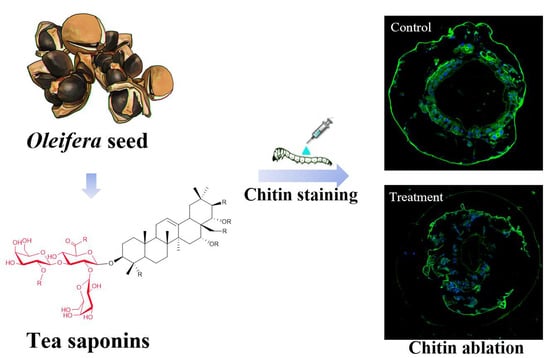
Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia oleifera
Abstract
:1. Introduction
2. Results
2.1. Saponins in Aqueous Ethanolic Extracts
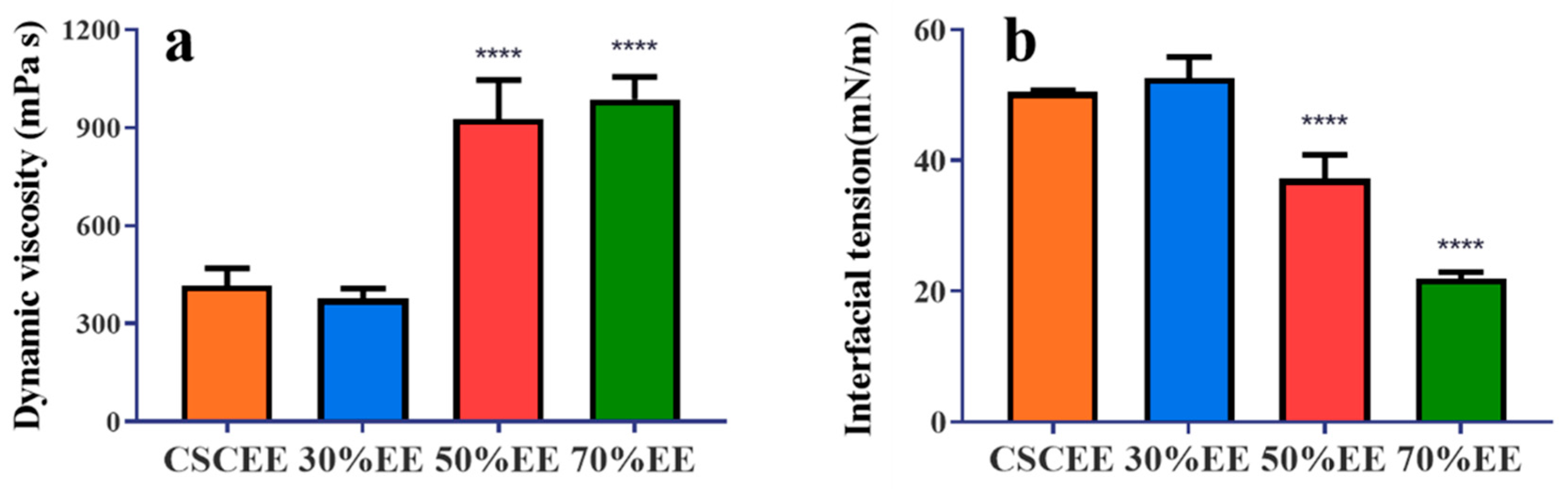
2.2. Dynamic Viscosity Coefficient and Interfacial Tension Values of Different Extracts
2.3. Toxicty of the Saponins on the Stomach of E. obliqua Larvae
2.4. Contact Toxicity of the Camellia Saponins on E. obliqua Larvae
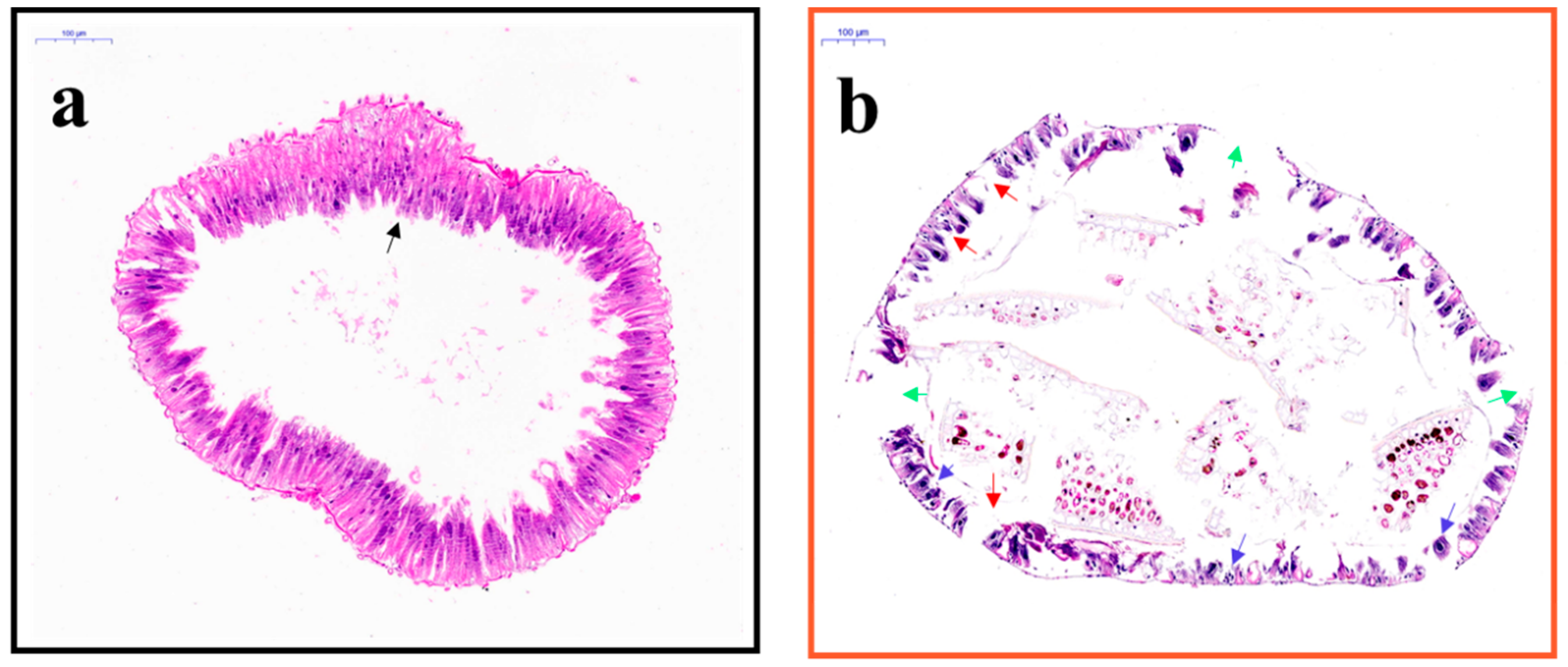
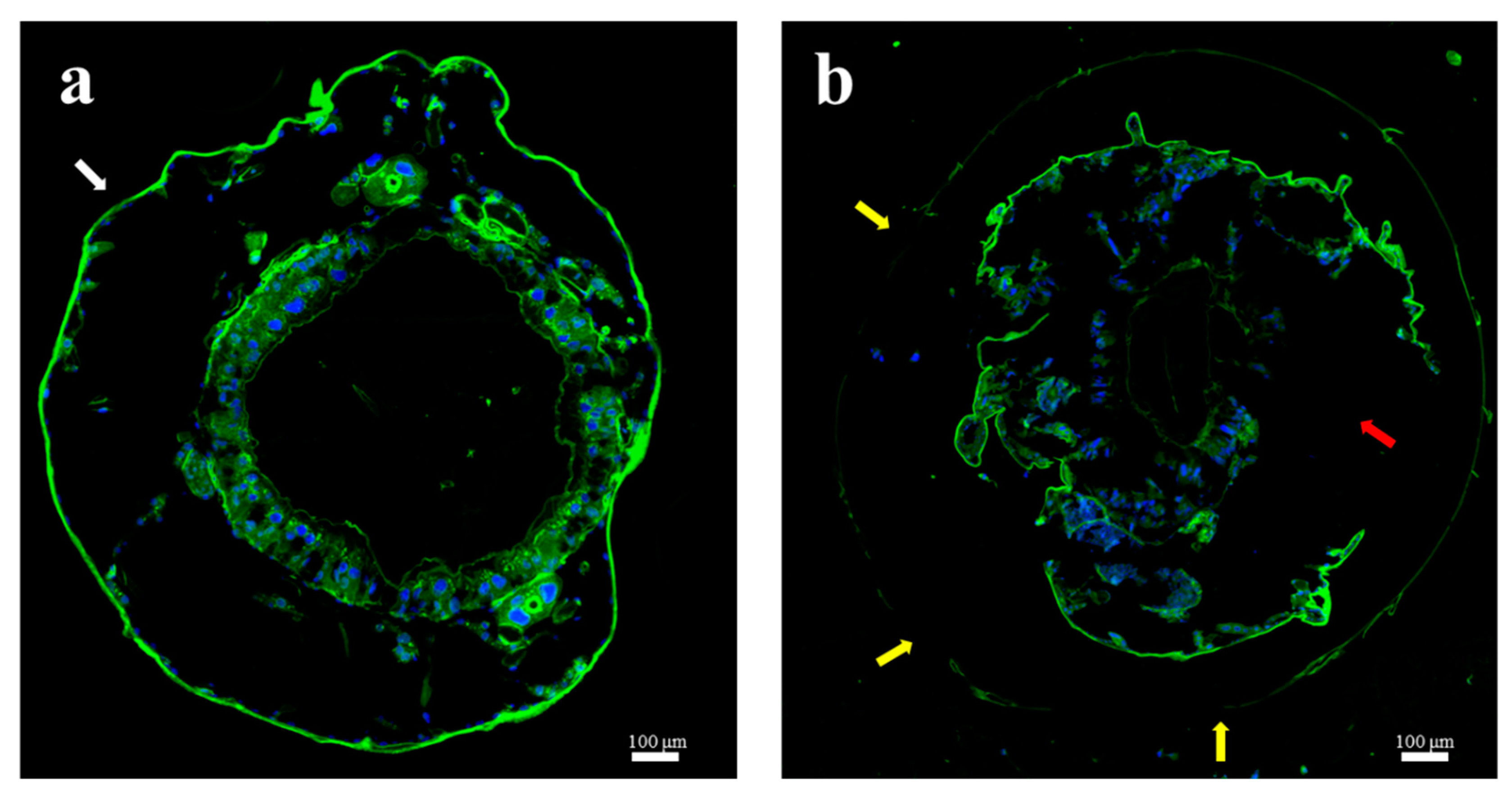
2.5. Midgut of E. obliqua Larvae and Morphological Changes after Tea Saponin Treatment
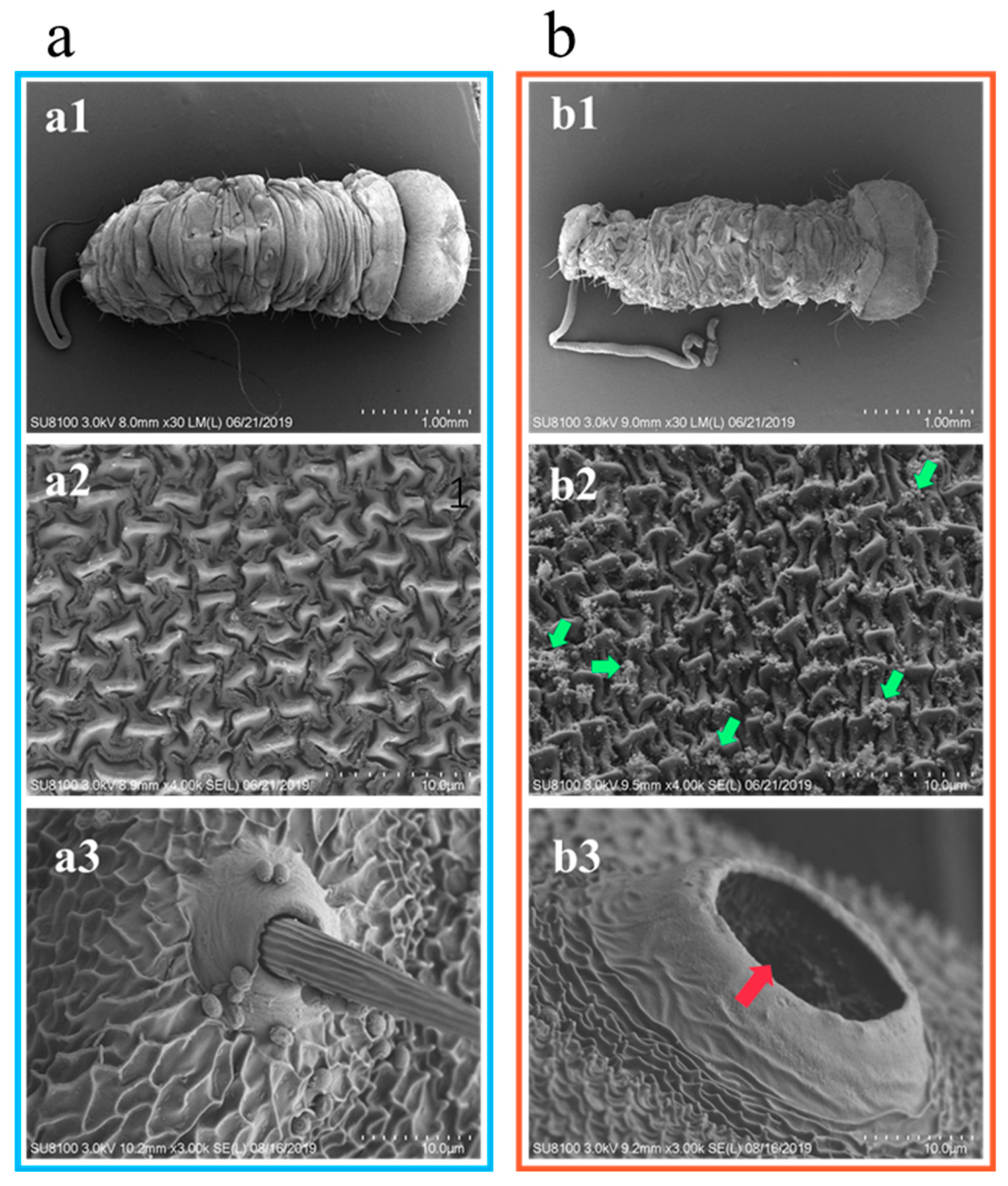
2.6. Observation of the Epidermis of E. obliqua Larvae Treated with Tea Saponin by Electron Microscopy
2.7. Chitin Staining of E. obliqua Larvae and Morphological Changes after Tea Saponin Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Analytical Method
4.3. Interfacial Tension and Dynamic Viscosity Coefficient of the Different Extracts
4.4. Bioassays
4.4.1. Evaluation of the Contact Toxicity of Camellia Saponins on E. obliqua
4.4.2. Evaluation of the Stomach Toxicity of Camellia Saponins on E. obliqua
4.4.3. Midgut Slices of E. obliqua
4.4.4. Scanning Electron Microscopy
4.4.5. Chitin Staining of E. obliqua Sections
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malongane, F.; Mcgaw, L.J.; Mudau, F.N. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: A review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Sinu, P.A.; Mandal, P.; Banerjee, D.; Mallick, S.; Talukdar, T.; Pathak, S.K. Moth pests collected in light traps of tea plantations in North East India: Species composition, seasonality and effect of habitat type. Curr. Sci. 2013, 104, 646–651. [Google Scholar]
- Ma, L.; Li, Z.-Q.; Bian, L.; Cai, X.-M.; Luo, Z.-X.; Zhang, Y.-J.; Chen, Z.-M. Identification and comparative study of chemosensory genes related to host selection by legs transcriptome analysis in the tea geometrid Ectropis obliqua. PLoS ONE 2016, 11, e0149591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Chen, L. Research of resistance mechanism to Ectropis oblique by tea plant. J. Tea Sci. 2014, 34, 541–547. [Google Scholar]
- Feng, J.; Tang, H.; Chen, D.; Li, L. Monitoring and risk assessment of pesticide residues in tea samples from China. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 169–183. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhao, S.C.; Deng, L.G.; Mao, J.S.; Guo, C.Y.; Yang, G.S.; Lu, X.; Aboul-Enein, H.Y. Rapid Determination of Organonitrogen, Organophosphorus and Carbamate Pesticides in Tea by Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry (UPLC-MS/MS). Food Anal. Methods 2013, 6, 497–505. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, Y.; Cao, H.; Tong, Z.; Xiao, J.; Liao, M.; Wu, X.; Hua, R. Degradation Dynamics and Dietary Risk Assessments of Two Neonicotinoid Insecticides during Lonicera japonica Planting, Drying, and Tea Brewing Processes. J. Agric. Food Chem. 2017, 65, 1483–1488. [Google Scholar] [CrossRef]
- Cui, C.; Zong, J.; Sun, Y.; Zhang, L.; Ho, C.-T.; Wan, X.; Hou, R. Triterpenoid saponins from the genus Camellia: Structures, biological activities, and molecular simulation for structure–Activity relationship. Food Funct. 2018, 9, 3069–3091. [Google Scholar] [CrossRef]
- Wu, H. Study on Influencing Factors and Trend Prediction of Camellia Industry Development in China; Chinese Academy of Forestry: Beijing, China, 2017. [Google Scholar]
- Dolma, S.K.; Sharma, E.; Gulati, A.; Reddy, S.E. Insecticidal activities of tea saponin against diamondback moth, Plutella xylostella and aphid, Aphis craccivora. Toxin Rev. 2018, 37, 52–55. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Bai, Y.; Cai, H.; Wei, H.; Tian, H.; Zhao, J.; Chen, Y.; Yang, G.; Gu, X.; et al. Effect of Tea Saponin-treated host plants on activities of antioxidant enzymes in larvae of the Diamondback Moth Plutella xylostella (Lepidoptera: Plutellidae). Env. Entomol. 2018, 47, 749–754. [Google Scholar] [CrossRef]
- Cai, H.; Bai, Y.; Wei, H.; Lin, S.; Chen, Y.; Tian, H.; Gu, X.; Murugan, K. Effects of tea saponin on growth and development, nutritional indicators, and hormone titers in diamondback moths feeding on different host plant species. Pestic. Biochem. Physiol. 2016, 131, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, I. Saponins as insecticides: A review. Tunis. J. Plant Prot. 2010, 5, 39–50. [Google Scholar]
- Zhang, X.-F.; Yang, S.-L.; Han, Y.-Y.; Zhao, L.; Lu, G.-L.; Xia, T.; Gao, L.-P. Qualitative and quantitative analysis of triterpene saponins from tea seed pomace (Camellia oleifera abel) and their activities against bacteria and fungi. Molecules 2014, 19, 7568–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devonshire, A.L.; Moores, G.D. A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate and pyrethroid resistance in peach-potato aphids (Myzus persicae). Pestic. Biochem. Physiol. 1982, 18, 235–246. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Lai, J.; Li, X.; Zhang, Y. Study of tea saponin TS-D insecticidal effects on cabbage butterfly. Plant Prot. 1996, 22, 27–28. [Google Scholar]
- Zeng, C.; Wu, L.; Zhao, Y.; Yun, Y.; Peng, Y. Tea saponin reduces the damage of Ectropis obliqua to tea crops, and exerts reduced effects on the spiders Ebrechtella tricuspidata and Evarcha albaria compared to chemical insecticides. PeerJ 2018, 6, e4534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaicharoenpong, C.; Petsom, A. Quantitative thin layer chromatographic analysis of the saponins in tea seed meal. Phytochem. Anal. 2009, 20, 253–255. [Google Scholar] [CrossRef]
- Li, N.; Ma, Z.-J.; Chu, Y.; Wang, Y.; Li, X. Phytochemical analysis of the triterpenoids with cytotoxicity and QR inducing properties from the total tea seed saponin of Camellia sinensis. Fitoterapia 2013, 84, 321–325. [Google Scholar] [CrossRef]
- Snodgrass, R.E. The abdominal mechanisms of a grasshopper. Smithson. Misc. Collect. 1935, 94, 6. [Google Scholar]
- Akai, H.; Kimura, K.; Kiuchi, M.; Shibukawa, A. Effects of anti-juvenoid treatment on cocoon and cocoon filaments in Bombyx mori. J. Sericultural Sci. Jpn. 1984, 53, 545–546. [Google Scholar]
- Serrão, J.E.; Da Cruz-Landim, C. Gut structures in adult workers of necrophorous Neotropical stingless bees (Hymenoptera: Apidae: Meliponinae). Entomol. Gen. 1995, 19, 261–265. [Google Scholar] [CrossRef]
- Cavalcante, V.; da Cruz-Landim, C. Types of cells present in the midgut of the insects: A review. Nat.-Sao Paulo. 1999, 24, 19–40. [Google Scholar]
- Agrawal, S.; Kelkenberg, M.; Begum, K.; Steinfeld, L.; Williams, C.E.; Kramer, K.J.; Beeman, R.W.; Park, Y.; Muthukrishnan, S.; Merzendorfer, H. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem. Mol. Biol. 2014, 49, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, B.R. The contact toxicity of some pesticide residues to hymenopterous parasites and coccinellid predators. J. Econ. Entomol. 1963, 56, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Thompson, H.M. Assessing the exposure and toxicity of pesticides to bumblebees (Bombus sp.). Apidologie 2001, 32, 305–321. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.Q.; Guo, S.S.; Wang, Y.; Pang, X.; Geng, Z.F.; Du, S.S. Toxicity and repellency of essential oil from Evodia lenticellata Huang fruits and its major monoterpenes against three stored-product insects. Ecotoxicol. Environ. Saf. 2018, 160, 342–348. [Google Scholar] [CrossRef]
- Wang, Y.; You, C.X.; Yang, K.; Wu, Y.; Chen, R.; Zhang, W.J.; Liu, Z.L.; Du, S.S.; Deng, Z.W.; Geng, Z.F. Bioactivity of Essential Oil of Zingiber purpureum Rhizomes and Its Main Compounds against Two Stored Product Insects. J. Econ. Entomol. 2015, 108, 925–932. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Lognay, G.; Bitume, E.; Hance, T.; Mailleux, A.C. A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J. Pest Sci. 2013, 86, 361–386. [Google Scholar] [CrossRef]
- Gil, Y.; Sinfort, C. Emission of pesticides to the air during sprayer application: A bibliographic review. Atmos. Environ. 2005, 39, 5183–5193. [Google Scholar] [CrossRef]
- Wang, B.; Song, J.; Zeng, A.; Liu, Y.; Zhang, J.; He, X. Effects of formulations and surfactants on the behavior of pesticide liquid spreading in the plant leaves. Chin. J. Pestic. Sci. 2012, 14, 334–340. [Google Scholar]
- Kirkwood, R.C. Recent developments in our understanding of the plant cuticle as a barrier to the foliar uptake of pesticides. Pestic. Sci. 1999, 55, 69–77. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Luzhen, W. Test on insecticidal effect of high chlorine and octyl oil. Agric. Technol. Equip. 2014, 22, 50–51. [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorganic Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Yahouédo, G.A.; Chandre, F.; Rossignol, M.; Ginibre, C.; Balabanidou, V.; Mendez, N.G.A.; Pigeon, O.; Vontas, J.; Cornelie, S. Contributions of cuticle permeability and enzyme detoxification to pyrethroid resistance in the major malaria vector Anopheles gambiae. Sci. Rep. 2017, 7, 11091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vontas, J.; David, J.P.; Nikou, D.; Hemingway, J.; Christophides, G.; Louis, C.; Ranson, H. Transcriptional analysis of insecticide resistance in Anopheles stephensi using cross-species microarray hybridization. Insect Mol. Biol. 2007, 16, 315–324. [Google Scholar] [CrossRef]
- Awolola, T.; Oduola, O.; Strode, C.; Koekemoer, L.; Brooke, B.; Ranson, H. Hygiene, Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1139–1145. [Google Scholar] [CrossRef]
- Vannini, L.; Reed, T.W.; Willis, J.H. Temporal and spatial expression of cuticular proteins of Anopheles gambiae implicated in insecticide resistance or differentiation of M/S incipient species. Parasites Vectors 2014, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, H. Synergistic interaction of tea saponin with mancozeb against Pestalotiopsis theae. Crop Prot. 2012, 40, 126–131. [Google Scholar] [CrossRef]
- Zong, J.; Wang, R.; Bao, G.; Ling, T.; Zhang, L.; Zhang, X.; Hou, R. Novel triterpenoid saponins from residual seed cake of Camellia oleifera Abel. show anti-proliferative activity against tumor cells. Fitoterapia 2015, 104, 7–13. [Google Scholar] [CrossRef]
- Beloti, V.H.; Alves, G.R.; Araújo, D.F.D.; Picoli, M.M.; de Andrade Moral, R.; Demétrio, C.G.B.; Yamamoto, P.T. Lethal and sublethal effects of insecticides used on citrus, on the ectoparasitoid Tamarixia radiata. PLoS ONE 2015, 10, e0132128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, P.; Gao, X.W.; Zheng, B.Z. Genetic basis of resistance and studies on cross-resistance in a population of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. Former. Pestic. Sci. 2003, 59, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Pesticides | Toxicity Regression Equation a | LC50 (mg/L) b | 95% Confidence Interval c | R2 |
|---|---|---|---|---|
| C. oleifera seed cake ethanol extract | y = −6.69 + 4.12x | 49.100 | 39.729–60.126 | 0.874 |
| 30% ethanol eluate | y = −6.4 + 3.84x | 53.239 | 42.799–66.291 | 0.862 |
| 50% ethanol eluate | y = −6.72 + 4.21x | 45.287 | 36.527–55.263 | 0.905 |
| 70% ethanol eluate | y = −4.52 + 3.39x | 22.395 | 16.489–28.338 | 0.943 |
| Pesticides | Toxicity Regression Equation a | LC50 (mg/L) b | 95% Confidence Interval c | R2 |
|---|---|---|---|---|
| C. oleifera seed cake ethanol extract | y = −5.00 + 3.43x | 27.380 | 21.428–36.969 | 0.977 |
| 30% ethanol eluate | y = −6.46 + 4.94x | 21.004 | 16.848–25.238 | 0.907 |
| 50% ethanol eluate | y = −3.75 + 3.14x | 15.732 | 8.677–20.814 | 0.834 |
| 70% ethanol eluate | y = −3.72 + 4.13x | 8.459 | 3.564–11.646 | 0.984 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; Yang, Y.; Zhao, T.; Zou, K.; Peng, C.; Cai, H.; Wan, X.; Hou, R. Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia oleifera. Molecules 2019, 24, 4518. https://doi.org/10.3390/molecules24244518
Cui C, Yang Y, Zhao T, Zou K, Peng C, Cai H, Wan X, Hou R. Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia oleifera. Molecules. 2019; 24(24):4518. https://doi.org/10.3390/molecules24244518
Chicago/Turabian StyleCui, Chuanjian, Yunqin Yang, Tianyu Zhao, Kangkang Zou, Chuanyi Peng, Huimei Cai, Xiaochun Wan, and Ruyan Hou. 2019. "Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia oleifera" Molecules 24, no. 24: 4518. https://doi.org/10.3390/molecules24244518
APA StyleCui, C., Yang, Y., Zhao, T., Zou, K., Peng, C., Cai, H., Wan, X., & Hou, R. (2019). Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia oleifera. Molecules, 24(24), 4518. https://doi.org/10.3390/molecules24244518