Simultaneous LC/MS Analysis of Carotenoids and Fat-Soluble Vitamins in Costa Rican Avocados (Persea americana Mill.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stationary Phase Selection and Green Chemistry
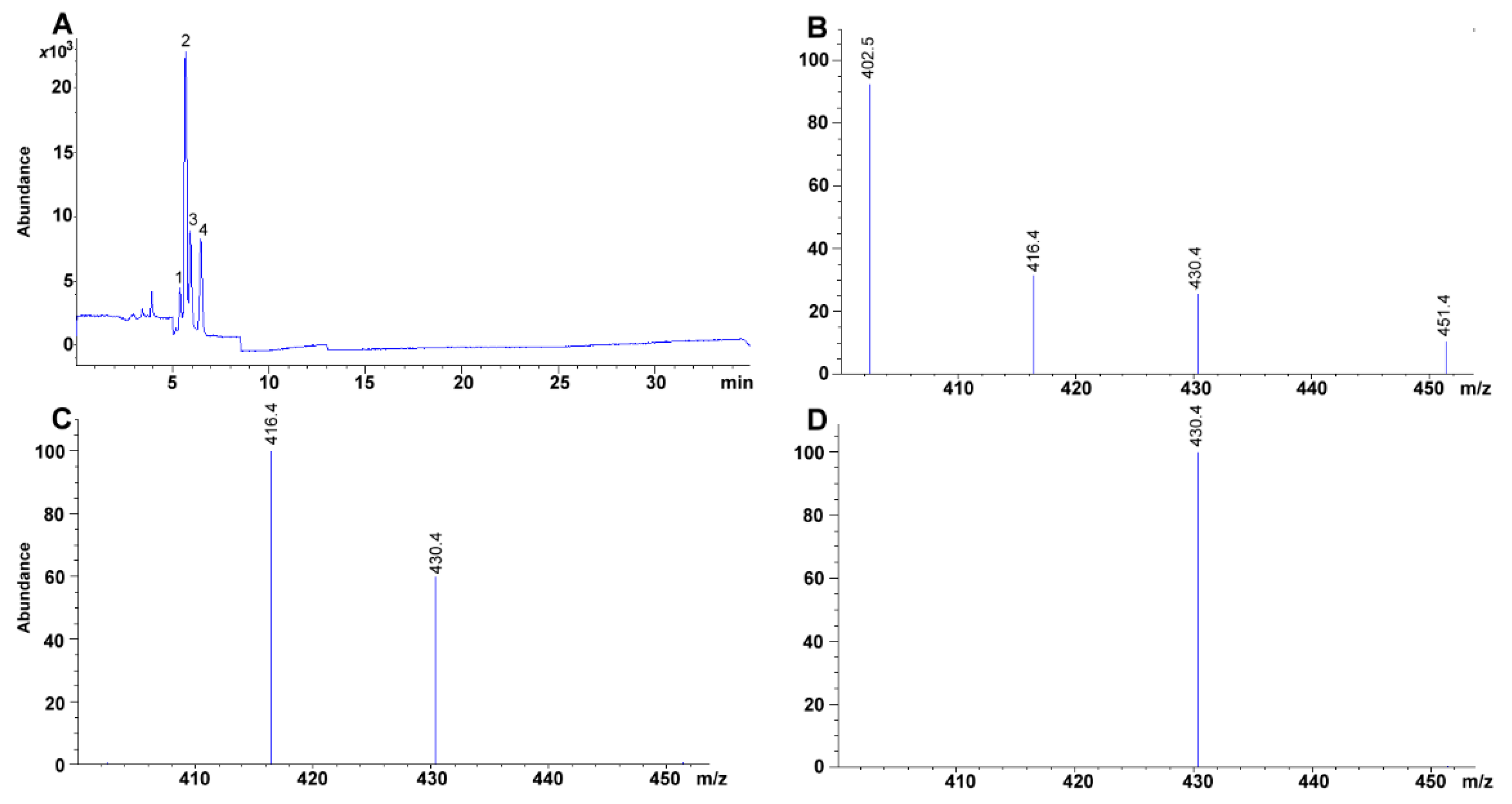
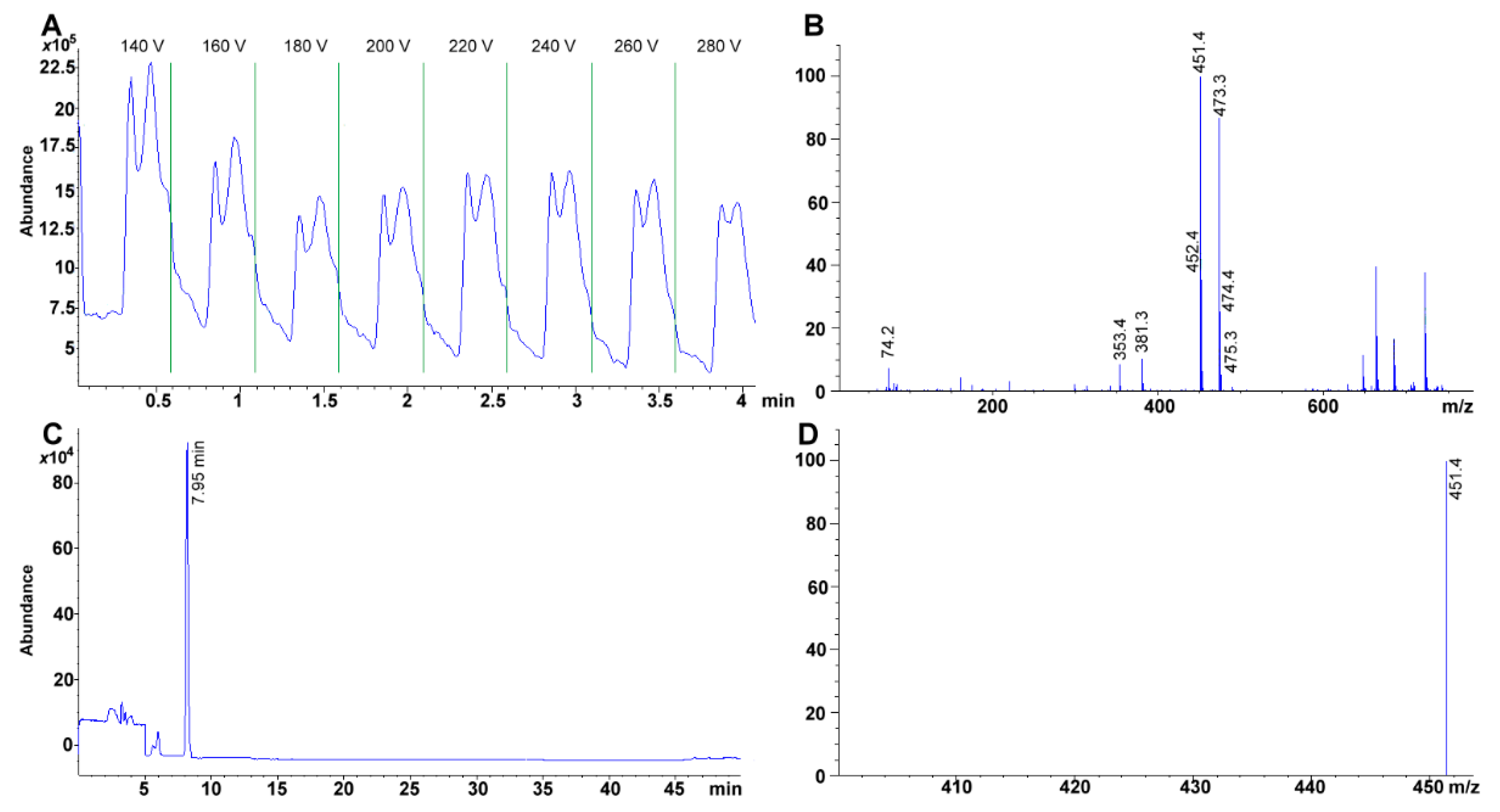
2.2. Singular Ion Monitoring Parameter Selection
2.3. Method Performance Data
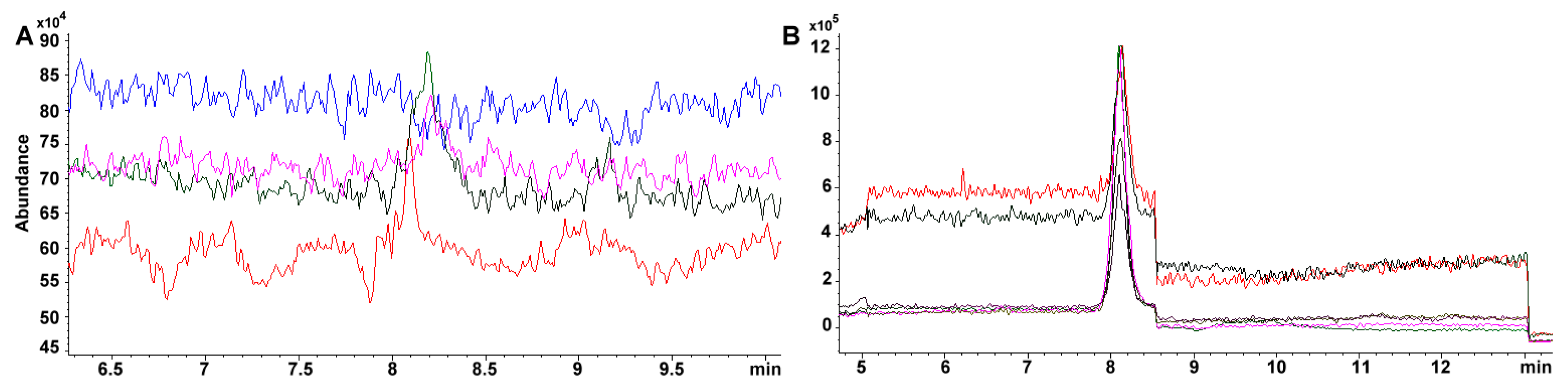
2.4. Performance during Saponification
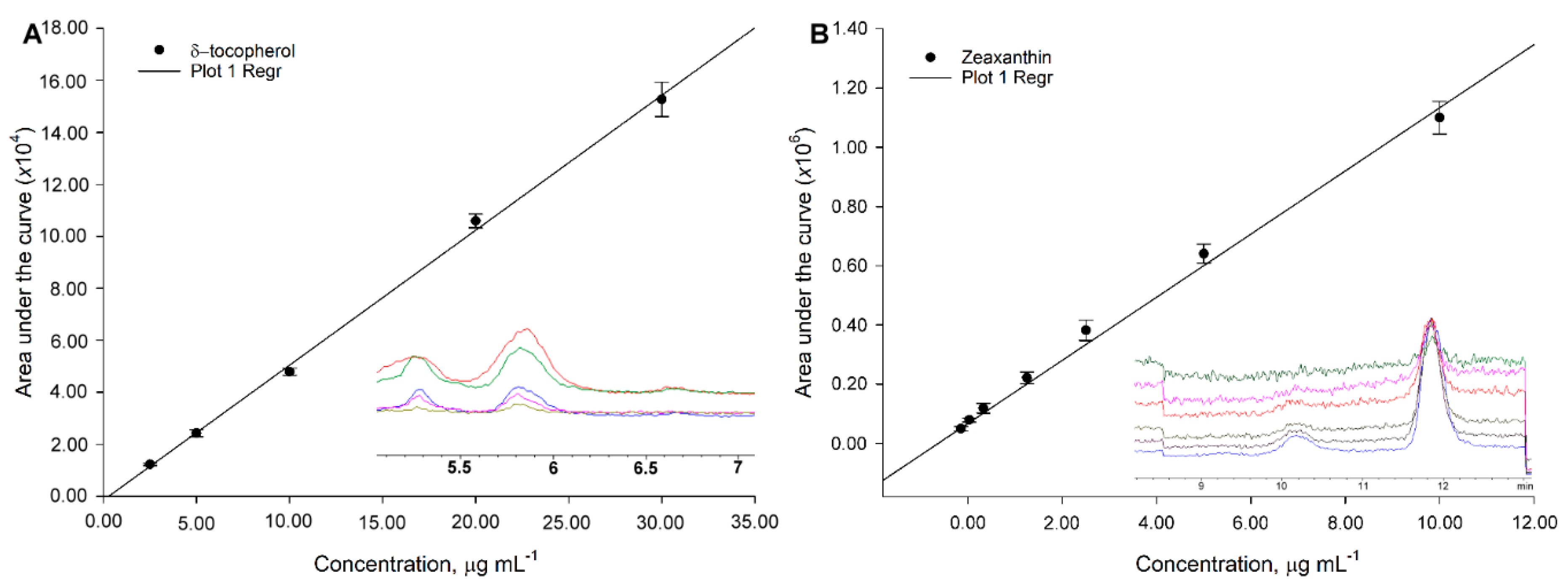
2.5. Quantification, Linearity, and Calibration Curve Construction
2.6. Analyte Recovery and Method Accuracy
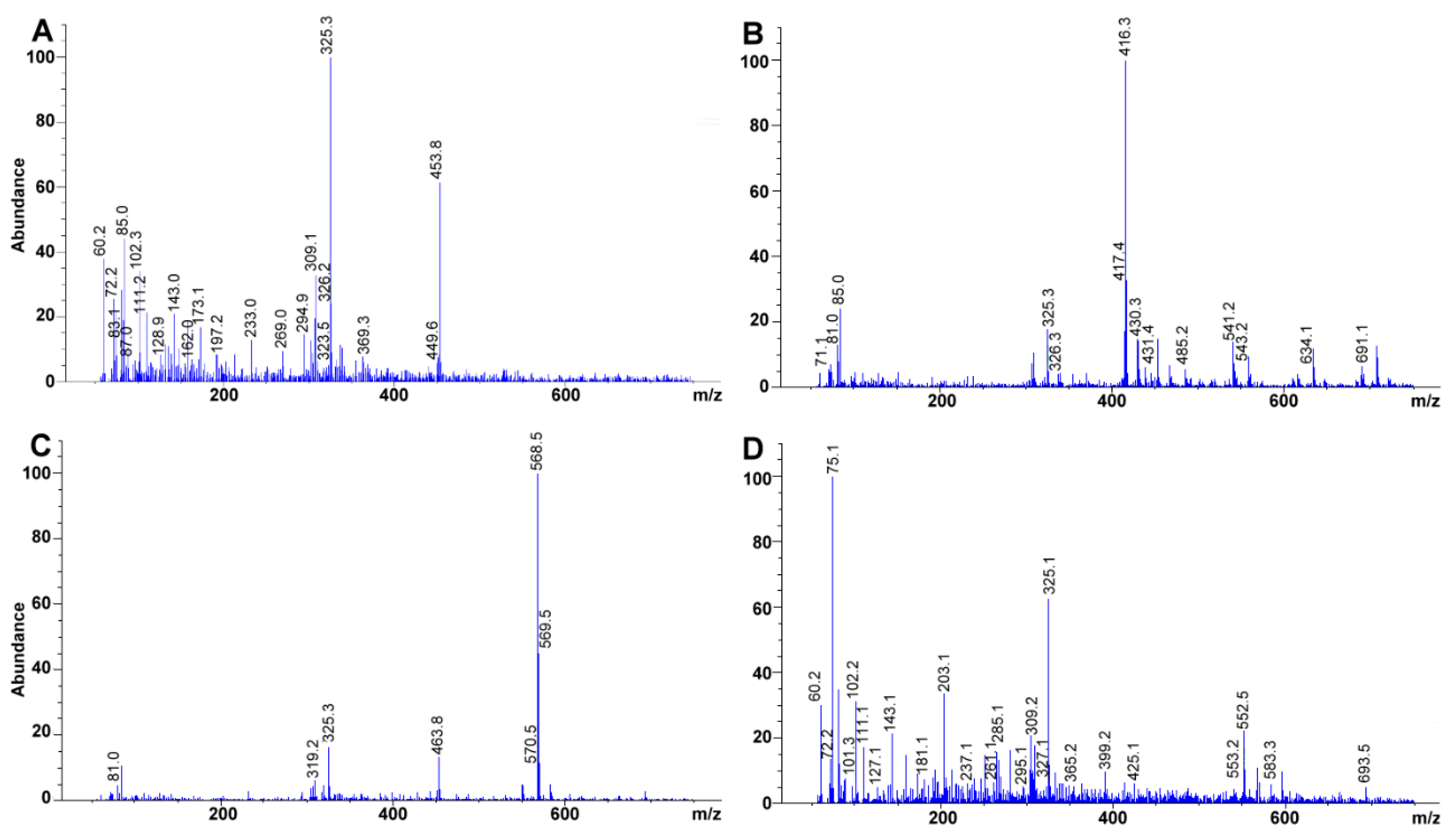
2.7. Mass Spectra Analysis
2.8. Chromatographic Separation of Tocopherol Isomers
2.9. Method Application in Real Samples
2.10. Method Application in Other Samples
3. Materials and Methods
3.1. Reagents
3.2. Sample Treatment and Preparation
3.3. Optimization of Saponification Conditions
3.4. Sample Saponification
3.5. Stationary Phase and Selection of Chromatographic Conditions
3.6. Chromatographic Conditions
3.7. MS Detection System Conditions
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arias, F.; Montoya, C.; Velásquez, O. Dinámica del Mercado mundial de aguacate. Rev. Virtual Univ. Católica Del Norte 2018, 55, 22–35. [Google Scholar] [CrossRef]
- Xiong, B.; Song, Y. Big data and dietary trend: The case of avocado imports in China. J. Int. Food Agribus. Mark. 2018, 30, 343–354. [Google Scholar] [CrossRef]
- Macías Macías, A. Mexico in the International Avocado Market. Rev. De Cienc. Soc. (RCS) 2011, 17, 517–532. [Google Scholar]
- Díaz Vasquez, J.; Ardila Lopez, C.; Guerra Aranguren, M.A. Case Study on the Eligibility of Colombian Hass Avocado in the US Market: Opportunities in East Asia. Online J. Mundo Asia Pac. 2019, 8, 5–27. [Google Scholar]
- PROCOMER [Promotora de Comercio Exterior de Costa Rica]. Anuario Estadístico 2017. PROCOMER: San José, Costa Rica, 2018. Available online: https://procomer.com/en/estudios/anuario_estadistico_2018 (accessed on 25 August 2019).
- Butnariu, M. Methods of analysis (extraction, separation, identification, and quantification) of carotenoids from natural products. J. Ecosys. Ecograph. 2016, 6, 2. [Google Scholar] [CrossRef]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid Absorption from Salad and Salsa by Humans Is Enhanced by the Addition of Avocado or Avocado Oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef]
- Brown, M.J.; Ferruzzi, M.G.; Nguyen, M.L.; Cooper, D.A.; Schwartz, S.J.; White, W.S. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat reduced salad dressings as measured with electrochemical detection. Am. J. Clin. Nutr. 2004, 80, 396–403. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.D.; Landahl, S.; Donetti, M.; Terry, L.A. Avocado. In Health-Promoting Properties of Fruits and Vegetables, 1st ed.; Terry, L.A., Ed.; CABI: Oxfordshire, UK, 2011; pp. 27–50. [Google Scholar]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effect. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Fulgoni, V.L.; Dreher, M.L.; Davenport, A.J. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: Results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Nutr. J. 2013, 12. [Google Scholar] [CrossRef] [Green Version]
- Comerford, K.B.; Ayoob, K.T.; Murray, R.D.; Atkinson, S.A. The role of Avocados in Maternal Diets during the periconceptional Period, Pregnancy, and Lactation. Nutrients 2016, 8, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, P.F.; Chaves, M.A.; Borges, C.D.; Mendoca, C.R.B. Avocado: Characteristics, Health Benefits and Uses. Ciência Rural 2016, 46, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Noorul, H.; Nesar, A.; Zafar, K.; Khalid, M.; Zeeshan, A.; Vartika, S. Health benefits and pharmacology of Persea americana mill. (Avocado). Int. J. Res. Pharmacol. Pharmacother. 2016, 5, 132–141. [Google Scholar]
- Scott, T.M.; Rasmussen, H.M.; Chen, O.; Johnson, E.J. Avocado Consumption Increases Macular Pigment Density in Older Adults: A Randomized, Controlled Trial. Nutrients 2016, 9, 919. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.-Y.; Arteaga, J.R.; Zhang, Q.; Huerta, S.; Go, V.L.W.; Heber, D. Inhibition of prostate cancer cell growth by an avocado extract: Role of lipid-soluble bioactive substances. J. Nutr. Biochem. 2005, 16, 23–30. [Google Scholar] [CrossRef]
- Gross, J.; Gabai, M.; Lifshitz, A. The carotenoid of the avocado pear. Persea americana, Nabal Variety. J. Food Sci. 1972, 37, 589–591. [Google Scholar] [CrossRef]
- Moran, N.E.; Johnson, E.J. Closer to clarity on the effect of lipid consumption on fat-soluble vitamin and carotenoid absorption: Do we need to close in further? Am. J. Clin. Nutr. 2017, 106, 969–970. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Catharine Ross, A.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Bielaski, H.K. β-Carotene is an important vitamin A source for human. J. Nutr. 2010, 140, 2269S–2285S. [Google Scholar] [CrossRef] [Green Version]
- Mardigan, L.P.; dos Santos, V.J.; da Silva, P.T.; Visentainer, J.V.; Gomes, S.T.M.; Matsuchita, M. Investigation of bioactive compounds from various avocado varieties (Persea americana Miller). Food Sci. Technol. 2018, 39 (Suppl. 1), 15–21. [Google Scholar] [CrossRef] [Green Version]
- Ashton, O.B.O.; Wong, M.; McGhie, T.K.; Vather, R.; Wang, Y.; Requejo-Jakcman, C.; Ramankutty, P.; Woolf, A.B. Pigments in Avocado Tissue and Oil. J. Agric. Food Chem. 2006, 54, 10151–10158. [Google Scholar] [CrossRef]
- Yano, M.; Kato, M.; Ikoma, Y.; Kawasaki, A.; Fukazawa, Y.; Sugiura, M.; Matsumo, H.; Oohara, Y.; Nagao, A.; Ogawa, K. Quantitation of Carotenoids in Raw and Processed Fruits in Japan. Food Sci. Technol. Res. 2005, 11, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A. Phytochemical analysis of avocado seeds (Persea amerciana Mill., c.v. Hass), 1st ed.; Cuvillier Verlag: Göttingen, Germany, 2007; pp. 26–28. [Google Scholar]
- Talabi, J.Y.; Osukoya, O.A.; Ajayi, O.O.; Adegoke, G.O. Nutritional and antinutritional compositions of processed Avocado (Persea americana Mill) seeds. Asian J. Plant. Sci. Res. 2016, 6, 6–12. [Google Scholar]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Roca, M. Recent Developments in the Analysis of Carotenoids by Mass Spectrometry. In Progress in Carotenoid Research, 1st ed.; Zepka, L.Q., Ed.; IntechOpen Limited: London, UK, 2018; pp. 17–44. [Google Scholar]
- Lu, Q.-Y.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.-P.; Gao, K.; Byrns, R.; Heber, D. California Hass Avocado: Profiling of Carotenoids, Tocopherol, and Fat Content during Maturation and from Different Growing Areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Stability of avocado paste carotenoids as affected by high hydrostatic pressure processing and storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 121–128. [Google Scholar] [CrossRef]
- Morera, J.A. El Aguacate, 1st ed.; Unidad de Recursos Fitogenéticos CATIE/GTZ: Turrialba, Costa Rica, 1983; pp. 10–19. [Google Scholar]
- McGraw, K.J.; Toomey, M.B. Carotenoid accumulation in the tissues of Zebra Finches: Predictors of Intergumentary Pigmentation and Implication for Carotenoid Allocation strategies. Physiol. Biochem. Zool. 2010, 83, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Taygerly, J.P.; Miller, L.M.; Yee, A.; Peterson, E.A. A convenient guide to help select replacement solvents for dichloromethane in chromatography. Green Chem. 2012, 14, 3020–3025. [Google Scholar] [CrossRef]
- Sander, L.C.; Sharpless, K.E.; Craft, N.E.; Wise, S.A. Development of Engineered Stationary Phase for the Separation of Carotenoid Isomers. Anal. Chem. 1994, 66, 1667–1674. [Google Scholar] [CrossRef]
- Van Breemer, R.B.; Huang, C.-H. High-performance liquid chromatography-electrospray mass spectrometry of retinoids. FASEB J. 1996, 10, 1098–1101. [Google Scholar] [CrossRef]
- Fraser, P.D.; Enfissi, E.M.A.; Goodfellow, M.; Eguchi, T.; Bramley, P.M. Metabolite profiling of plant carotenoids using the matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Plant J. 2007, 49, 552–564. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, Q.P.; Dong, H.R. Determination of lycopene and β-carotene by high-performance liquid chromatography using sudan I as internal standard. J. Chrom. B 2006, 838, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Hui, B. Quantification of all-trans-lycopene and β-carotene from tomato and its products by internal standard method on C30-HPLC. Food Sci. 2010, 31, 348–354. [Google Scholar]
- Tan, J.; Leong Neo, J.G.; Setiawati, T.; Zhang, C. Determination of carotenoids in human serum and breast milk using high performance liquid chromatography coupled with a Diode Array Detector (HPLC-DAD). Separations 2017, 4, 19. [Google Scholar] [CrossRef]
- Van Breemen, R.B. Liquid chromatography/mass spectrometry of carotenoids. Pure Appl. Chem. 1997, 69, 2061–2066. [Google Scholar]
- Borman, P.; Elder, D. Q2 (R1) Validation of Analytical Procedures. In ICH Quality Guidelines: An Implementation Guide; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 127–166. [Google Scholar]
- Kumar Saini, R.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Binnal, P.; Nirguna Babu, P. Production of high biodiesel through direct saponification of wet biomass of Chlorella protothecoides in a low cost microwave reactor: Kinetic and thermodynamic studies. Korean J. Chem. Eng. 2017, 34, 1027–1036. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wu, H.; Wang, G.; Dai, S.; Fan, J.; He, H.; Xiang, W. A saponification method for chlorophyll removal from microalgae biomass as oil feedstock. Mar. Drugs 2016, 14, 162. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Pénicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef]
- Hadjal, T.; Dhuique-Mayer, C.; Madani, K.; Dornier, M.; Achir, N. Thermal degrdation kinetics of xanthophylls from blood orange in model and real food systems. Food Chem. 2013, 138, 2442–2450. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.; Gandul-Rojas, B. Thermal degradation kinetics of lutein, β-carotene and β-cryptoxanthin. J. Food Comp. Anal. 2011, 24, 811–820. [Google Scholar] [CrossRef]
- Silva Fernades, A.; Casagrande do Nascimento, T.; Jacob-Lopes, E.; Vera de Roso, V.; Queiroz Zepka, L. Carotenoids: A Brief Overview on Its Structure, Biosynthesis, Synthesis, and Applications. In Progress in Carotenoid Research, 1st ed.; Queiroz Zepka, L., Jacob-Lopes, E., Vera de Roso, V., Eds.; IntechOpen: London, UK, 2018; pp. 1–15. [Google Scholar]
- Li, D.; Xiao, Y.; Zhang, Z.; Liu, C. Light-induced oxidation and isomeration of all-trans-β-cryptoxanthin in a model system. J. Photochem. Photobiol. B Biol. 2015, 142, 51–58. [Google Scholar] [CrossRef] [PubMed]
- AOAC [Association of Official Analytical Chemists]. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. 2002. Available online: https://www.aoac.org/aoac_prod_imis/AOAC_Docs/StandardsDevelopment/SLV_Guidelines_Dietary_Supplements.pdf (accessed on 25 August 2019).
- Scheppele, S.E.; Mitchum, R.K.; Rudolph, C.J.; Kinneberg, K.F.; Odell, G.V. Mass Spectra of Tocopherols. Lipids 1971, 7, 297–304. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Pon, A.; Karu, N.; Zheng, J.; Li, C.; Arndt, D.; Gautam, M.; Allen, F.; Wishart, D.S. CFM-ID 3.0: Significanlty improved ESI-MS/MS prediction and compound identification. Metbolites 2019, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Rivera, S.M.; Christou, P.; Canela-Garyoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2013, 9999, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Liigand, J.; Laaniste, A.; Kruve, A. pH effects on electrospray ionization efficiency. J. Am. Soc. Mass Spectrom. 2017, 28, 461–469. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA_MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Morera, J.A. Caracterización agronómica de una colección de variedades de aguacate (Persea americana Miller) en la subestación fraijanes, Alajuela, Costa Rica. Rev. Agr. Trop. 2004, 34, 19–25. [Google Scholar]
- Toti, E.; Oliver Chen, C.-Y.; Palmery, M.; Villañ Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Inmunomodulators: Recommneded Diatary Allowance, Therapeutic Index, or Personalized Nutrition? Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Aremu, S.O.; Nweze, C.C. Determination of vitamin content form selected Nigerian fruits using spectrophotometric method. Bangladesh J. Sci. Ind. Res. 2017, 52, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Anary, P.M.; Santos, A.C. Nutritional Value of the Pulp of Different Sugar Apple Cultivars (Annona squamosa L.). In Nutritional composition of fruit cultivars, 1st ed.; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; p. 208. [Google Scholar]
- Marinova, M.; Lütjohann, D.; Westhofen, P.; Watzka, M.; Breuer, O.; Oldenburg, J. A validated HPLC Method for the determination of vitamin K in human serum–First Application in a Pharmocological Study. Open Clin. Chem. J. 2011, 4, 17–27. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the fat-soluble vitamins and carotenoids are available from the authors. |


| Stationary Phase | C8 | C18 | C30 | |||
|---|---|---|---|---|---|---|
| Solvent System | 95:5 MeOH:H2O | 95:5 MeOH:H2O | 90:7:3 MeOH:CH3CN:2-propanol | |||
| Flow, mL min−1 | 0.75 | 1.00 | 0.50 | |||
| Temperature, °C | 50 | 50 | 35 | |||
| Compound | tR, min | Rs | tR, min | Rs | tR, min | Rs |
| Retinyl acetate | 3.50 | 4.75 | 5.42 | |||
| Ergocalciferol | 4.74 | 0 | 8.41 | 8.96 | 0.78 | |
| Cholecalciferol | 4.74 | 0 | 8.81 | 1.12 | 9.27 | 0.78 |
| δ-tocopherol | 4.67 | 1.47 | 8.05 | 1.20 | 7.42 | 2.96 |
| γ-tocopherol | 5.17 | 1.47 | 9.52 | 2.07 | 8.28 | 1.85 |
| α-tocopherol | 7.05 | 15.14 | 12.19 | |||
| Phylloquinone | 7.84 | 19.87 | 13.17 | |||
| Retinyl palmitate | 13.09 | 48.33 | 35.22 | |||
| Detector Set Time, min | Compound | tR, min | Selected SIM Ion, m/z | Fragmentor, V | Dwell Time, ms |
|---|---|---|---|---|---|
| From 0 to 5 | Retinyl acetate | 2.99 | 269.3 [C20H29]•+/325.2 [C20H29OH + K]+ | 100 | 95 |
| Ergocalciferol | 3.59 | 398.3 [M + H]+ | 220 | ||
| Cholecalciferol | 4.02 | 385.3 [M + H]+ | 160 | ||
| From 5 to 8 | δ-tocopherol | 5.26 | 402.5 [M+] | 220 | 71 |
| γ-tocopherol | 5.78 | 416.4 [M+] | 140 | ||
| α-tocopherol | 6.61 | 430.4 [M+] | 80 | ||
| From 8 to 13 | Phylloquinone | 8.07 | 451.4 [M + H]+ | 140 | 56 |
| Astaxanthin | 9.07 | 597.4 [M + H]+ | 160 | ||
| Lutein | 9.97 | 569.4 [M + H]+ | 140 | ||
| Zeaxanthin | 11.49 | 568.4 [M+] | 140 | ||
| After 13 | Retinyl palmitate | 11.19 | 269.3 [C20H29]•+/563.4 [M + K]+ | 100 | 95 |
| β-cryptoxanthin | 16.58 | 552.6 [M+] | 120 | ||
| β-carotene | 22.51 | 536.4 [M+] | 120 | ||
| Lycopene | 37.39 | 536.1 [M+] | 160 |
| Sensitivity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | LoD, μg L−1 | LoQ, μg L−1 | LoD, μg/100 g fat | LoQ, μg/100 g fat | LoD, μg/100 g dry matter | LoQ, μg/100 g dry matter | ||||||
| Retinyl acetate | 3.00 × 102 | 9.20 × 102 | 1.00 × 102 | 3.07 × 102 | 1.50 × 101 | 4.60 × 101 | ||||||
| Ergocalciferol | 1.00 × 102 | 2.90 × 102 | 3.30 × 101 | 9.70 × 101 | 0.50 × 101 | 1.50 × 101 | ||||||
| Cholecalciferol | 2.70 × 102 | 8.20 × 102 | 9.00 × 101 | 2.73 × 102 | 1.40 × 101 | 4.10 × 101 | ||||||
| δ-tocopherol | 1.70 × 102 | 5.10 × 102 | 5.70 × 101 | 1.70 × 102 | 0.90 × 101 | 2.60 × 101 | ||||||
| γ-tocopherol | 1.30 × 101 | 3.80 × 101 | 0.40 × 101 | 1.30 × 101 | 0.10 × 101 | 0.20 × 101 | ||||||
| α-tocopherol | 0.70 × 101 | 2.40 × 101 | 0.20 × 101 | 0.80 × 101 | 0.10 × 101 | 0.10 × 101 | ||||||
| Phylloquinone | 4.30 × 102 | 1.29 × 103 | 1.43 × 102 | 4.30 × 102 | 2.20 × 101 | 6.50 × 101 | ||||||
| Astaxanthin | 2.20 × 101 | 1.25 × 102 | 0.70 × 101 | 4.20 × 101 | 0.10 × 101 | 0.60 × 101 | ||||||
| Lutein | 1.00 × 101 | 2.90 × 101 | 0.30 × 101 | 1.00 × 101 | 0.10 × 101 | 0.10 × 101 | ||||||
| Zeaxanthin | 0.90 × 101 | 2.80 × 101 | 0.30 × 101 | 0.90 × 101 | 0.10 × 101 | 0.10 × 101 | ||||||
| Retinyl palmitate | 2.40 × 101 | 7.40 × 102 | 0.80 × 101 | 2.47 × 102 | 0.10 × 101 | 3.70 × 101 | ||||||
| β-cryptoxanthin | 3.30 × 102 | 9.90 × 102 | 1.10 × 102 | 3.30 × 102 | 1.70 × 101 | 5.00 × 101 | ||||||
| β-carotene | 8.80 × 102 | 2.67 × 103 | 2.93 × 102 | 8.90 × 102 | 4.40 × 101 | 1.34 × 102 | ||||||
| Lycopene | 1.56 × 102 | 4.71 × 102 | 5.20 × 101 | 1.57 × 102 | 0.80 × 101 | 2.40 × 101 | ||||||
| Chromatographic Parameters | ||||||||||||
| Compound | tR, min | [[], mg L−1 | Area, ×105 | Height, ×104 | Peak Width | Symmetry | Rs | k | α | N, ×104 | ||
| Retinyl acetate | 3.00 | 5.00 | 8.98 | 12.96 | 0.12 | 0.71 | 3.67 | 0.35 | 1.61 | 1.08 | ||
| Ergocalciferol | 3.48 | 0.50 | 5.34 | 5.12 | 0.15 | 1.71 | 3.54 | 0.57 | 1.39 | 0.92 | ||
| Cholecalciferol | 3.97 | 1.00 | 1.76 | 1.78 | 0.13 | 0.91 | 7.96 | 0.79 | 1.71 | 1.45 | ||
| δ-tocopherol | 5.21 | 1.00 | 0.67 | 0.63 | 0.18 | 0.95 | 2.85 | 1.35 | 1.16 | 1.34 | ||
| γ-tocopherol | 5.71 | 1.00 | 9.07 | 7.65 | 0.17 | 0.77 | 3.52 | 1.57 | 1.22 | 1.87 | ||
| α-tocopherol | 6.49 | 1.00 | 21.99 | 13.27 | 0.28 | 0.99 | 7.20 | 1.92 | 1.33 | 0.88 | ||
| Phylloquinone | 7.89 | 1.00 | 0.29 | 0.32 | 0.11 | 0.80 | 4.75 | 2.56 | 1.18 | 7.77 | ||
| Astaxanthin | 8.94 | 0.05 | 0.78 | 0.40 | 0.33 | 0.88 | 2.18 | 3.03 | 1.11 | 1.19 | ||
| Lutein | 9.68 | 0.05 | 4.19 | 1.95 | 0.36 | 1.35 | 4.59 | 3.37 | 1.19 | 1.17 | ||
| Zeaxanthin | 11.09 | 1.00 | 0.66 | 0.44 | 0.25 | 1.06 | 2.87 | 3.40 | 1.07 | 3.09 | ||
| Retinyl palmitate | 11.75 | 0.20 | 5.68 | 4.52 | 0.21 | 1.08 | 26.91 | 4.30 | 1.49 | 5.03 | ||
| β-cryptoxanthin | 16.40 | 0.20 | 0.03 | 0.04 | 0.14 | 0.80 | 27.10 | 6.40 | 1.40 | 23.14 | ||
| β-carotene | 22.14 | 1.00 | 0.38 | 0.22 | 0.29 | 0.76 | 71.90 | 8.98 | 1.76 | 9.54 | ||
| Lycopene | 37.39 | 1.00 | 0.28 | 0.34 | 0.14 | 1.04 | - | - | - | 118.31 | ||
| Temperature, °C | 60 | 80 | 95 | ||||
|---|---|---|---|---|---|---|---|
| Base Concentration, mol KOH L−1 | 1 | 2 | 1 | 2 | 1 | 2 | |
| Compound | tR, min | mg/100 g a | |||||
| Ergocalciferol | 3.59 | 18.89 (−0.14) | 10.98 (−0.50) | 22.06 | 8.38 (−0.62) | 1.74 (−0.92) | ND (−1.00) |
| δ-tocopherol | 5.26 | 9.65 (−0.89) | 88.22 (−0.02) | 90.06 | 67.32 (−0.25) | 0.24 (−1.00) | 88.17 (−0.02) |
| γ-tocopherol | 5.78 | 0.45 (−0.93) | 0.50 (−0.92) | 6.14 | 2.48 (−0.60) | 0.11 (−0.98) | 9.62 (0.57) |
| α-tocopherol | 6.61 | 180.96 (−0.32) | 102.35 (−0.62) | 267.77 | 90.77 (−0.66) | 74.00 (−0.72) | 70.63 (−0.74) |
| Astaxanthin | 9.07 | 18.69 (−0.26) | 16.82 (−0.33) | 25.14 | 17.12 (−0.32) | 7.64 (−0.70) | 14.16 (−0.44) |
| Lutein | 9.97 | 0.08 (0.00) | 0.07 (−0.13) | 0.08 | 0.12 (0.50) | 0.02 (−0.75) | 0.12 (0.50) |
| Zeaxanthin | 11.49 | 26.60 (−0.13) | 37.70 (0.23) | 30.73 | 47.44 (0.54) | 7.85 (−0.74) | 20.38 (−0.34) |
| β-cryptoxanthin | 16.58 | 24.66 (−0.22) | 12.13 (−0.61) | 31.50 | 27.02 (−0.14) | 5.54 (−0.82) | 16.93 (−0.46) |
| β-carotene | 22.51 | 18.89 (−0.14) | 10.98 (−0.50) | 22.06 | 8.38 (−0.62) | 1.74 (−0.92) | ND (−1.00) |
| p values | 0.044 | 0.040 | - | 0.037 | 0.011 | 0.028 | |
| Compound | tR, min | Concentration, µmol | Recovery, % a | |
|---|---|---|---|---|
| Theoretical/Added | Experimental/Obtained | |||
| Ergocalciferol | 3.59 | 5.00 × 101 | 4.00 × 101 | 81.21 (70–110) |
| α-tocopherol | 6.61 | 4.60 × 101 | 3.70 × 101 | 80.43 (70–110) |
| Phylloquinone | 8.07 | 3.50 × 101 | 4.20 × 101 | 117.02 (70–110) |
| Astaxanthin | 9.07 | 1.70 × 101 | 1.00 × 101 | 62.27 (60–120) |
| Lutein | 9.97 | 0.35 × 101 | 0.23 × 101 | 63.68 (60–120) |
| Zeaxanthin | 11.49 | 0.23 × 101 | 0.10 × 101 | 43.80 (60–120) |
| β-cryptoxanthin | 16.58 | 1.09 × 102 | 6.50 × 101 | 59.51 (70–110) |
| β-carotene | 22.51 | 1.23 × 101 | 1.33 × 101 | 108.63 (60–120) |
| Compound | Simmonds | Hass | Guatemala |
|---|---|---|---|
| Concentration, mg/100 g dry Weight Basis | |||
| Ergocalciferol | 1.84 ± 0.18 | 0.31 ± 0.16 | 0.53 ± 0.33 |
| Cholecalciferol | 119.50 ± 1.20 | 103.50 ± 15.50 | 108.50 ± 33.40 |
| δ-tocopherol | 42.48 ±7.96 | 8.31 ±1.63 | 6.16 ± 2.88 |
| γ-tocopherol | 4.81 ± 0.48 | 2.42 ± 0.37 | 0.92 ± 0.16 |
| α-tocopherol | 34.80 ± 14.50 | 8.16 ± 0.97 | 8.20 ± 3.67 |
| Astaxanthin | 2.23 ± 0.14 | 0.64 ± 0.23 | 0.98 ± 0.55 |
| Lutein | 6.41 ± 2.03 | 15.13 ± 8.66 | 10.79 ± 2.77 |
| Zeaxanthin | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| β-cryptoxanthin | 6.10 ± 1.39 | 3.37 ± 0.20 | 1.66 ± 0.85 |
| β-carotene | 3.43 ± 2.09 | 2.28 ± 1.56 | 1.71 ± 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Herrera, C.; Chacón, A.; Artavia, G.; Granados-Chinchilla, F. Simultaneous LC/MS Analysis of Carotenoids and Fat-Soluble Vitamins in Costa Rican Avocados (Persea americana Mill.). Molecules 2019, 24, 4517. https://doi.org/10.3390/molecules24244517
Cortés-Herrera C, Chacón A, Artavia G, Granados-Chinchilla F. Simultaneous LC/MS Analysis of Carotenoids and Fat-Soluble Vitamins in Costa Rican Avocados (Persea americana Mill.). Molecules. 2019; 24(24):4517. https://doi.org/10.3390/molecules24244517
Chicago/Turabian StyleCortés-Herrera, Carolina, Andrea Chacón, Graciela Artavia, and Fabio Granados-Chinchilla. 2019. "Simultaneous LC/MS Analysis of Carotenoids and Fat-Soluble Vitamins in Costa Rican Avocados (Persea americana Mill.)" Molecules 24, no. 24: 4517. https://doi.org/10.3390/molecules24244517
APA StyleCortés-Herrera, C., Chacón, A., Artavia, G., & Granados-Chinchilla, F. (2019). Simultaneous LC/MS Analysis of Carotenoids and Fat-Soluble Vitamins in Costa Rican Avocados (Persea americana Mill.). Molecules, 24(24), 4517. https://doi.org/10.3390/molecules24244517






