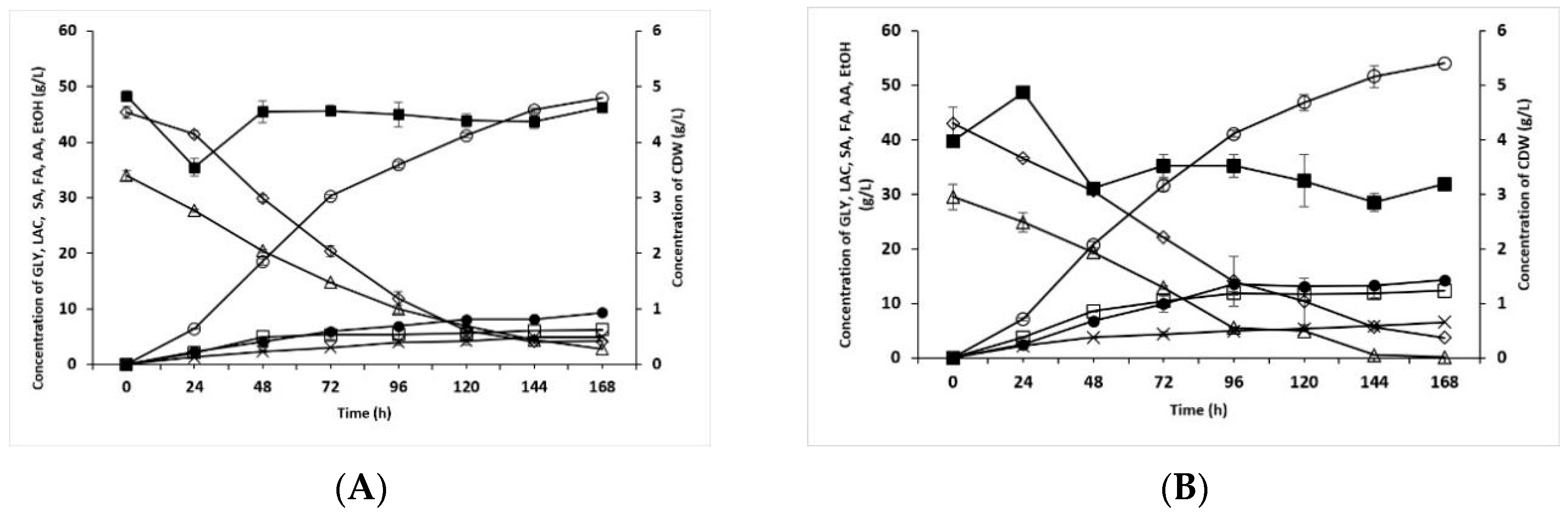
3.1. Increase of Lactose Content in Fermentation Medium and Usage of Crude Glycerol and Whey Permeate
3.2. Reduced YE Content and Different Methods of Inoculum Preparation
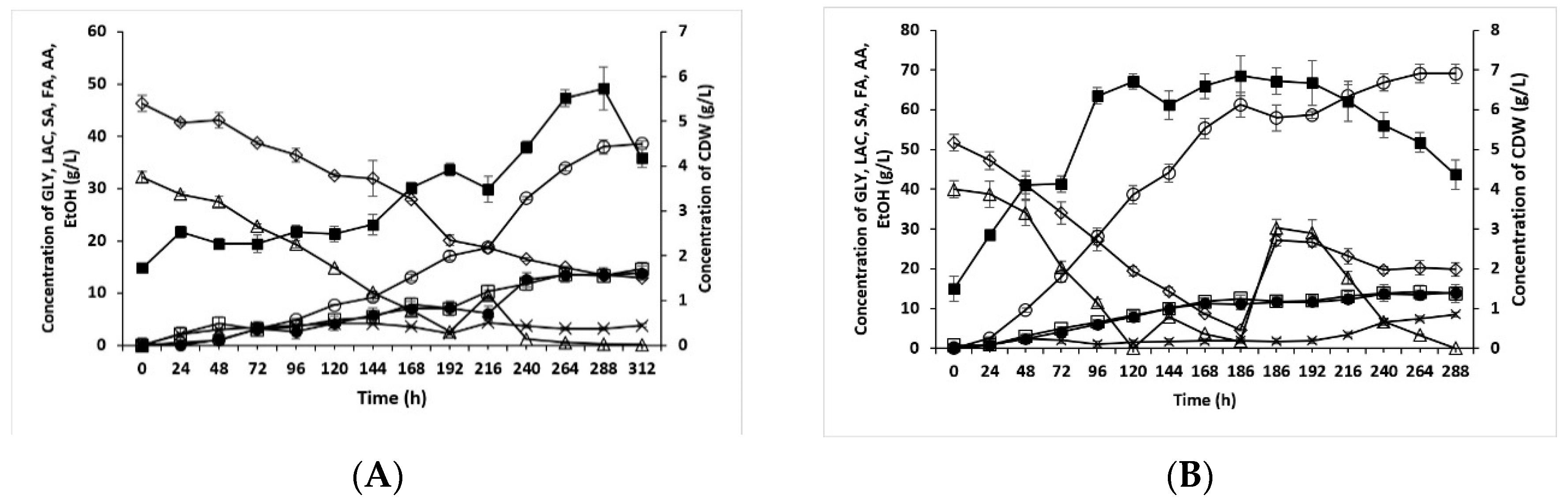
3.3. Microaerobic Cultivation on Crude Glycerol and Whey Permeate
3.4. Microaerobic Cultivation on Glycerol as the Sole Carbon Source
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.C.; Lee, W.G.; Lee, S.Y.; Chang, H.N. Succinic acid production with reduced by-product formation in the fermentation of Anaerobiospirillum succiniciproducens using glycerol as a carbon source. Biotechnol. Bioeng. 2001, 72, 41–48. [Google Scholar] [CrossRef]
- Wilkens, E.; Ringel, A.K.; Hortig, D.; Willke, T.; Vorlop, K.D. High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012, 93, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Barbirato, F.; Chedaille, D.; Bories, A. Propionic acid fermentation from glycerol: Comparison with conventional substrates. Appl. Microbiol. Biotechnol. 1997, 47, 441–446. [Google Scholar] [CrossRef]
- Priya, A.; Lal, B. Efficient valorization of waste glycerol to 2,3-butanediol using Enterobacter cloacae TERI BD 18 as a biocatalyst. Fuel 2019, 250, 292–305. [Google Scholar] [CrossRef]
- Cintolesi, A.; Clomburg, J.M.; Rigou, V.; Zygourakis, K.; Gonzalez, R. Quantitative analysis of the fermentative metabolism of glycerol in Escherichia coli. Biotechnol. Bioeng. 2012, 109, 187–198. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Davison, B.H.; Suttle, B.E.; Richardson, G.R. Production of succinic acid by Anaerobiospirillum succiniciproducens. Appl. Biochem. Biotechnol. 1997, 63, 565–576. [Google Scholar] [CrossRef]
- Vlysidis, A.; Binns, M.; Webb, C.; Theodoropoulos, C. Glycerol utilisation for the production of chemicals: Conversion to succinic acid, a combined experimental and computational study. Biochem. Eng. J. 2011, 58, 1–11. [Google Scholar] [CrossRef]
- Podleśny, M.; Jarocki, P.; Wyrostek, J.; Czernecki, T.; Kucharska, J.; Nowak, A.; Targoński, Z. Enterobacter sp. LU1 as a novel succinic acid producer – co-utilization of glycerol and lactose. Microb. Biotechnol. 2017, 10, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Guettler, M.V.; Jain, M.K.; Rumler, D. Method for making succinic acid, bacterial variants for use in the process, and method for obtaining variants. U.S. Patent 5573931, 1996. [Google Scholar]
- Jantama, K.; Haupt, M.J.; Svoronos, S.A.; Zhang, X.; Moore, J.C.; Shanmugam, K.T.; Ingram, L.O. Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol. Bioeng. 2008, 99, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Samuelov, N.S.; Datta, R.; Jain, M.K.; Zeikus, J.G. Whey fermentation by Anaerobiospirillum succiniciproducens for production of a succinate-based animal feed additive. Appl. Environ. Microbiol. 1999, 65, 2260–2263. [Google Scholar] [PubMed]
- Liu, Y.P.; Zheng, P.; Sun, Z.H.; Ni, Y.; Dong, J.J.; Wei, P. Strategies of pH control and glucose-fed batch fermentation for production of succinic acid by Actinobacillus succinogenes CGMCC1593. J. Chem. Technol. Biotechnol. 2008, 83, 722–729. [Google Scholar] [CrossRef]
- Lee, S.J.; Song, H.; Lee, S.Y. Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl. Environ. Microbiol. 2006, 72, 1939–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Li, Z.M.; Zhou, L.; Ye, Q. Improved succinic acid production in the anaerobic culture of an Escherichia coli pflB ldhA double mutant as a result of enhanced anaplerotic activities in the preceding aerobic culture. Appl. Environ. Microbiol. 2007, 73, 7837–7843. [Google Scholar] [CrossRef] [Green Version]
- Bu, J.; Yan, X.; Wang, Y.-T.; Zhu, S.-M.; Zhu, M.-J. Co-production of high-gravity bioethanol and succinic acid from potassium peroxymonosulfate and deacetylation sequentially pretreated sugarcane bagasse by simultaneous saccharification and co-fermentation. Energy Convers. Manag. 2019, 186, 131–139. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhu, L.W.; Li, H.M.; Tang, Y.J. Performance analyses of a neutralizing agent combination strategy for the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Bioprocess Biosyst. Eng. 2012, 35, 659–664. [Google Scholar] [CrossRef]
- Lee, P.C.; Lee, W.G.; Lee, S.Y.; Chang, H.N. Effects of medium components on the growth of Anaerobiospirillum succiniciproducens and succinic acid production. Process Biochem. 1999, 35, 49–55. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.W.; Choi, S.; You, J.K.; Hong, W.H.; Lee, S.Y. Effects of dissolved CO2 levels on the growth of Mannheimia succiniciproducens and succinic acid production. Biotechnol. Bioeng. 2007, 98, 1296–1304. [Google Scholar] [CrossRef]
- Bazaes, S.; Toncio, M.; Laivenieks, M.; Zeikus, J.G.; Cardemil, E. Comparative kinetic effects of Mn (II), Mg (II) and the ATP/ADP ratio on phosphoenolpyruvate carboxykinases from Anaerobiospirillum succiniciproducens and Saccharomyces cerevisiae. Protein J. 2007, 26, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Helmerius, J.; Hodge, D.; Berglund, K.A.; Rova, U. Inhibition of succinic acid production in metabolically engineered Escherichia coli by neutralizing agent, organic acids, and osmolarity. Biotechnol. Prog. 2009, 25, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Okino, S.; Noburyu, R.; Suda, M.; Jojima, T.; Inui, M.; Yukawa, H. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl. Microbiol. Biotechnol. 2008, 81, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Haefner, S.; Von Abendroth, G.; Hollmann, R.; Raddatz, A.; Ernst, H.; Gurski, H. Novel microbial succinic acid producers and purification of succinic acid. Patent WO2010092155 A1, 2010. [Google Scholar]
- Dharmadi, Y.; Murarka, A.; Gonzalez, R. Anaerobic fermentation of glycerol by Escherichia coli: A new platform for metabolic engineering. Biotechnol. Bioeng. 2006, 94, 821–829. [Google Scholar] [CrossRef]
- Podleśny, M.; Kubik-Komar, A.; Kucharska, J.; Wyrostek, J.; Jarocki, P.; Targoński, Z. Media optimization for economic succinic acid production by Enterobacter sp. LU1. AMB Express 2017, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Beauprez, J.J.; De Mey, M.; Soetaert, W.K. Microbial succinic acid production: Natural versus metabolic engineered producers. Process Biochem. 2010, 45, 1103–1114. [Google Scholar] [CrossRef]
- Fan, S.; Xiao, Z.; Tang, X.; Chen, C.; Zhang, Y.; Deng, Q.; Yao, P.; Li, W. Inhibition effect of secondary metabolites accumulated in a pervaporation membrane bioreactor on ethanol fermentation of Saccharomyces cerevisiae. Bioresour. Technol. 2014, 162, 8–13. [Google Scholar] [CrossRef]
- Ren, Q.; Henes, B.; Fairhead, M.; Thöny-Meyer, L. High level production of tyrosinase in recombinant Escherichia coli. BMC Biotechnol. 2013, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Zheng, P.; Tao, S.T.; Dong, J.J. Fermentation process for continuous production of succinic acid in a fibrous bed bioreactor. Biochem. Eng. J. 2014, 91, 92–98. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Zhang, J.; Wang, H.; Zhao, M.; Wang, N.; Dong, L.; Zhou, X.; Wang, D. Enhanced succinic acid production and magnesium utilization by overexpression of magnesium transporter mgtA in Escherichia coli mutant. Bioresour. Technol. 2014, 170, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.L.A.; Segueilha, L.; Verwaal, R.; Louchart, M. Dicarboxylic acid production process. Patent WO2012038390 A1, 2012. [Google Scholar]
- Durnin, G.; Clomburg, J.; Yeates, Z.; Alvarez, P.J.J.; Zygourakis, K.; Campbell, P.; Gonzalez, R. Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol. Bioeng. 2009, 103, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shanmugam, K.T.; Ingram, L.O. Fermentation of glycerol to succinate by metabolically engineered strains of Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 2397–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuzbashev, T.V.; Yuzbasheva, E.Y.; Sobolevskaya, T.I.; Laptev, I.A.; Vybornaya, T.V.; Larina, A.S.; Matsui, K.; Fukui, K.; Sineoky, S.P. Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol. Bioeng. 2010, 107, 673–682. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
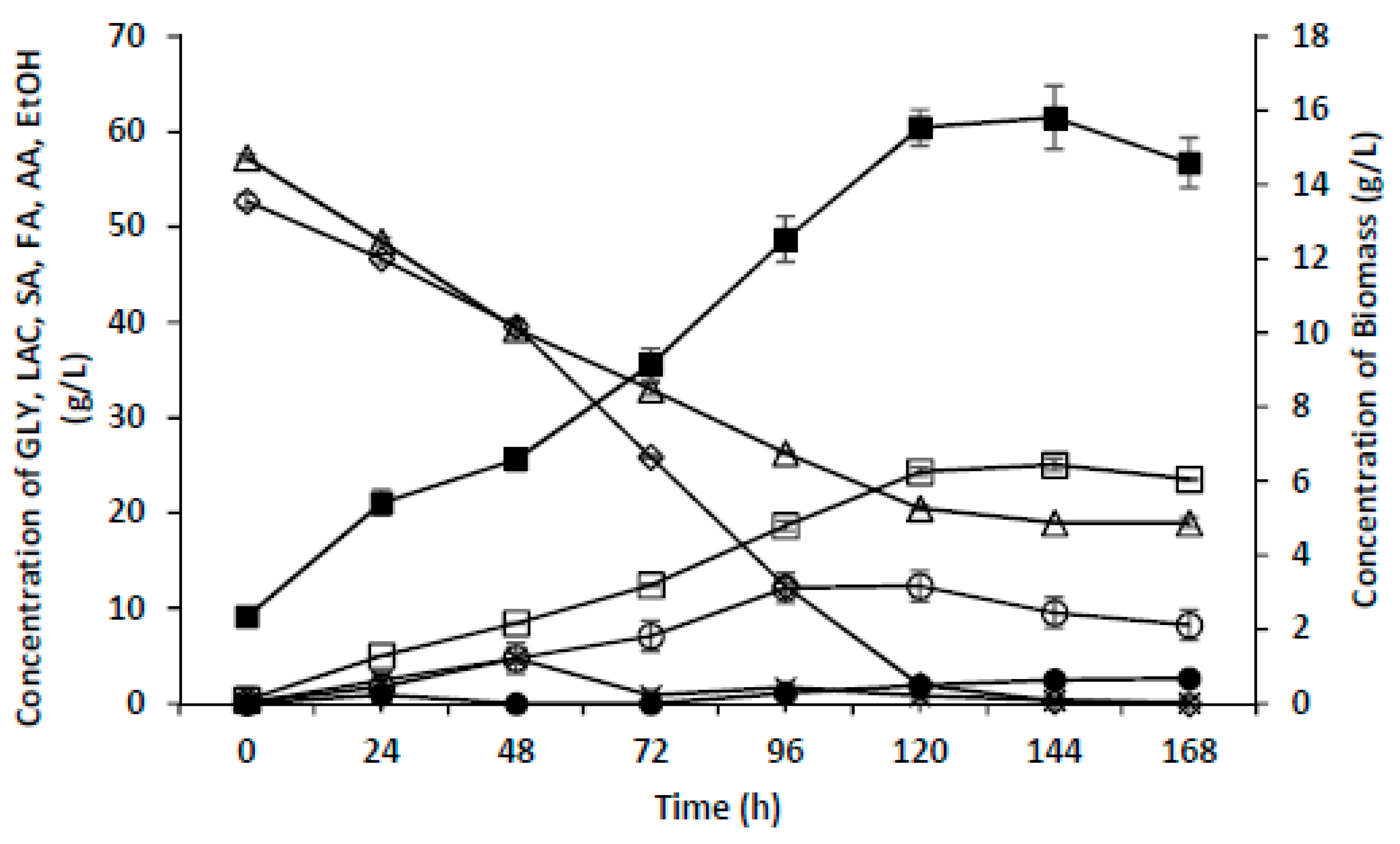
| Time (h) | GLY (g/L) | Products (g/L) | ||||
|---|---|---|---|---|---|---|
| SA | FA | AA | EtOH | CDW | ||
| 0 | 51.1 ± 0.49 | 0.26 ± 0.01 | 0.10 ± 0.02 | 0.30 ± 0.24 | n.d. | 1.45 ± 0.21 |
| 24 | 37.95 ± 0.62 | 2.50 ± 0.01 | 2.18 ± 0.05 | 5.20 ± 0.01 | n.d. | 4.88 ± 0.32 |
| 48 | 25.5 ± 0.47 | 6.77 ± 0.18 | 3.30 ± 0.14 | 9.10 ± 0.35 | n.d. | 6.90 ± 0.15 |
| 72 | 67.5 ± 0.03 | 11.51 ± 0.05 | 4.78 ± 0.05 | 12.96 ± 0.11 | n.d. | 7.94 ± 0.38 |
| 96 | 57.71 ± 1.28 | 15.69 ± 0.27 | 6.67 ± 0.14 | 16.68 ± 0.26 | n.d. | 8.89 ± 0.44 |
| 120 | 44.76 ± 0.63 | 21.73 ± 0.34 | 7.45 ± 0.02 | 19.95 ± 0.28 | n.d. | 8.31 ± 0.26 |
| 144 | 33.5 ± 0.19 | 28.19 ± 0.18 | 8.10 ± 0.03 | 23.00 ± 0.18 | n.d. | 11.18 ± 0.13 |
| 168 | 25.4 ± 0.29 | 33.12 ± 0.39 | 7.80 ± 0.16 | 25.60 ± 0.30 | n.d. | 11.40 ± 0.54 |
| 192 | 21.6 ± 0.48 | 37.37 ± 0.73 | 3.00 ± 0.06 | 28.80 ± 0.49 | n.d. | 10.23 ± 0.39 |
| Strain | Fermentation Conditions | Concentration (g/L) | Yield (g/g) | Productivity (g/L/h) | Reference |
|---|---|---|---|---|---|
| Actinobacillus succinogenes NCIMB 41825 | Anaerobic, batch | 29.3 | 1.23 | 0.270 | [9] |
| Anaerobiospirillum succiniciproducens ATCC 53488 | Anaerobic, fed-batch (feeding with YE and glycerol) | 19 | 1.60 | 0.157 | [3] |
| Escherichia coli pck+ Δpfl | Anaerobic, batch | 12.1 | 1.03 | 0.084 | [34] |
| Yarrowia lipolytica Y3314 Δsdh2 | Aerobic, batch | 45 | 0.36 | 0.271 | [35] |
| Basfia succiniciproducens DD1 | Anaerobic, batch (without disaccharide addition) | 19.5 | 1.12 | 0.81 | [24] |
| Basfia succiniciproducens DD1 Δpfl Δldh | Anaerobic, batch (without disaccharide addition) | 36.2 | 1.26 | 1.50 | [24] |
| Basfia succiniciproducens DD1 Δpfl Δldh | Anaerobic, batch (maltose addition) | 69.8 | 1.11 | 2.90 | [24] |
| Enterobacter sp. LU1 | Microaerobic, fed-batch | 37.3 ± 0.73 | 0.38 ± 0.21 | 0.19 ± 0.09 | This study |
| Enterobacter sp. LU1 | Anaerobic, fed-batch (lactose addition) | 69.0 ± 2.50 | 0.58 ± 0.11 | 0.24 ± 0.12 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podleśny, M.; Wyrostek, J.; Kucharska, J.; Jarocki, P.; Komoń-Janczara, E.; Targoński, Z. A New Strategy for Effective Succinic Acid Production by Enterobacter sp. LU1 Using a Medium Based on Crude Glycerol and Whey Permeate. Molecules 2019, 24, 4543. https://doi.org/10.3390/molecules24244543
Podleśny M, Wyrostek J, Kucharska J, Jarocki P, Komoń-Janczara E, Targoński Z. A New Strategy for Effective Succinic Acid Production by Enterobacter sp. LU1 Using a Medium Based on Crude Glycerol and Whey Permeate. Molecules. 2019; 24(24):4543. https://doi.org/10.3390/molecules24244543
Chicago/Turabian StylePodleśny, Marcin, Jakub Wyrostek, Jagoda Kucharska, Piotr Jarocki, Elwira Komoń-Janczara, and Zdzisław Targoński. 2019. "A New Strategy for Effective Succinic Acid Production by Enterobacter sp. LU1 Using a Medium Based on Crude Glycerol and Whey Permeate" Molecules 24, no. 24: 4543. https://doi.org/10.3390/molecules24244543
APA StylePodleśny, M., Wyrostek, J., Kucharska, J., Jarocki, P., Komoń-Janczara, E., & Targoński, Z. (2019). A New Strategy for Effective Succinic Acid Production by Enterobacter sp. LU1 Using a Medium Based on Crude Glycerol and Whey Permeate. Molecules, 24(24), 4543. https://doi.org/10.3390/molecules24244543





