Phytochemical Profiling and Fingerprint Analysis of Chinese Jujube (Ziziphus jujuba Mill.) Leaves of 66 Cultivars from Xinjiang Province
Abstract
:1. Introduction
2. Results and Discussion
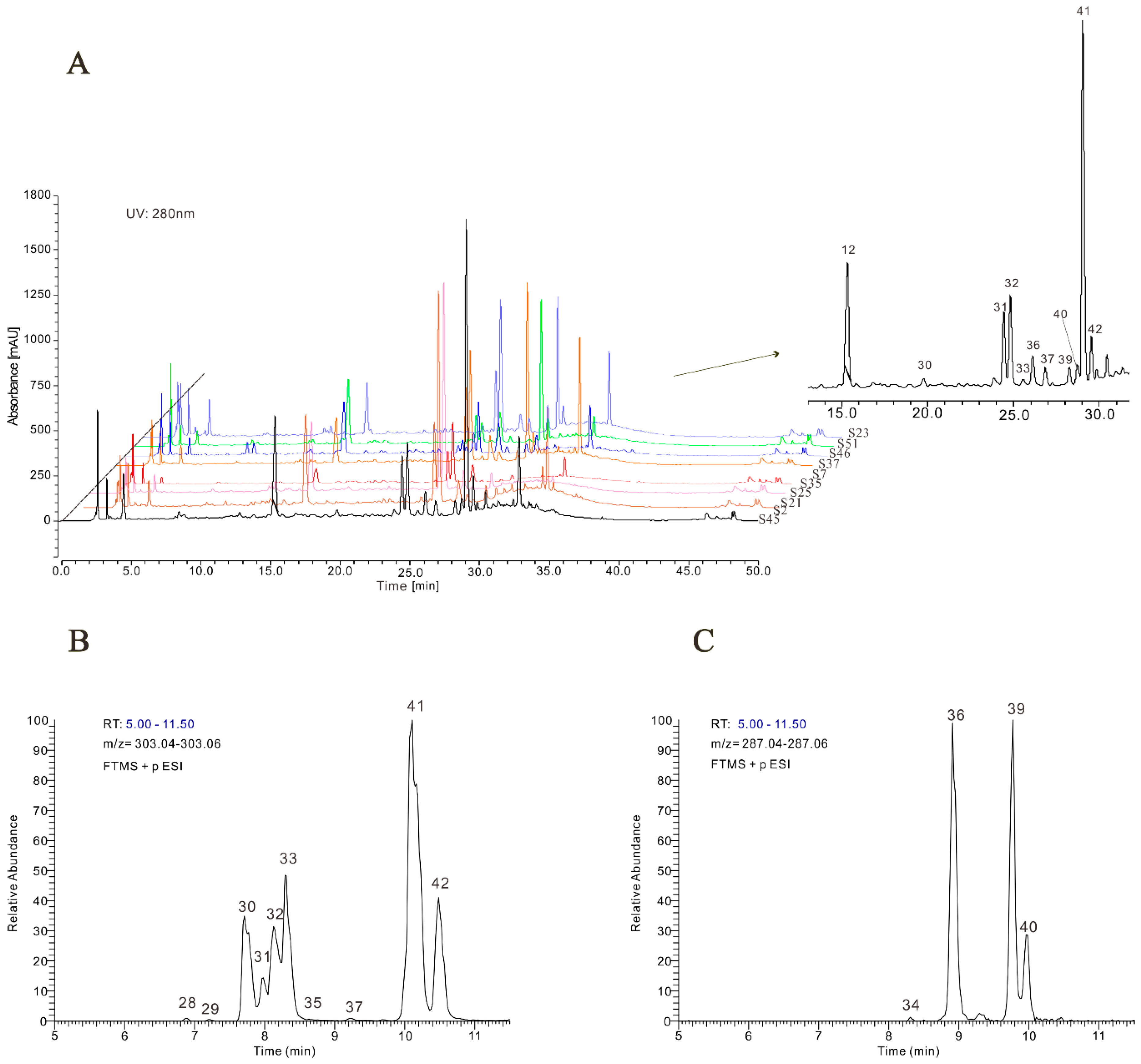
2.1. Identification of Phenolic Compounds
2.1.1. Flavonols
2.1.2. Flavanols and Flavanone
2.1.3. Phenolic Acids
2.1.4. Other Compounds
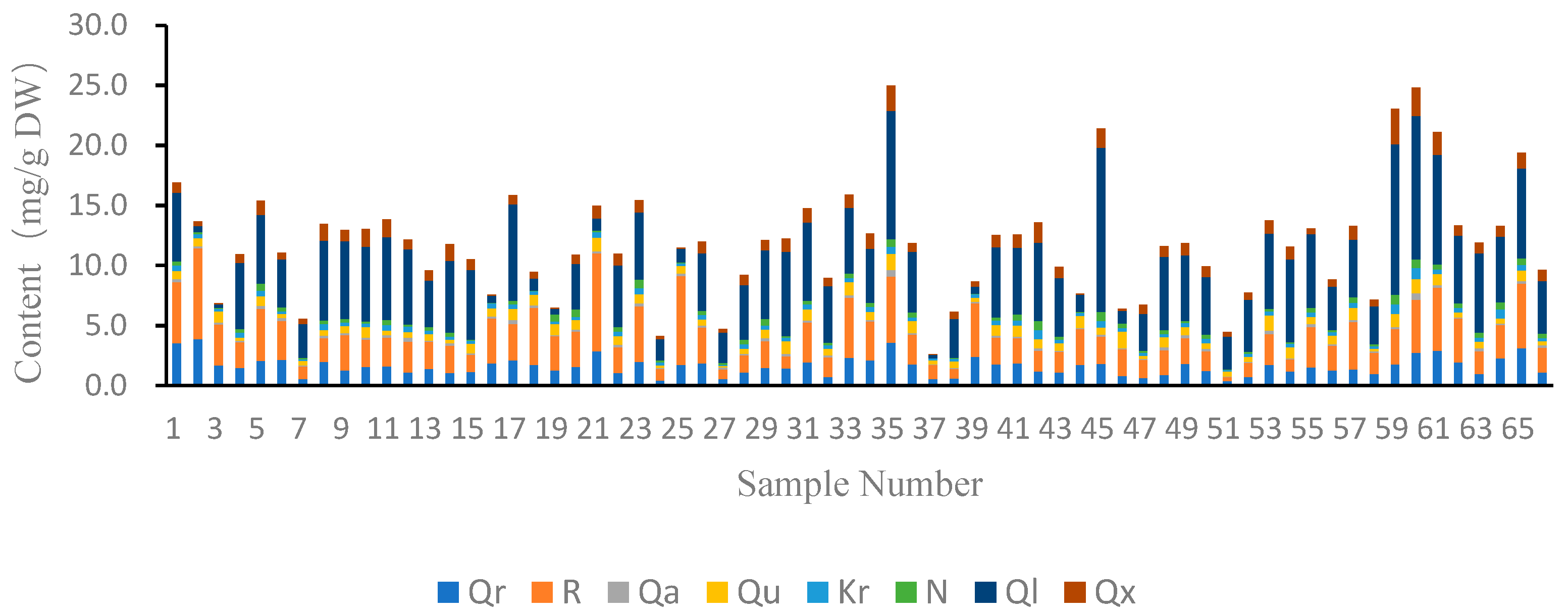
2.2. Quantitative Analysis of Flavonols in Jujube Leaves
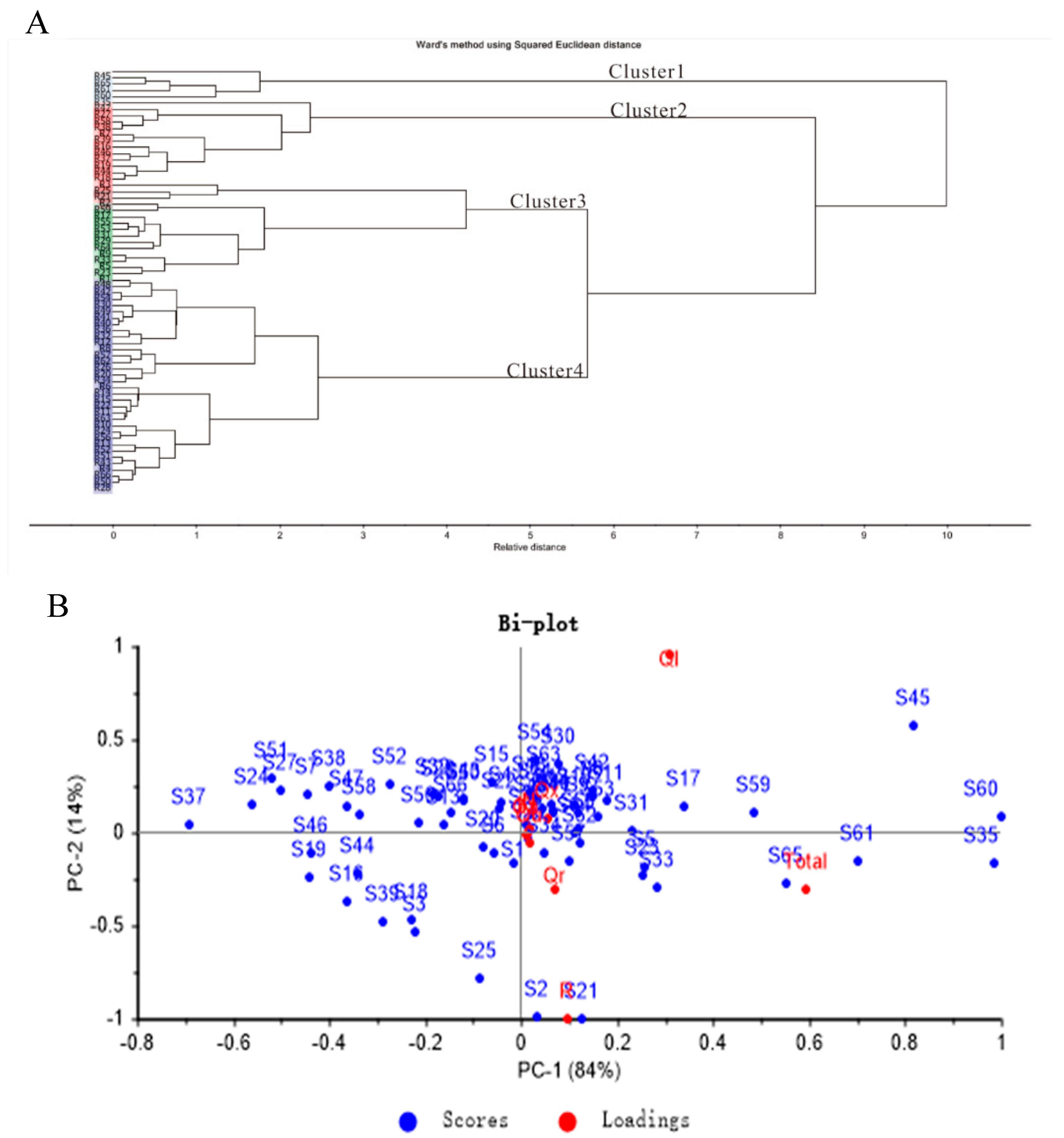
2.3. Hierarchical Cluster Analysis (HCA)
2.4. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plant Materials
3.2. Reagents
3.3. Sample Extraction
3.4. HPLC-ESI-MS Spectrometry
3.5. HPLC-UV
3.6. Qualitative and Quantitative Analyses
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, R.; Ding, S.; Zhao, D.; Wang, Z.; Wu, J.; Hu, X. Effect of dehydration methods on antioxidant activities, phenolic contents, cyclic nucleotides, and volatiles of jujube fruits. Food Sci. Biotechnol. 2016, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.; Maiwulanjiang, M.; Zhang, W.L.; Zhan, J.Y.X.; Lam, C.T.W.; Zhu, K.Y.; Yao, P.; Choi, R.C.Y.; Lau, D.T.W.; et al. Chemical and biological assessment of Ziziphus jujuba fruits from China: Different geographical sources and developmental stages. J. Agri. Food Chem. 2013, 61, 7315–7324. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nakano, K.; Ohashi, S.; Kubota, Y.; Takizawa, K.; Sasaki, Y. Detection of external insect infestations in jujube fruit using hyperspectral reflectance imaging. Biosyst. Eng. 2011, 108, 345–351. [Google Scholar] [CrossRef]
- Mahajan, R.T.; Chopda, M.Z. Phyto-Pharmacology of Ziziphus jujuba Mill-A plant review. Pharmacogn. Rev. 2009, 3, 320–329. [Google Scholar]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agri. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, M.; Tu, P. Characterization of water soluble polysaccharides from organs of Chinese jujube (Ziziphus jujuba Mill. cv. Dongzao). Eur. Food Res. Technol. 2008, 226, 985–989. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Shi, Q.; Peng, Z.; Zheng, Z.; Li, Z.; Wang, X. Quality control method for commercially available wild jujube leaf tea based on HPLC characteristic fingerprint analysis of flavonoid compounds. J. Sep. Sci. 2014, 37, 45–52. [Google Scholar] [CrossRef]
- The Committee of Chinese Materia. Chinese Materia; Shanghai Scientific and Technical Education Publishing House: Shanghai, China, 1999; Volume 5, No. 13; pp. 1–260. [Google Scholar]
- Akhmedov, U.A.; Khalmatov, K.K. Isolation of rutin from the leaves of Zizyphus jujuba Mill. Farmatsiia 1967, 16, 34–35. [Google Scholar]
- Guo, S.; Duan, J.; Tang, Y.; Qian, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC-PDA-MS/ELSD. J. Pharmaceut. Biomed. 2011, 56, 264–270. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Shimono, N.; Arihara, S. Antisweet Natural Products. VI. Jujuba saponins IV, V and VI from Zizyphus jujuba Mill. Chem. Pharm. Bull. 1992, 40, 2275–2278. [Google Scholar] [CrossRef] [Green Version]
- Damiano, S.; Forino, B.; De, A.; Vitalia, L.A.; Lupidia, G.; Taglialatela-Scafati, O. Antioxidant and antibiofilm activities of secondary metabolites from Ziziphus jujube leaves used for infusion preparation. Food Chem. 2017, 230, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Cui, X.; Cheng, N.; Cao, W.; Wu, Y.; Guo, S.; Zhang, L.; Ho, C.T.; Bai, N. Hepatoprotective standardized EtOH–water extract of the leaves of Ziziphus jujuba. Food Funct. 2017, 8, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Hovaneţ, M.-V.; Oprea, E.; Ancuceanu, R.V.; Duţu, L.E.; Budura, E.A.; Şeremet, O.; Ancu, I.; Moroşan, E. Wound healing properties of Ziziphus jujuba Mill. leaves. Rom. Biotech. Lett. 2016, 21, 11842–11849. [Google Scholar]
- Liu, H.X.; Xu, M.Q.; Li, S.P.; Tian, S.; Guo, M.X.; Qi, J.Y.; He, C.J.; Zhao, X.S. Jujube leaf green tea extracts inhibits hepatocellular carcinoma cells by activating AMPK. Oncotarget 2017, 8, 110566–110575. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Wiczkowski, W.; Piskuła, M.K. Food flavonoids. Pol. J. Food Nutr. Sci. 2004, 13, 101–114. [Google Scholar]
- Ju, W.T.; Kwon, O.C.; Kim, H.B.; Sung, G.B.; Kim, H.W.; Kim, Y.S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol. 2018, 55, 1789–1796. [Google Scholar] [CrossRef] [Green Version]
- Elless, M.P.; Blaylock, M.J.; Huang, J.W.; Gussman, C.D. Plants as a natural source of concentrated mineral nutritional supplements. Food Chem. 2000, 71, 181–188. [Google Scholar] [CrossRef]
- Liu, P.; Yang, B.; Kallio, H. Characterization of phenolic compounds in Chinese hawthorn (Crataegus pinnatifida bge. var. major) fruit by high performance liquid chromatography–electrospray ionization mass spectrometry. Food Chem. 2010, 121, 1188–1197. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019, 295, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Ullah, F. Reversed phase HPLC-DAD profiling of carotenoids, chlorophylls and phenolic compounds in Adiantum capillus-veneris leaves. Front. Chem. 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Oliveira, M.; Ibáñez, E.; Herrero, M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A 2014, 1327, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.Z.; Harnly, J.M. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agri. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Li, L.; Song, L.; Sun, X.; Yan, S.; Huang, W. Characterisation of phenolics in fruit septum of Juglans regia Linn. by ultra performance liquid chromatography coupled with Orbitrap mass spectrometer. Food Chem. 2019, 286, 669–677. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Identification/Tentative Identification a | Positive Ion Mode | Negative Ion Mode | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RT1 b | RT2 c | Formula | Δ mmu | [M − H]− | MS2 | [M + H]+ | MS2 | ||
| (min) | (min) | (m/z) | (m/z) | (m/z) | (m/z) | ||||
| 1 | Unknown hexoside | 0.46 | C18H18O9 | 0.62 | 377.0859 | 215.0328 | |||
| 2 | Malic acid * | 0.50 | C4H6O5 | 0.80 | 133.0139 | ||||
| 3 | Citric acid * | 0.57 | C6H8O7 | 1.13 | 191.0197 | ||||
| 4 | Aconitic acid | 0.59 | C6H6O6 | 0.97 | 173.0090 | ||||
| 5 | Gallic acid * | 0.82 | C7H6O5 | 0.99 | 169.0141 | ||||
| 6 | Syringic acid | 1.03 | C9H10O5 | 1.27 | 197.0457 | ||||
| 7 | Protocatechuic acid | 1.27 | C7H6O4 | 1.03 | 153.0193 | ||||
| 8 | Caffeic acid-O-hexoside | 1.81 | C15H18O9 | 1.24 | 341.0882 | 179.0348 | |||
| 9 | Caffeic acid-O-hexoside | 2.17 | C15H18O9 | 1.24 | 341.0882 | 179.0348 | |||
| 10 | Catechin * | 2.54 | C15H14O6 | 0.09 | 289.0721 | 291.0864 | |||
| 11 | Caffeic acid-O-hexoside | 2.60 | C15H18O9 | 1.24 | 341.0882 | 179.0348 | |||
| 12 | Chlorogenic acid * | 2.98 | 15.08 | C16H18O9 | 1.52 | 353.0883 | 191.0560 | ||
| 13 | Caffeic acid-O-hexoside | 3.59 | C15H18O9 | 1.24 | 341.0882 | 179.0348 | |||
| 14 | Ferulic acid-O-hexoside | 3.79 | C16H18O9 | 1.31 | 355.1038 | 193.0506 | |||
| 15 | Quercetin-(di-hexose)-rhamnose | 3.91 | C33H40O21 | 0.50 | 773.2140 | 627.1553; 465.1028; 303.0495 | |||
| 16 | Naringenin-C-di-hexoside | 4.04 | C27H32O15 | 0.43 | 595.1680 | 505.1356; 385.0935; 355.0829 | 597.1814 | ||
| 17 | Chlorogenic acid isomer | 4.09 | C16H18O9 | 1.52 | 353.0883 | 191.0560 | |||
| 18 | Epicatechin * | 4.12 | C15H14O6 | 0.09 | 289.0721 | 291.0864 | |||
| 19 | Quercetin-O-(di-hexose)-rhamnose | 4.29 | C33H40O21 | 0.50 | 773.2140 | 627.1553; 465.1028; 303.0495 | |||
| 20 | Ferulic acid-O-hexoside | 4.33 | C16H18O9 | 1.46 | 355.1039 | 193.0506 | |||
| 21 | Coumaroylqunic acid | 4.59 | C16H17O8 | 0.10 | 337.0933 | 191.0561 | 339.1075 | 147.0442 | |
| 22 | Kaempferol-O-(di-hexose)-rhamnose | 5.45 | C33H40O20 | 1.20 | 757.2197 | 595.1664; 449.1094; 287.0549 | |||
| 23 | Coumaroylqunic acid | 5.69 | C16H17O8 | 0.10 | 337.0933 | 191.0561 | 339.1075 | 147.0442 | |
| 24 | Quercetin-O-hexose-rhamnose-pentose | 5.75 | C32H38O20 | 0.70 | 743.2036 | 611.1594; 465.1030; 303.0498 | |||
| 25 | Quercetin-O-(di-hexose)-rhamnose | 6.18 | C33H40O21 | 0.30 | 773.2138 | 627.1557; 465.1027; 303.0500 | |||
| 26 | Myricetin-O-hexose-rhamnose | 6.56 | C27H30O17 | 0.06 | 625.1420 | 463.0887; 317.0226 | 627.1557 | 465.1030; 319.0447 | |
| 27 | Quercetin-O-(di-hexose)-rhamnose | 6.59 | C33H40O21 | 0.50 | 773.2140 | 627.1553; 465.1028; 303.0495 | |||
| 28 | Quercetin-O-hexose-rhamnose-pentose | 6.83 | 20.96 | C32H38O20 | 0.30 | 743.2032 | 611.1611; 465.1023; 303.0499 | ||
| 29 | Quercetin-O-(di-hexose)-rhamnose | 7.36 | C33H40O21 | 1.20 | 773.2147 | 627.1557; 465.1029; 303.0498 | |||
| 30 | Quercetin-3-O-robinobioside * | 7.71 | 23.93 | C27H30O16 | 1.23 | 611.1599 | 465.1023; 303.0496 | ||
| 31 | Rutin (Quercetin-3-O-rutinoside) * | 7.94 | 24.38 | C27H30O16 | 0.68 | 611.1605 | 465.1028; 303.0498 | ||
| 32 | Hyperoside (Quercetin-3-O-galactoside) * | 8.11 | 24.98 | C21H20O12 | 0.12 | 465.1026 | 303.0460 | ||
| 33 | Quercetin-3-O-glucoside * | 8.33 | 25.32 | C21H20O12 | 0.12 | 465.1026 | 303.0495 | ||
| 34 | Kaempferol-O-hexose-rhamnose-pentose | 8.38 | C32H38O19 | 1.40 | 727.2094 | 595.1666; 449.1085; 287.0554 | |||
| 35 | Quercetin-O-hexose-rhamnose-pentose | 8.52 | C32H38O20 | 0.30 | 743.2032 | 597.1440; 465.1028;303.0497 | |||
| 36 | Kaempferol-3-O-robinobioside * | 8.89 | 25.62 | C27H30O15 | 0.20 | 595.1660 | 449.1080; 287.0549 | ||
| 37 | Quercetin-O-pentose-pentose | 9.22 | 26.13 | C25H26O15 | 0.85 | 567.1353 | 435.0922; 303.0503 | ||
| 38 | Quercetin-O-hexose-rhamnose-pentose | 9.41 | C32H38O20 | 0.10 | 743.2030 | 611.1636; 465.1028; 303.0496 | |||
| 39 | Nicotiflorin(Kaempferol-3-O-rutinoside) * | 9.71 | 27.90 | C27H30O15 | 0.45 | 595.1660 | 449.1080; 287.0549 | ||
| 40 | Kaempferol-3-O-glucoside * | 9.96 | 28.23 | C21H20O11 | 0.16 | 449.1109 | 287.0549 | ||
| 41 | Quercetin-3-O-arabinosyl-rhamnoside | 10.11 | 28.73 | C26H28O15 | 0.05 | 581.1503 | 449.1109; 303.0469 | ||
| 42 | Quercetin-3-O-xylosyl-rhamnoside | 10.42 | 29.25 | C26H28O15 | 0.53 | 581.1505 | 449.1082; 303.0500 | ||
| NO. | Cultivars | Qr | R | Qa | Qu | Kr | N | Ql | Qx | T |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Binglang | 3.53 ± 0.57 | 5.05 ± 0.56 | 0.28 ± 0.11 | 0.68 ± 0.03 | 0.43 ± 0.03 | 0.35 ± 0.05 | 5.71 ± 0.55 | 0.86 ± 0.01 | 16.90 ± 1.83 |
| S2 | Chahu | 3.88 ± 0.38 | 7.54 ± 0.80 | 0.16 ± 0.01 | 0.70 ± 0.11 | 0.31 ± 0.03 | 0.20 ± 0.10 | 0.48 ± 0.19 | 0.39 ± 0.07 | 13.66 ± 1.48 |
| S3 | Dadongling | 1.70 ± 0.05 | 3.41 ± 0.06 | 0.16 ± 0.01 | 0.89 ± 0.05 | 0.20 ± 0.00 | 0.10 ± 0.06 | 0.28 ± 0.00 | 0.11 ± 0.00 | 6.86 ± 0.23 |
| S4 | Guanyin | 1.49 ± 0.16 | 2.11 ± 0.21 | 0.00 ± 0.00 | 0.27 ± 0.02 | 0.39 ± 0.04 | 0.30 ± 0.03 | 5.52 ± 0.49 | 0.69 ± 0.10 | 10.84 ± 1.11 |
| S5 | Haba | 2.07 ± 0.19 | 4.32 ± 0.37 | 0.23 ± 0.02 | 0.82 ± 0.06 | 0.47 ± 0.04 | 0.56 ± 0.05 | 5.73 ± 0.20 | 1.19 ± 0.18 | 15.39 ± 0.71 |
| S6 | Hengyangzhenzhu | 2.16 ± 0.01 | 3.24 ± 0.03 | 0.25 ± 0.01 | 0.30 ± 0.00 | 0.20 ± 0.00 | 0.34 ± 0.00 | 4.01 ± 0.02 | 0.54 ± 0.04 | 11.06 ± 0.11 |
| S7 | Jikangyihao | 0.55 ± 0.01 | 1.05 ± 0.05 | 0.09 ± 0.01 | 0.40 ± 0.04 | 0.00 ± 0.01 | 0.11 ± 0.00 | 2.82 ± 0.27 | 0.43 ± 0.07 | 5.55 ± 0.20 |
| S8 | Jinchangyihao | 1.96 ± 0.11 | 2.01 ± 0.09 | 0.20 ± 0.00 | 0.45 ± 0.02 | 0.51 ± 0.04 | 0.29 ± 0.05 | 6.66 ± 0.22 | 1.36 ± 0.41 | 13.43 ± 0.95 |
| S9 | Jinmangguo | 1.25 ± 0.12 | 2.97 ± 0.27 | 0.17 ± 0.01 | 0.57 ± 0.06 | 0.28 ± 0.02 | 0.30 ± 0.02 | 6.46 ± 0.61 | 0.95 ± 0.09 | 12.96 ± 1.21 |
| S10 | Jinzan | 1.57 ± 0.31 | 2.26 ± 0.38 | 0.19 ± 0.05 | 0.87 ± 0.12 | 0.29 ± 0.04 | 0.18 ± 0.04 | 6.19 ± 0.92 | 1.47 ± 0.48 | 13.02 ± 2.33 |
| S11 | Jing39 | 1.61 ± 0.34 | 2.40 ± 0.47 | 0.21 ± 0.10 | 0.39 ± 0.08 | 0.43 ± 0.21 | 0.45 ± 0.08 | 6.86 ± 1.48 | 1.48 ± 0.56 | 13.83 ± 3.11 |
| S12 | Lajiaozao | 1.09 ± 0.00 | 2.59 ± 0.01 | 0.31 ± 0.14 | 0.49 ± 0.03 | 0.46 ± 0.21 | 0.16 ± 0.01 | 6.27 ± 0.19 | 0.80 ± 0.02 | 12.16 ± 0.18 |
| S13 | Lejinsanhao | 1.38 ± 0.08 | 2.27 ± 0.12 | 0.10 ± 0.01 | 0.55 ± 0.06 | 0.3 ± 0.00 | 0.26 ± 0.02 | 3.88 ± 0.25 | 0.81 ± 0.21 | 9.56 ± 0.75 |
| S14 | Linyilajiao | 1.05 ± 0.03 | 2.26 ± 0.06 | 0.25 ± 0.10 | 0.27 ± 0.11 | 0.25 ± 0.06 | 0.36 ± 0.07 | 5.95 ± 0.35 | 1.38 ± 0.34 | 11.77 ± 0.91 |
| S15 | Linxianyazao | 1.16 ± 0.00 | 1.43 ± 0.03 | 0.11 ± 0.01 | 0.82 ± 0.03 | 0.23 ± 0.01 | 0.11 ± 0.00 | 5.78 ± 0.06 | 0.88 ± 0.01 | 10.51 ± 0.13 |
| S16 | Mohu | 1.87 ± 0.02 | 3.74 ± 0.05 | 0.14 ± 0.02 | 0.69 ± 0.02 | 0.4 ± 0.00 | 0.03 ± 0.00 | 0.6 ± 0.01 | 0.08 ± 0.00 | 7.55 ± 0.02 |
| S17 | Popo | 2.12 ± 0.03 | 3.01 ± 0.05 | 0.34 ± 0.05 | 0.92 ± 0.17 | 0.36 ± 0.01 | 0.30 ± 0.01 | 8.03 ± 0.22 | 0.75 ± 0.01 | 15.83 ± 0.41 |
| S18 | Shanximutou | 1.75 ± 0.32 | 4.71 ± 0.81 | 0.20 ± 0.03 | 0.90 ± 0.21 | 0.3 ± 0.04 | 0.03 ± 0.01 | 1.03 ± 0.47 | 0.57 ± 0.43 | 9.47 ± 2.32 |
| S19 | Shanxiniunaicui | 1.26 ± 0.03 | 2.86 ± 0.08 | 0.12 ± 0.04 | 0.90 ± 0.07 | 0.22 ± 0.00 | 0.59 ± 0.32 | 0.44 ± 0.08 | 0.06 ± 0.01 | 6.44 ± 0.01 |
| S20 | Shengli | 1.55 ± 0.07 | 2.95 ± 0.08 | 0.19 ± 0.00 | 0.76 ± 0.06 | 0.28 ± 0.01 | 0.61 ± 0.26 | 3.80 ± 0.06 | 0.75 ± 0.15 | 10.88 ± 0.18 |
| S21 | Tailihong | 2.88 ± 0.13 | 8.13 ± 0.08 | 0.17 ± 0.03 | 1.14 ± 0.20 | 0.46 ± 0.07 | 0.12 ± 0.03 | 1.00 ± 0.20 | 1.06 ± 0.51 | 14.96 ± 1.18 |
| S22 | Xiangfen | 1.05 ± 0.04 | 2.16 ± 0.06 | 0.18 ± 0.00 | 0.75 ± 0.02 | 0.35 ± 0.00 | 0.39 ± 0.06 | 5.11 ± 0.34 | 0.96 ± 0.27 | 10.97 ± 0.78 |
| S23 | Xiaodaxiao | 1.99 ± 0.06 | 4.61 ± 0.21 | 0.25 ± 0.02 | 0.74 ± 0.04 | 0.52 ± 0.03 | 0.72 ± 0.03 | 5.57 ± 0.15 | 1.00 ± 0.69 | 15.41 ± 0.49 |
| S24 | Xinzhengxiaoyuan | 0.43 ± 0.35 | 0.98 ± 0.76 | 0.07 ± 0.02 | 0.23 ± 0.19 | 0.23 ± 0.08 | 0.18 ± 0.14 | 1.77 ± 1.42 | 0.24 ± 0.17 | 4.13 ± 3.14 |
| S25 | Xuanchengyuanzao | 1.72 ± 0.01 | 7.38 ± 0.02 | 0.22 ± 0.06 | 0.63 ± 0.07 | 0.24 ± 0.00 | 0.05 ± 0.00 | 1.14 ± 0.31 | 0.11 ± 0.06 | 11.49 ± 0.4 |
| S26 | Yanjiamaoyuan | 1.87 ± 0.10 | 2.99 ± 0.15 | 0.15 ± 0.04 | 0.48 ± 0.04 | 0.38 ± 0.02 | 0.32 ± 0.01 | 4.81 ± 0.32 | 0.96 ± 0.18 | 11.96 ± 0.86 |
| S27 | Yongchengchanghong | 0.55 ± 0.00 | 0.78 ± 0.00 | 0.17 ± 0.01 | 0.15 ± 0.00 | 0.00 ± 0.00 | 0.18 ± 0.00 | 2.51 ± 0.01 | 0.3 ± 0.00 | 4.72 ± 0.05 |
| S28 | Yujing | 1.09 ± 0.03 | 1.44 ± 0.03 | 0.13 ± 0.01 | 0.43 ± 0.09 | 0.38 ± 0.00 | 0.35 ± 0.00 | 4.53 ± 0.09 | 0.84 ± 0.17 | 9.19 ± 0.18 |
| S29 | Zan3 | 1.47 ± 0.11 | 2.25 ± 0.22 | 0.24 ± 0.03 | 0.69 ± 0.14 | 0.36 ± 0.05 | 0.55 ± 0.26 | 5.7 ± 0.59 | 0.85 ± 0.23 | 12.11 ± 0.9 |
| S30 | Zanjing | 1.44 ± 0.11 | 1.01 ± 0.11 | 0.20 ± 0.03 | 1.04 ± 0.07 | 0.31 ± 0.01 | 0.12 ± 0.00 | 6.99 ± 0.29 | 1.10 ± 0.10 | 12.22 ± 0.72 |
| S31 | Zanping | 1.93 ± 0.12 | 3.32 ± 0.16 | 0.18 ± 0.03 | 0.91 ± 0.07 | 0.41 ± 0.01 | 0.33 ± 0.01 | 6.51 ± 0.32 | 1.16 ± 0.15 | 14.74 ± 0.81 |
| S32 | Zhongzaoyihao | 0.74 ± 0.27 | 1.62 ± 0.61 | 0.16 ± 0.04 | 0.58 ± 0.15 | 0.27 ± 0.05 | 0.22 ± 0.09 | 4.67 ± 1.76 | 0.71 ± 0.24 | 8.96 ± 3.20 |
| S33 | Bayuezuoguo | 2.33 ± 0.10 | 4.97 ± 0.13 | 0.20 ± 0.01 | 1.11 ± 0.10 | 0.36 ± 0.02 | 0.36 ± 0.03 | 5.45 ± 0.22 | 1.12 ± 0.23 | 15.89 ± 0.15 |
| S34 | Banzao | 2.12 ± 0.07 | 3.22 ± 0.14 | 0.13 ± 0.03 | 0.66 ± 0.06 | 0.42 ± 0.03 | 0.33 ± 0.01 | 4.48 ± 0.56 | 1.27 ± 0.49 | 12.64 ± 1.4 |
| S35 | Fushuai | 3.60 ± 0.17 | 5.48 ± 0.01 | 0.68 ± 0.13 | 1.36 ± 0.12 | 0.58 ± 0.07 | 0.64 ± 0.18 | 10.69 ± 0.2 | 2.08 ± 0.17 | 25.11 ± 0.32 |
| S36 | Minzao | 1.78 ± 0.07 | 2.46 ± 0.06 | 0.12 ± 0.01 | 1.01 ± 0.10 | 0.31 ± 0.01 | 0.40 ± 0.11 | 5.07 ± 0.34 | 0.71 ± 0.01 | 11.85 ± 0.01 |
| S37 | Heigeda | 0.54 ± 0.44 | 1.18 ± 0.92 | 0.04 ± 0.00 | 0.34 ± 0.27 | 0.16 ± 0.02 | 0.04 ± 0.03 | 0.27 ± 0.21 | 0.04 ± 0.01 | 2.59 ± 1.89 |
| S38 | Hameizao | 0.59 ± 0.03 | 0.80 ± 0.01 | 0.09 ± 0.00 | 0.56 ± 0.00 | 0.19 ± 0.00 | 0.08 ± 0.01 | 3.23 ± 0.05 | 0.58 ± 0.07 | 6.13 ± 0.05 |
| S39 | Jinlingyuanzao | 2.42 ± 0.07 | 4.44 ± 0.13 | 0.11 ± 0.02 | 0.00 ± 0.01 | 0.28 ± 0.01 | 0.06 ± 0.00 | 0.56 ± 0.07 | 0.45 ± 0.12 | 8.66 ± 0.42 |
| S40 | Jiuqingfu | 1.76 ± 0.01 | 2.23 ± 0.04 | 0.00 ± 0.00 | 0.91 ± 0.05 | 0.36 ± 0.01 | 0.27 ± 0.01 | 5.82 ± 0.29 | 1.01 ± 0.21 | 12.45 ± 0.61 |
| S41 | Jinzaoyihao | 1.84 ± 0.07 | 2.11 ± 0.07 | 0.11 ± 0.01 | 0.93 ± 0.18 | 0.44 ± 0.04 | 0.49 ± 0.16 | 5.56 ± 0.11 | 1.09 ± 0.15 | 12.58 ± 0.75 |
| S42 | Lingbao | 1.17 ± 0.09 | 1.72 ± 0.12 | 0.25 ± 0.07 | 0.72 ± 0.16 | 0.79 ± 0.12 | 0.74 ± 0.20 | 6.48 ± 0.51 | 1.72 ± 0.43 | 13.59 ± 1.7 |
| S43 | Lejinyhihao | 1.10 ± 0.00 | 1.71 ± 0.04 | 0.12 ± 0.02 | 0.62 ± 0.02 | 0.28 ± 0.01 | 0.28 ± 0.03 | 4.86 ± 0.03 | 0.92 ± 0.21 | 9.88 ± 0.21 |
| S44 | Lantiandazao | 1.71 ± 0.14 | 2.99 ± 0.21 | 0.10 ± 0.04 | 1.01 ± 0.14 | 0.24 ± 0.04 | 0.09 ± 0.00 | 1.41 ± 0.49 | 0.09 ± 0.00 | 7.64 ± 1.04 |
| S45 | Mopanzao | 1.80 ± 0.08 | 2.29 ± 0.07 | 0.15 ± 0.01 | 0.61 ± 0.12 | 0.54 ± 0.04 | 0.72 ± 0.23 | 13.66 ± 0.21 | 1.60 ± 0.23 | 21.38 ± 0.72 |
| S46 | Mayabai | 0.82 ± 0.05 | 2.19 ± 0.02 | 0.09 ± 0.02 | 1.37 ± 0.11 | 0.33 ± 0.01 | 0.34 ± 0.00 | 1.03 ± 0.03 | 0.18 ± 0.04 | 6.37 ± 0.18 |
| S47 | Naitouzao | 0.63 ± 0.03 | 1.51 ± 0.05 | 0.08 ± 0.00 | 0.00 ± 0.01 | 0.24 ± 0.01 | 0.17 ± 0.00 | 3.06 ± 0.08 | 0.75 ± 0.11 | 6.72 ± 0.31 |
| S48 | Ningyanglingzao | 0.91 ± 0.02 | 2.03 ± 0.04 | 0.27 ± 0.03 | 0.81 ± 0.11 | 0.27 ± 0.00 | 0.32 ± 0.00 | 6.08 ± 0.01 | 0.88 ± 0.02 | 11.57 ± 0.18 |
| S49 | Shanxibaizao | 1.81 ± 0.11 | 2.17 ± 0.06 | 0.23 ± 0.01 | 0.66 ± 0.09 | 0.35 ± 0.02 | 0.17 ± 0.00 | 5.46 ± 0.23 | 0.99 ± 0.11 | 11.83 ± 0.61 |
| S50 | Tengzhoutangzao | 1.21 ± 0.03 | 1.68 ± 0.04 | 0.19 ± 0.06 | 0.45 ± 0.04 | 0.37 ± 0.06 | 0.34 ± 0.07 | 4.76 ± 0.14 | 0.89 ± 0.25 | 9.9 ± 0.69 |
| S51 | Xupufuzao | 0.38 ± 0.31 | 0.34 ± 0.24 | 0.04 ± 0.00 | 0.41 ± 0.33 | 0.13 ± 0.11 | 0.06 ± 0.04 | 2.72 ± 2.2 | 0.39 ± 0.30 | 4.46 ± 3.52 |
| S52 | Xiangzao | 0.72 ± 0.04 | 1.20 ± 0.08 | 0.08 ± 0.00 | 0.42 ± 0.03 | 0.26 ± 0.00 | 0.17 ± 0.01 | 4.3 ± 0.41 | 0.56 ± 0.04 | 7.71 ± 0.6 |
| S53 | Zan2 | 1.73 ± 0.02 | 2.57 ± 0.02 | 0.29 ± 0.00 | 1.28 ± 0.07 | 0.29 ± 0.01 | 0.24 ± 0.01 | 6.26 ± 0.07 | 1.1 ± 0.16 | 13.76 ± 0.26 |
| S54 | ZL4Chen | 1.20 ± 0.09 | 0.98 ± 0.05 | 0.11 ± 0.02 | 0.92 ± 0.08 | 0.31 ± 0.01 | 0.12 ± 0.01 | 6.88 ± 0.07 | 1.02 ± 0.00 | 11.54 ± 0.34 |
| S55 | Zaoshipo | 1.50 ± 0.09 | 3.39 ± 0.15 | 0.24 ± 0.01 | 0.60 ± 0.02 | 0.35 ± 0.00 | 0.36 ± 0.02 | 6.15 ± 0.52 | 0.49 ± 0.33 | 13.08 ± 0.06 |
| S56 | Zan1 | 1.27 ± 0.00 | 2.06 ± 0.00 | 0.14 ± 0.00 | 0.71 ± 0.10 | 0.27 ± 0.01 | 0.16 ± 0.00 | 3.6 ± 0.06 | 0.62 ± 0.06 | 8.84 ± 0.01 |
| S57 | Zhongzaosanhao | 1.35 ± 0.13 | 3.94 ± 0.16 | 0.17 ± 0.00 | 1.01 ± 0.28 | 0.43 ± 0.13 | 0.46 ± 0.13 | 4.79 ± 0.21 | 1.14 ± 0.31 | 13.29 ± 1.35 |
| S58 | Baodeyouzao | 0.97 ± 0.02 | 1.78 ± 0.04 | 0.11 ± 0.01 | 0.20 ± 0.02 | 0.24 ± 0.01 | 0.16 ± 0.00 | 3.1 ± 0.10 | 0.55 ± 0.13 | 7.12 ± 0.34 |
| S59 | Goutouzao | 1.76 ± 0.27 | 2.95 ± 0.45 | 0.19 ± 0.03 | 1.08 ± 0.46 | 0.79 ± 0.30 | 0.79 ± 0.32 | 12.55 ± 2.13 | 2.94 ± 1.5 | 23.04 ± 5.46 |
| S60 | Jinsixiaozao | 2.75 ± 0.18 | 4.39 ± 0.26 | 0.52 ± 0.21 | 1.18 ± 0.27 | 0.95 ± 0.26 | 0.72 ± 0.03 | 11.93 ± 1.89 | 2.38 ± 0.92 | 24.82 ± 4.02 |
| S61 | Longzao | 2.89 ± 0.05 | 5.24 ± 0.08 | 0.23 ± 0.04 | 0.93 ± 0.06 | 0.36 ± 0.01 | 0.43 ± 0.09 | 9.12 ± 0.09 | 1.91 ± 0.35 | 21.11 ± 0.51 |
| S62 | Qiding | 1.94 ± 0.30 | 3.65 ± 0.49 | 0.1 ± 0.00 | 0.41 ± 0.11 | 0.37 ± 0.04 | 0.35 ± 0.02 | 5.66 ± 0.54 | 0.83 ± 0.03 | 13.31 ± 1.52 |
| S63 | Zunyi | 0.96 ± 0.04 | 1.91 ± 0.09 | 0.25 ± 0.02 | 0.55 ± 0.05 | 0.32 ± 0.02 | 0.42 ± 0.13 | 6.61 ± 0.95 | 0.85 ± 0 | 11.87 ± 1.3 |
| S64 | Xiaozi | 2.26 ± 0.24 | 2.79 ± 0.27 | 0.10 ± 0.00 | 0.46 ± 0.02 | 0.72 ± 0.05 | 0.57 ± 0.06 | 5.47 ± 0.39 | 0.9 ± 0.05 | 13.28 ± 1.04 |
| S65 | Donglingwuhe | 3.12 ± 0.32 | 5.37 ± 0.51 | 0.2 ± 0.03 | 0.89 ± 0.07 | 0.46 ± 0.03 | 0.55 ± 0.04 | 7.49 ± 0.68 | 1.28 ± 0.07 | 19.37 ± 1.7 |
| S66 | Bayangzao | 1.12 ± 0.01 | 2.03 ± 0.03 | 0.23 ± 0.11 | 0.31 ± 0.02 | 0.28 ± 0.09 | 0.35 ± 0.01 | 4.36 ± 0.21 | 0.95 ± 0.26 | 9.63 ± 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Zheng, J.; Zhang, L.; Yan, S.; Huang, W.; He, J.; Liu, P. Phytochemical Profiling and Fingerprint Analysis of Chinese Jujube (Ziziphus jujuba Mill.) Leaves of 66 Cultivars from Xinjiang Province. Molecules 2019, 24, 4528. https://doi.org/10.3390/molecules24244528
Song L, Zheng J, Zhang L, Yan S, Huang W, He J, Liu P. Phytochemical Profiling and Fingerprint Analysis of Chinese Jujube (Ziziphus jujuba Mill.) Leaves of 66 Cultivars from Xinjiang Province. Molecules. 2019; 24(24):4528. https://doi.org/10.3390/molecules24244528
Chicago/Turabian StyleSong, Lijun, Jie Zheng, Li Zhang, Shijuan Yan, Wenjie Huang, Jun He, and Pengzhan Liu. 2019. "Phytochemical Profiling and Fingerprint Analysis of Chinese Jujube (Ziziphus jujuba Mill.) Leaves of 66 Cultivars from Xinjiang Province" Molecules 24, no. 24: 4528. https://doi.org/10.3390/molecules24244528






