Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System
Abstract
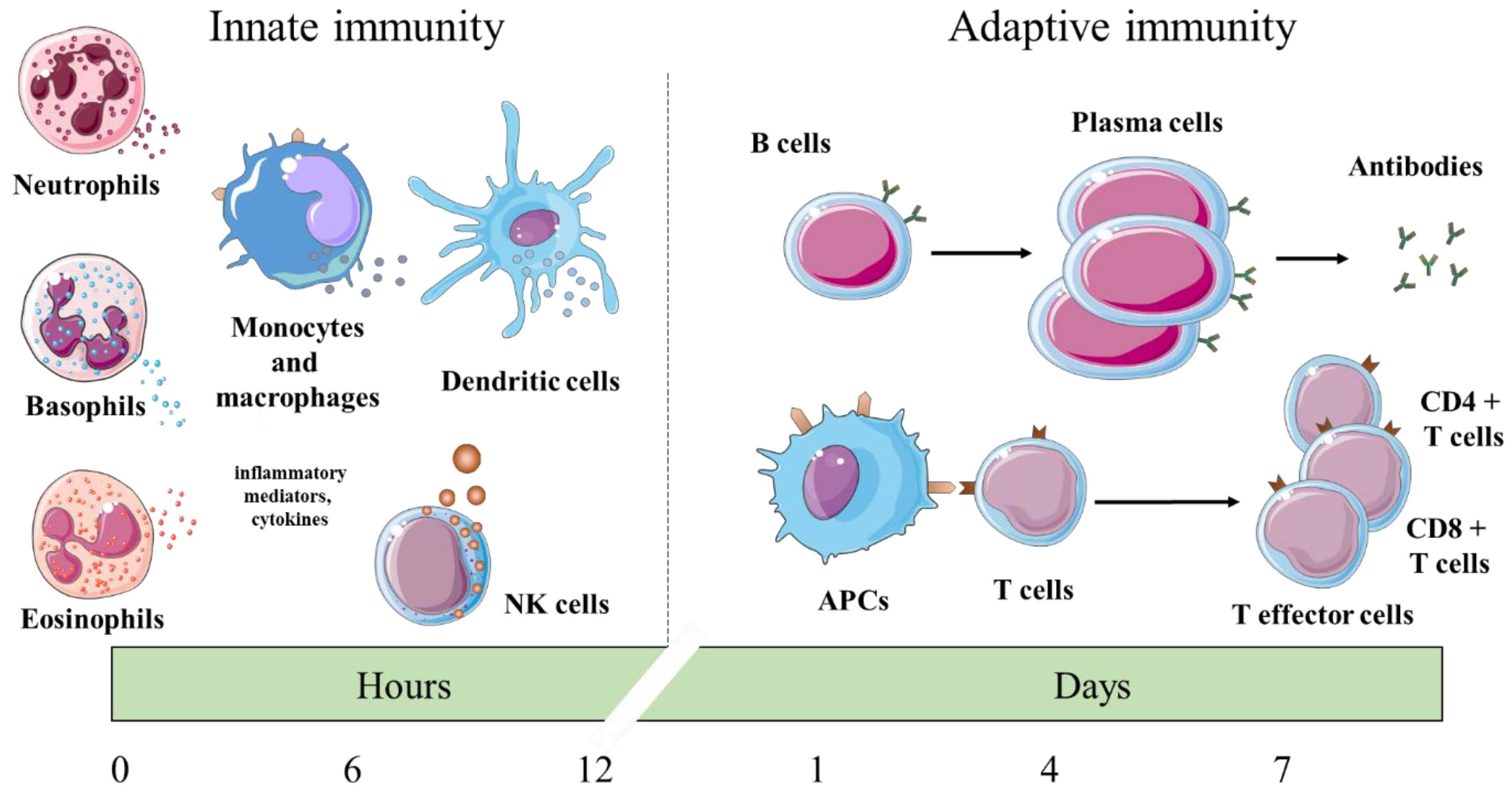
:1. Introduction
2. In Vitro Investigations
3. In Vivo Pre-Clinical Studies
4. Dietary Supplementation for Livestock and Fish
5. Human Studies
6. Effects of Essential Oils from Trees—Forest Bathing
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.F.; Lin, S.S.; Liao, P.H.; Young, S.C.; Yang, C.C. The immunopharmaceutical effects and mechanisms of herb medicine. Cell. Mol. Immunol. 2008, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Delves, P.; Roitt, I.M. The immune system. First of two parts. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [PubMed]
- Delves, P.J.; Roitt, I.M. The immune system. Second of two parts. N. Engl. J. Med. 2000, 343, 108–117. [Google Scholar] [PubMed]
- Smyth, M.J.; Kelly, J.M.; Sutton, V.R.; Davis, J.E.; Browne, K.A.; Sayers, T.J.; Trapani, J.A. Unlocking the secrets of cytotoxic granule proteins. J. Leukoc. Biol. 2001, 70, 18–29. [Google Scholar] [PubMed]
- Chowdhury, D.; Lieberman, J. Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008, 26, 389–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SaiRam, M.; Sharma, S.K.; Ilavazhagan, G.; Kumar, D.; Selvamurthy, W. Immunomodulatory effects of NIM-76, a volatile fraction from Neem oil. J. Ethnopharmacol. 1997, 55, 133–139. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Kaur, R.; Bhat, Z.A.; Gupta, V.K.; Kumar, V. A review of immunomodulators in the Indian traditional health care system. J. Microbiol. Immunol. Infect. 2012, 45, 165–184. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491s–499s. [Google Scholar] [CrossRef]
- Barrett, B. Medicinal properties of Echinacea: A critical review. Phytomedicine 2003, 10, 66–86. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phyther. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, C.; Fang, L.; Min, W. Protein Hydrolyzates from Changbai Mountain Walnut (Juglans mandshurica Maxim.) Boost Mouse Immune System and Exhibit Immunoregulatory Activities. Evid. Based Complement. Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manion, C.R.; Widder, R.M. Essentials of essential oils. Am. J. Heal. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phyther. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Haran, G.H.; da Silva Calisto, V.K.; Jothi, G.; de Souza Siqueira Quintans, J.; Cuevas, L.E.; Narain, N.; Júnior, L.J.Q.; Cipolotti, R.; et al. Essential oils and its bioactive compounds modulating cytokines: A systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine 2019, 31, 152854. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils - A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- de Lavor, É.M.; Cavalcante Fernandes, A.W.; de Andrade Teles, R.B.; Pereira Leal, A.E.B.; de Oliveira, R.G.; Gama e Silva, M.; de Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Melo Coutinho, H.D.; et al. Essential oils and their major compounds in the treatment of chronic inflammation: A review of antioxidant potential in preclinical studies and molecular mechanisms. Oxid. Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, D.P.; De Almeida Soares Hocayen, P.; Andrade, L.N.; Andreatini, R. A systematic review of the anxiolytic-like effects of essential oils in animal models. Molecules 2015, 20, 18620–18660. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef]
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef]
- Standen, M.D.; Myers, S.P. The roles of essential oils in the modulation of immune function and inflammation: Survey of aromatherapy educators. Int. J. Aromather. 2004, 14, 150–161. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Balasubramanian, N.; Quinn, M.T. Neutrophil immunomodulatory activity of natural organosulfur compounds. Molecules 2019, 24, 1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafino, A.; Vallebona, P.; Andreola, F.; Zonfrillo, M.; Mercuri, L.; Federici, M.; Rasi, G.; Garaci, E.; Pierimarchi, P. Stimulatory effect of Eucalyptus essential oil on innate cell-mediated immune response. BMC Immunol. 2008, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadlon, A.E.; Lamson, D.W. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern. Med. Rev. J. Clin. Ther. 2010, 15, 33–47. [Google Scholar]
- Dibazar, S.P.; Fateh, S.; Daneshmandi, S. Immunomodulatory effects of clove (Syzygium aromaticum) constituents on macrophages: In vitro evaluations of aqueous and ethanolic components. J. Immunotoxicol. 2015, 12, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Dibazar, S.P.; Fateh, S.; Daneshmandi, S. Clove (Syzygium aromaticum) ingredients affect lymphocyte subtypes expansion and cytokine profile responses: An in vitro evaluation. J. Food Drug Anal. 2014, 22, 448–454. [Google Scholar] [CrossRef]
- Özek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Özek, T.; Başer, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Mikhaeil, B.R.; Maatooq, G.T.; Badria, F.A.; Amer, M.M.A. Chemistry and immunomodulatory activity of frankincense oil. Zeitschrift Fur Naturforsch. C J. Biosci. 2003, 58, 230–238. [Google Scholar] [CrossRef]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. Essential Oil Exerts Antibacterial and Anti-Inflammatory Effect in Macrophage Mediated Immune Response to Staphylococcus aureus. Immunol. Invest. 2016, 45, 11–28. [Google Scholar] [CrossRef]
- Krifa, M.; El Mekdad, H.; Bentouati, N.; Pizzi, A.; Ghedira, K.; Hammami, M.; El Meshri, S.E.; Chekir-Ghedira, L. Immunomodulatory and anticancer effects of Pituranthos tortuosus essential oil. Tumor Biol. 2015, 36, 5165–5170. [Google Scholar] [CrossRef]
- Duarte, J.A.; de Bairros Zambrano, L.A.; Quintana, L.D.; Rocha, M.B.; Schmitt, E.G.; Boligon, A.A.; Anraku de Campos, M.M.; De Oliveira, L.F.S.; Machado, M.M. Immunotoxicological Evaluation of Schinus molle L. (Anacardiaceae) Essential Oil in Lymphocytes and Macrophages. Evid. Based Complement. Altern. Med. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Franca Rodrigues, K.A.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.C.; Carneiro, S.M.P.; de Amorim Carvalho, F.A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullio, V.; Mandras, N.; Allizond, V.; Nostro, A.; Roana, J.; Merlino, C.; Banche, G.; Scalas, D.; Cuffini, A.M. Positive interaction of thyme (red) essential oil with human polymorphonuclear granulocytes in eradicating intracellular Candida albicans. Planta Med. 2012, 78, 1633–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonar, P.K.; Singh, R.; Saraf, S.K. Phytochemical, chromatographic and spectroscopic investigation of Carum copticum seeds and their potential as immunomodulatory agents. Pharm. Biol. 2015, 54, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Halder, S.; Mehta, A.K.; Mediratta, P.K.; Sharma, K.K. Essential oil of clove (Eugenia caryophyllata) augments the humoral immune response but decreases cell mediated immunity. Phyther. Res. 2011, 25, 1254–1256. [Google Scholar] [CrossRef]
- Hertiani, T.; Yuswanto, A.; Utami Tunjung Pratiwi, S.; Muthma’innah Mashar, H. Effect of massoia (Massoia aromatica becc.) bark on the phagocytic activity of wistar rat macrophages. Sci. Pharm. 2018, 86, 19. [Google Scholar] [CrossRef] [Green Version]
- Hertiani, T.; Pratiwi, S.; Yuswanto, A.; Permanasari, P. Potency of Massoia bark in combating immunosuppressed-related infection. Pharmacogn. Mag. 2016, 12, 363. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.Y.; Chang, M.H.; Do, J.S.; Seo, H.J.; Oh, H.K. Essential oil of niaouli preferentially potentiates antigen-specific cellular immunity and cytokine production by macrophages. Immunopharmacol. Immunotoxicol. 2008, 30, 459–474. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Fan, G.; Ren, J.N.; Zhang, L.L.; Pan, S.Y. Effects of orange essential oil on intestinal microflora in mice. J. Sci. Food Agric. 2019, 99, 4019–4028. [Google Scholar] [CrossRef]
- Reyes, A.W.B.; Hop, H.T.; Arayan, L.T.; Huy, T.X.N.; Park, S.J.; Kim, K.D.; Min, W.G.; Lee, H.J.; Rhee, M.H.; Kwak, Y.S.; et al. The host immune enhancing agent Korean red ginseng oil successfully attenuates Brucella abortus infection in a murine model. J. Ethnopharmacol. 2017, 198, 5–14. [Google Scholar] [CrossRef]
- Carrasco, F.R.; Schmidt, G.; Romero, A.L.; Sartoretto, J.L.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: Evidence for humor- and cell-mediated responses. J. Pharm. Pharmacol. 2009, 61, 961–967. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.L.; Figueiredo, P.M.S.; Yano, T. Chemotherapeutic potential of the volatile oils from Zanthoxylum rhoifolium Lam leaves. Eur. J. Pharmacol. 2007, 576, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hesabi Nameghi, A.; Edalatian, O.; Bakhshalinejad, R. Effects of a blend of thyme, peppermint and eucalyptus essential oils on growth performance, serum lipid and hepatic enzyme indices, immune response and ileal morphology and microflora in broilers. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Hu, J.; Liu, W.; Liu, Z.; He, S.; Dan, B.C.T.; Diem, N.N.; Ooi, E.L.; Zhou, Z. Thymol and Carvacrol Affect Hybrid Tilapia through the Combination of Direct Stimulation and an Intestinal Microbiota-Mediated Effect: Insights from a Germ-Free Zebrafish Model. J. Nutr. 2016, 146, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian Australas. J. Anim. Sci. 2012, 25, 1617–1626. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Ru, Y.J.; Liu, M.; Xu, B.; Péron, A.; Shi, X.G. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest. Sci. 2012, 145, 119–123. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef]
- Su, G.; Zhou, X.; Wang, Y.; Chen, D.; Chen, G.; Li, Y.; He, J. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.Z.; Benchaar, C.; Ametaj, B.N.; Chaves, A.V.; He, M.L.; McAllister, T.A. Effects of Garlic and Juniper Berry Essential Oils on Ruminal Fermentation and on the Site and Extent of Digestion in Lactating Cows. J. Dairy Sci. 2007, 90, 5671–5681. [Google Scholar] [CrossRef] [Green Version]
- Vazirzadeh, A.; Dehghan, F.; Kazemeini, R. Changes in growth, blood immune parameters and expression of immune related genes in rainbow trout (Oncorhynchus mykiss) in response to diet supplemented with Ducrosia anethifolia essential oil. Fish. Shellfish Immunol. 2017, 69, 164–172. [Google Scholar] [CrossRef]
- Farhadi, D.; Karimi, A.; Sadeghi, G.; Sheikhahmadi, A.; Habibian, M.; Raei, A.; Sobhani, K. Effects of using eucalyptus (Eucalyptus globulus L.) leaf powder and its essential oil on growth performance and immune response of broiler chickens. Iran. J. Vet. Res. 2017, 18, 60–62. [Google Scholar] [PubMed]
- Sutili, F.J.; Velasquez, A.; Pinheiro, C.G.; Heinzmann, B.M.; Gatlin, D.M.; Baldisserotto, B. Evaluation of Ocimum americanum essential oil as an additive in red drum (Sciaenops ocellatus) diets. Fish. Shellfish Immunol. 2016, 56, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Basmacioğlu Malayoğlu, H.; Baysal, Ş.; Misirlioğlu, Z.; Polat, M.; Yilmaz, H.; Turan, N. Effects of oregano essential oil with or without feed enzymes on growth performance, digestive enzyme, nutrient digestibility, lipid metabolism and immune response of broilers fed on wheat–soybean meal diets. Br. Poult. Sci. 2010, 51, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Nieto, C.; Bandrick, M.; Baidoo, S.K.; Anil, L.; Molitor, T.W.; Hathaway, M.R. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J. Anim. Sci. 2011, 89, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Ghafari Farsani, H.; Gerami, M.H.; Farsani, M.N.; Rashidiyan, G.; Mehdipour, N.; Ghanad, M.; Faggio, C. Effect of different levels of essential oils (Satureja hortensis) in diet on improvement growth, blood biochemical and immunity of Angelfish (Pterophyllum scalare Schultze, 1823). Nat. Prod. Res. 2018, 1–6. [Google Scholar] [CrossRef]
- Placha, I.; Takacova, J.; Ryzner, M.; Cobanova, K.; Laukova, A.; Strompfova, V.; Venglovska, K.; Faix, S. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br. Poult. Sci. 2014, 55, 105–114. [Google Scholar] [CrossRef]
- Kuriyama, H.; Watanabe, S.; Nakaya, T.; Shigemori, I.; Kita, M.; Yoshida, N.; Masaki, D.; Tadai, T.; Ozasa, K.; Fukui, K.; et al. Immunological and psychological benefits of aromatherapy massage. Evid. Based Complement. Altern. Med. 2005, 2, 179–184. [Google Scholar] [CrossRef]
- Takeda, H.; Tsujita, J.; Kaya, M.; Takemura, M.; Oku, Y. Differences Between the Physiologic and Psychologic Effects of Aromatherapy Body Treatment. J. Altern. Complement. Med. 2008, 14, 655–661. [Google Scholar] [CrossRef]
- Imanishi, J.; Kuriyama, H.; Shigemori, I.; Watanabe, S.; Aihara, Y.; Kita, M.; Sawai, K.; Nakajima, H.; Yoshida, N.; Kunisawa, M.; et al. Anxiolytic effect of aromatherapy massage in patients with breast cancer. Evid. Based Complement. Altern. Med. 2009, 6, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Komori, T.; Fujiwara, R.; Tanida, M.; Nomura, J.; Yokoyama, M.M. Effects of citrus fragrance on immune function and depressive states. Neuroimmunomodulation 1995, 2, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Khiewkhern, S.; Promthet, S.; Sukprasert, A.; Eunhpinitpong, W.; Bradshaw, P. Effectiveness of aromatherapy with light thai massage for cellular immunity improvement in colorectal cancer patients receiving chemotherapy. Asian Pac. J. Cancer Prev. 2013, 14, 3903–3907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trellakis, S.; Fischer, C.; Rydleuskaya, A.; Tagay, S.; Bruderek, K.; Greve, J.; Lang, S.; Brandau, S. Subconscious olfactory influences of stimulant and relaxant odors on immune function. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Chou, C.-C.; Yang, L.; Tsai, Y.-L.; Chang, Y.-C.; Liaw, J.-J. Effects of Aromatherapy Massage on Pregnant Women’s Stress and Immune Function: A Longitudinal, Prospective, Randomized Controlled Trial. J. Altern. Complement. Med. 2017, 23, 778–786. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Graham, J.E.; Malarkey, W.B.; Porter, K.; Lemeshow, S.; Glaser, R. Olfactory influences on mood and autonomic, endocrine, and immune function. Psychoneuroendocrinology 2008, 33, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Lang, M.; Ferron, P.-J.; Bursztyka, J.; Montjarret, A.; Duteil, E.; Bazire, A.; Bedoux, G. Evaluation of immunomodulatory activities of essential oils by high content analysis. J. Biotechnol. 2019, 303, 65–71. [Google Scholar] [CrossRef]
- Kang, S.-W.; Min, H.-Y. Ginseng, the “immunity boost”: The effects of panax ginseng on immune system. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef] [Green Version]
- Riahi-Zanjani, B.; Balali-Mood, M.; Mohammadi, E.; Badie-Bostan, H.; Memar, B.; Karimi, G. Safranal as a safe compound to mice immune system. Avicenna J. Phytomedicine 2015, 5, 441–449. [Google Scholar]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Sabino, M.; Carmelo, V.A.O.; Mazzoni, G.; Cappelli, K.; Capomaccio, S.; Ajmone-Marsan, P.; Verini-Supplizi, A.; Trabalza-Marinucci, M.; Kadarmideen, H.N. Gene co-expression networks in liver and muscle transcriptome reveal sex-specific gene expression in lambs fed with a mix of essential oils. BMC Genomics 2018, 19, 236. [Google Scholar] [CrossRef]
- Li, Q.; Nakadai, A.; Matsushima, H.; Miyazaki, Y.; Krensky, A.M.; Kawada, T.; Morimoto, K. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunopharmacol. Immunotoxicol. 2006, 28, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.I.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.; Wakayama, Y.; et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Li, Y.J.; Wakayama, T.; et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in femal subjects. J. Biol. Regul. Homeost. Agents 2008, 22, 45–55. [Google Scholar] [PubMed]
- Li, Q.; Kobayashi, M.; Inagaki, H.; Hirata, Y.; Li, Y.J.; Hirata, K.; Shimizu, T.; Suzuki, H.; Katsumata, M.; Wakayama, Y.; et al. A day trip to a forest park increases human natural killer activity and the expression of anti-cancer proteins in male subjects. J. Biol. Regul. Homeost. Agents 2010, 24, 157–165. [Google Scholar]
- Han, J.W.; Choi, H.; Jeon, Y.H.; Yoon, C.H.; Woo, J.M.; Kim, W. The effects of forest therapy on coping with chronic widespread pain: Physiological and psychological differences between participants in a forest therapy program and a control group. Int. J. Environ. Res. Public Health 2016, 13, 255. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Jeong, H.; Park, S.; Lee, S. Forest adjuvant anti-cancer therapy to enhance natural cytotoxicity in urban women with breast cancer: A preliminary prospective interventional study. Eur. J. Integr. Med. 2015, 7, 474–478. [Google Scholar] [CrossRef]
- Im, S.G.; Choi, H.; Jeon, Y.H.; Song, M.K.; Kim, W.; Woo, J.M. Comparison of effect of two-hour exposure to forest and urban environments on cytokine, anti-oxidant, and stress levels in young adults. Int. J. Environ. Res. Public Health 2016, 13, 625. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.-X.; Cao, Y.-B.; Lan, X.-G.; He, Z.-H.; Chen, Z.-M.; Wang, Y.-Z.; Hu, X.-L.; Lv, Y.-D.; Wang, G.-F.; Yan, J. Therapeutic effect of forest bathing on human hypertension in the elderly. J. Cardiol. 2012, 60, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Cao, Y.; Wang, B.; Wang, S.; Chen, Z.; Wang, J.; Xing, W.; Ren, X.; Lv, X.; Dong, J.; et al. The salutary influence of forest bathing on elderly patients with chronic heart failure. Int. J. Environ. Res. Public Health 2017, 14, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsunetsugu, Y.; Park, B.-J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) in an old-growth broadleaf forest in Yamagata prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haluza, D.; Schönbauer, R.; Cervinka, R. Green perspectives for public health: A narrative review on the physiological effects of experiencing outdoor nature. Int. J. Environ. Res. Public Health 2014, 11, 5445–5461. [Google Scholar] [CrossRef]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-yoku (forest bathing) and nature therapy: A state-of-the-art review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef]
- Oh, B.; Lee, K.J.; Zaslawski, C.; Yeung, A.; Rosenthal, D.; Larkey, L.; Back, M. Health and well-being benefits of spending time in forests: Systematic review. Environ. Health Prev. Med. 2017, 22, 71. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of nature therapy: A review of the research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef]
- Antonelli, M.; Barbieri, G.; Donelli, D. Effects of forest bathing (shinrin-yoku) on levels of cortisol as a stress biomarker: A systematic review and meta-analysis. Int. J. Biometeorol. 2019, 63, 1117–1134. [Google Scholar] [CrossRef]
- Franco, L.S.; Shanahan, D.F.; Fuller, R.A. A review of the benefits of nature experiences: More than meets the eye. Int. J. Environ. Res. Public Health 2017, 14, 864. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M. How might contact with nature promote human health? Promising mechanisms and a possible central pathway. Front. Psychol. 2015, 6, 1093. [Google Scholar] [CrossRef]
- Rook, G.A. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proc. Natl. Acad. Sci. USA 2013, 110, 18360–18367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, R.; Honnay, O.; Van Nieuwenhuyse, A. Biodiversity and human health: Mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med. Bull. 2018, 127, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanahan, D.F.; Bush, R.; Gaston, K.J.; Lin, B.B.; Dean, J.; Barber, E.; Fuller, R.A. Health benefits from nature experiences depend on dose. Sci. Rep. 2016, 6, 28551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.P.; Alcock, I.; Grellier, J.; Wheeler, B.W.; Hartig, T.; Warber, S.L.; Bone, A.; Depledge, M.H.; Fleming, L.E. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci. Rep. 2019, 9, 7730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera, C.; Valenzuela, R.; Rincón, M.Á.; Espinosa, A.; Echeverria, F.; Romero, N.; Gonzalez-Mañan, D.; Videla, L.A. Molecular mechanisms related to the hepatoprotective effects of antioxidant-rich extra virgin olive oil supplementation in rats subjected to short-term iron administration. Free Radic. Biol. Med. 2018, 126, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; Gonzalez-Mañan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019, 63, 35–43. [Google Scholar] [CrossRef]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-γ and NF-κB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef]
- Valenzuela, R.; Illesca, P.; Echeverría, F.; Espinosa, A.; Rincón-Cervera, M.Á.; Ortiz, M.; Hernandez-Rodas, M.C.; Valenzuela, A.; Videla, L.A. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef]
| Essential Oils or EO Constituents Used in Different Experimental Settings | References |
|---|---|
| In Vitro Investigations | |
| Allium sativum (garlic) | [22] |
| Eucalyptus globulus (eucalyptus) | [23,24] |
| eugenol (from Syzygium aromaticum, clove) | [25,26] |
| Ferula iliensis | [27] |
| frankincense EO (derived from Boswellia carterii) | [28] |
| Lavandula angustifolia (lavender) | [29] |
| Pituranthos tortuosus | [30] |
| Schinus molle | [31] |
| Syzygium cumini (jambolão) | [32] |
| Thymus vulgaris (thyme) | [33] |
| In Vivo Pre-Clinical Studies | |
| Carum copticum (bishop’s weed) seeds EO compounds: α-pinene and carvacrol | [34] |
| Eucalyptus globulus (eucalyptus) | [23,24] |
| Eugenia caryophyllata | [35] |
| Massoia aromatica bark EO and massoialactone | [36,37] |
| Melaleuca viridiflora (niaouli) | [38] |
| orange EO, and its components: Limonene, linalool, and citral | [39] |
| Panax ginseng (ginseng) | [40] |
| Salvia officinalis (sage) | [41] |
| Syzygium aromaticum (clove) | [41] |
| Zanthoxylum rhoifolium | [42] |
| Zingiber officinale (ginger) | [41] |
| Dietary Supplementation for Livestock and Fish | |
| a blend of thyme (Thymus vulgaris), peppermint (Mentha piperita), and eucalyptus (Eucalyptus globulus) | [43] |
| a blend of thymol and carvacrol | [44] |
| a blend of thymol and cinnamaldehyde | [45,46,47,48] |
| Allium sativum (garlic) | [49] |
| Ducrosia anethifolia | [50] |
| Eucalyptus globulus (eucalyptus) | [51] |
| Juniperus communis (juniper) | [49] |
| Ocimum americanum (American basil) | [52] |
| Origanum onites | [53] |
| Origanum vulgare (oregano) | [54] |
| Satureja hortensis (savoury) | [55] |
| Thymus vulgaris (thyme) | [56] |
| Human Studies | |
| a blend of lavender (Lavandula angustifolia), cypress (Cupressus sempervirens), and sweet marjoram (Origanum majorana) | [57] |
| a blend of orange sweet (Citrus sinensis), true lavender, and marjoram sweet (Origanum majorana) | [58] |
| a blend of sweet orange (Citrus aurantium), lavender (Lavandula angustifolia), and sandalwood (Santalum album) | [59] |
| Chamaecyparis obtusa (hinoki cypress) | [60] |
| Citrus fragrance (lemon oil+orange oil+bergamot oil+cis-4-hexenol) | [61] |
| ginger EO | [62] |
| grapefruit, fennel, pepper, lavender, patchouli, and rose | [63] |
| Lavandula angustifolia (lavender) | [64] |
| Lavandula angustifolia (lavender), Citrus limonum (lemon) | [65] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterfalvi, A.; Miko, E.; Nagy, T.; Reger, B.; Simon, D.; Miseta, A.; Czéh, B.; Szereday, L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules 2019, 24, 4530. https://doi.org/10.3390/molecules24244530
Peterfalvi A, Miko E, Nagy T, Reger B, Simon D, Miseta A, Czéh B, Szereday L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules. 2019; 24(24):4530. https://doi.org/10.3390/molecules24244530
Chicago/Turabian StylePeterfalvi, Agnes, Eva Miko, Tamas Nagy, Barbara Reger, Diana Simon, Attila Miseta, Boldizsár Czéh, and Laszlo Szereday. 2019. "Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System" Molecules 24, no. 24: 4530. https://doi.org/10.3390/molecules24244530
APA StylePeterfalvi, A., Miko, E., Nagy, T., Reger, B., Simon, D., Miseta, A., Czéh, B., & Szereday, L. (2019). Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules, 24(24), 4530. https://doi.org/10.3390/molecules24244530





