Abstract
A series of triazolo-thiadiazepines 4a–k were synthesized with excellent yields using dehydrated PTSA as a catalyst in toluene. Two triazolo-thiadiazines were obtained; 8a was formed directly by reflux in ethanol, whereas, PTSA promoted the formation of 8b. The molecular structure of the formed triazolo-thiadiazepines is identical to the imine-form 4a–k and not the enamine-tautomer 6a–k. The structures of the newly synthesized triazolo-thiadiazepines 4a–k and triazolo-thiadiazines 8a–b were elucidated using NMR (1H, and 13C), 2D NMR, HRMS, and X-ray single crystal. Furthermore, 4a was deduced using X-ray single crystal diffraction analysis. These new thiadiazepine hits represent an optimized series of previously synthesized indole-triazole derivatives for the inhibition of EGFR. The cytotoxicity activity against two cancer cell lines including human liver cancer (HEPG-2) and breast cancer (MCF-7) was promising, with IC50 between 12.9 to 44.6 µg/mL and 14.7 to 48.7 µg/mL for the tested cancer cell lines respectively, compared to doxorubicin (IC50 4.0 µg/mL). Docking studies revealed that the thiadiazepine scaffold presented a suitable anchor, allowing good interaction of the various binding groups with the enzyme binding regions and sub-pockets.
1. Introduction
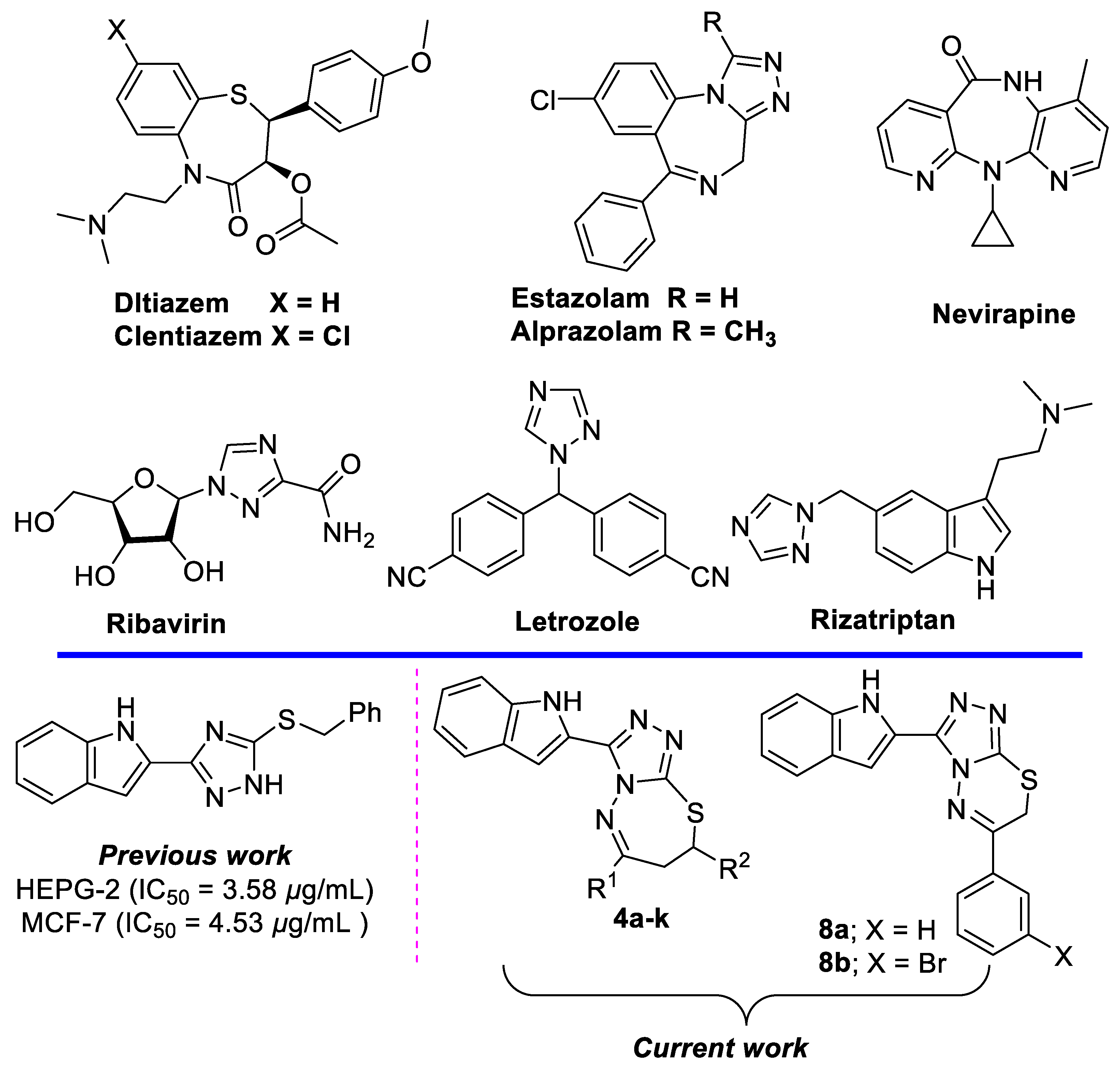
Indole and 1,2,4-triazoles heterocycles exhibit important biological activity and are present in many drugs [1,2]. The search for model drugs for different targets has led to the identification of ribavirin (an anti-viral drug), rizatriptan (used in the treatment of migraine headaches), and letrozole (for the treatment of hormonally-responsive breast cancer after surgery) [3,4,5].
Seven-membered heterocycles, like thiazepines, have occupied a unique place in pharmaceutical chemistry as they act as calcium channel blockers and are known to be applicable as cardiovascular drugs; these include diltiazem, clentiazem, and siratiazem [6,7,8,9]. Conversely, many examples of heterocycles have been reported with the benzodiazepine motif, such as estazolam, used for treatment of insomnia; alprazolam, commonly used for the treatment of panic disorder and anxiety disorders; flurazepam, which is anticonvulsant, anxiolytic and sedative, and enhanced the skeletal muscle relaxant responses; and nevirapine, used to treat HIV-1 infection and AIDS (Figure 1) [10,11].
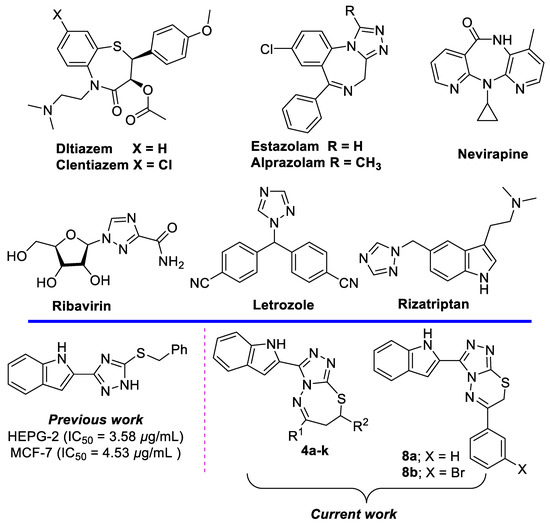
Figure 1.
Selected drugs containing benzothiazepine, benzodiazepine, 1,2,4-triazole and indole scaffolds, and our designed new compounds.
Additionally, thiadiazepines are expected to play a vital role in the biological system. Recently, researchers have increased the focus towards the synthesis and biological importance of thiadiazepines [12,13,14,15,16].
The synthesis of annulated heterocyclic systems, which incorporate biologically active nuclei, is expected to show interesting new pharmacological and biological activities which are highly challenging.
Recently, Boraei, A.T.A., et al., reported the cytotoxicity activity of a set of indole triazole derivatives, as potential EGFR inhibitors [17]. Therefore, we decided to combine the two previously discussed privileged structures as a potential scaffold to optimize the interaction of the indole-triazole series with the EGFR receptor.
Herein, PTSA was used as a catalyst for the synthesis of 11 indolo-triazolo-thiadiazepines and two indolo-triazolo-thiadiazenes, via reaction of 4-amino-1,2,4-triazole-3-thione with various chalcones and two phenacyl bromides. The newly introduced indolo-triazolo-thiadiazepine scaffolds, which possess better EGFR activity, selectivity, and greater potential, are promising for future preclinical and clinical development.
2. Results
2.1. Synthesis of 4a–k
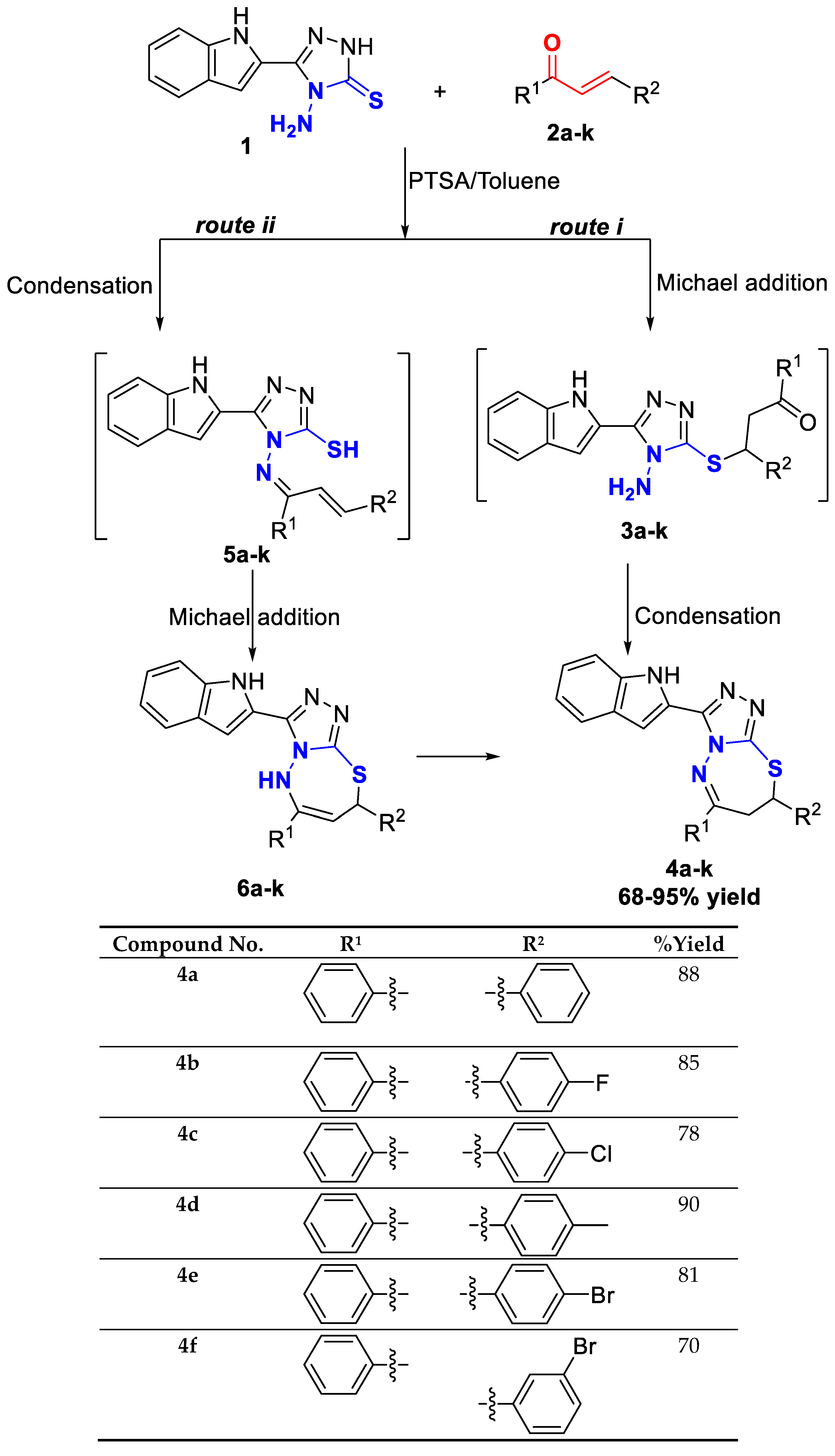
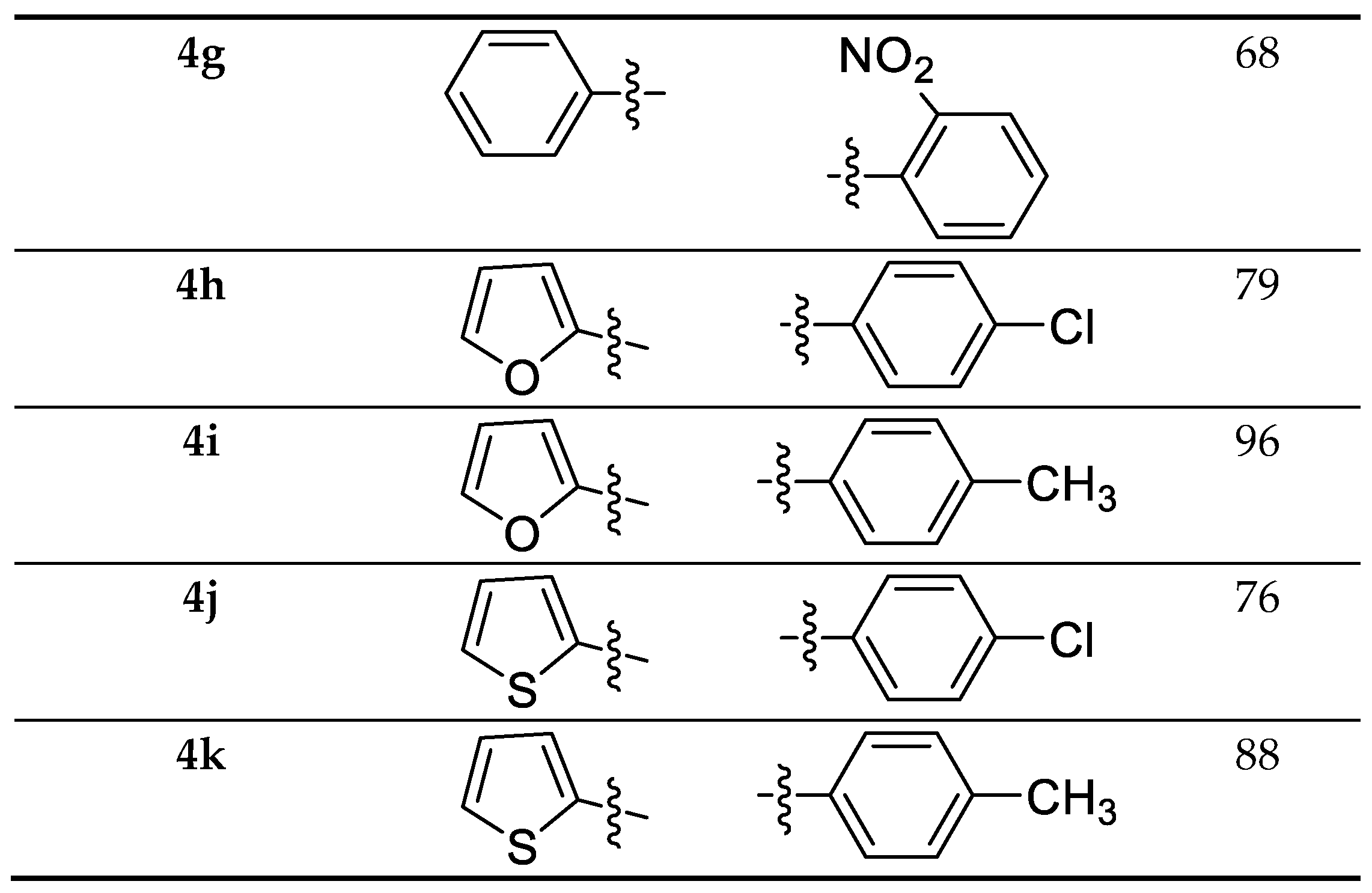
The required precursor chalcones were synthesized via the reaction of aromatic aldehydes and ketones in the presence of either aqueous KOH or NaOH in ethanol [18]. Chalcones 2a–k were reacted with 4-amino-1,2,4-triazole-3-thione having indole moiety 1, using p-toluenesulfonic acid (pTsOH or PTSA) as a reaction promoter, in dry toluene for 30 min under reflux, to yield the target compounds 4a–k with high yield (Scheme 1).
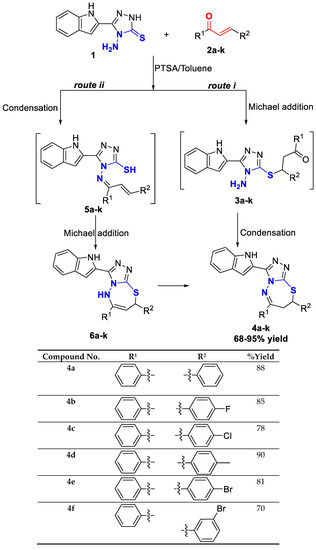
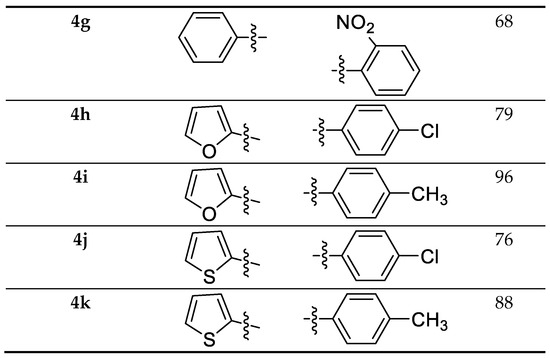
Scheme 1.
Synthesis and substrate scope of the target molecules 4a–k.
The mechanism of the reaction is expected to follow one of the following pathways: route (i), which involved Michael addition of the sulfhydryl group of the 4-amino-1,2,4-triazole-3-thione derivative 1 to the α,β-unsaturated carbonyl compounds 2a–k [18] to form the intermediate thia-Michael adducts 3a–k, followed by condensation and dehydration due to the intramolecular nucleophilic attack of the amino group on the carbonyl carbon, or route (ii), which involves, firstly, the removal of a water molecule due to the condensation of the amino and carbonyl groups to form the intermediate aza-dienes 5a–k, and then a subsequent intramolecular conjugate addition of the sulfhydryl group to produce triazolo-thiadiazepine [19].
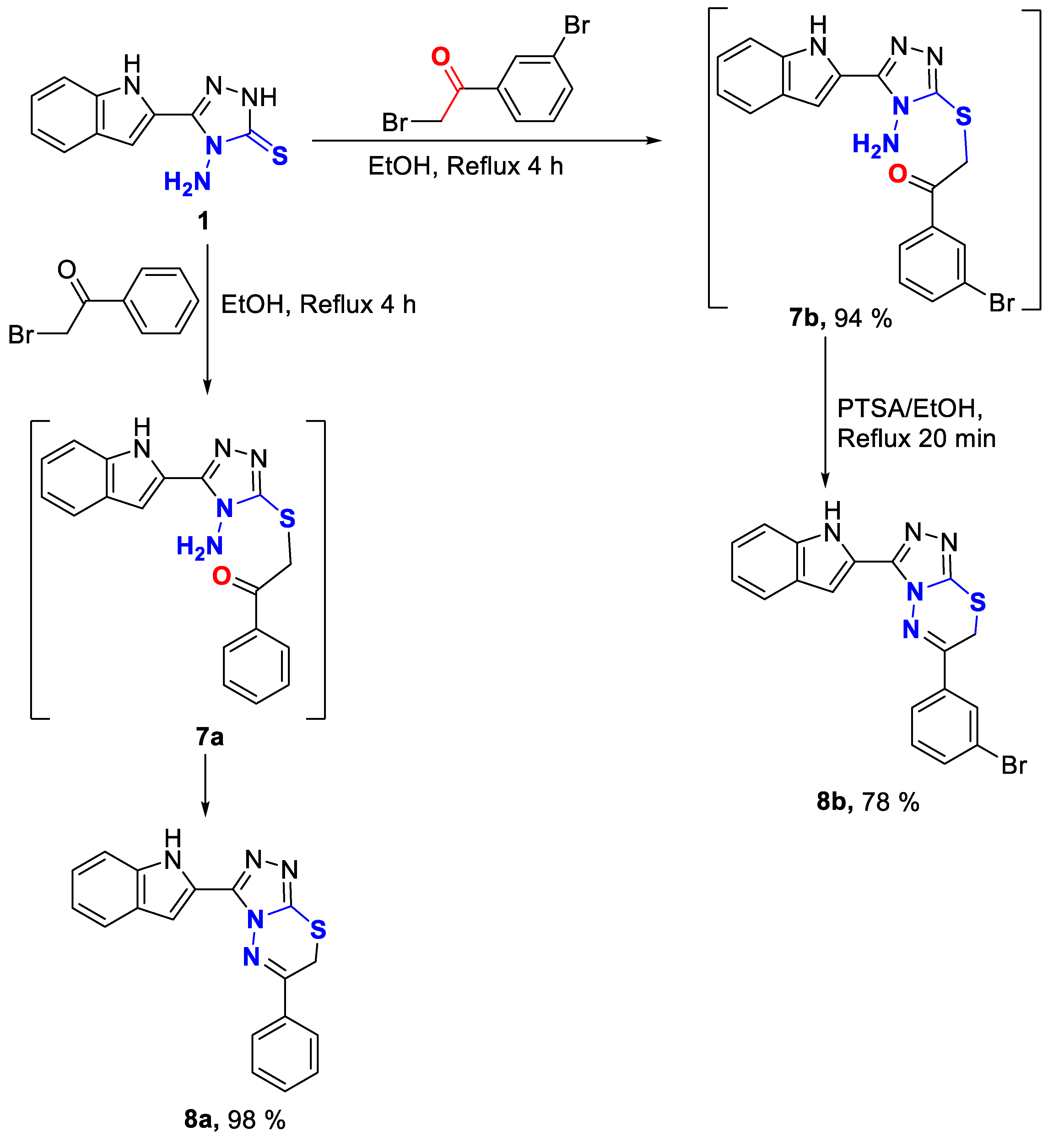
Triazolo-thiadiazines 8a, and 8b were obtained via the reaction of 4-amino-1,2,4-triazole-3-thione derivative 1 with phenacyl bromide and m-bromophenacyl bromide, respectively. We noticed that in the case of phemacyl bromide, 8a was obtained directly by reflux in ethanol for 4 h whereas, in the case of m-bromophenacyl bromide at the same conditions, the intermediate 7b was isolated which then cyclized by reflux in the presence of PTSA in ethanol for 20 min to afford product 8b. The formation of the intermediate 7b is suggested to be due to the deactivation caused by the electron withdrawing effect of bromine atom (Scheme 2).
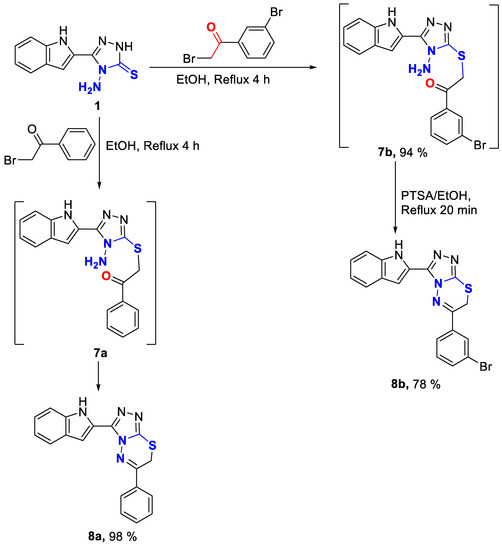
Scheme 2.
Synthesis of triazolo-thiadiazines 8a–b.
2.1.1. Structural Analysis
1H NMR of the triazole-thiadiazepines 4a–k showing that the three protons of the thiadiazepine ring are not equivalent and appeared mostly as doublet of doublets at different chemical shifts. For example, one of the methylene protons (described as trans proton) appeared as doublet of doublets around 3.45 ppm, with coupling constants 2J ≈ 13.5 Hz and 3J ≈ 13.2 Hz, whereas, the second methylene proton (described as cis proton) appeared around 3.74 ppm with 2J ≈ 13.5 Hz, and 3J ≈ 4.7 Hz; and the methine proton (CH) attached to the asymmetric carbon was identified between δ 5.33 and 5.60 ppm with a 3J around 13.0 and 4.7 Hz respectively. The remaining aromatic protons were observed between δ 7.00 and 8.30 ppm, while the indole NH was observed around δ 12.00 ppm. 13C NMR displayed the thiadiazepine methylene carbon (CH2) around δ 36.6 ppm and methine carbon (CH) around δ 53.5 ppm. The quaternary carbon resulted from the carbonyl condensation, appeared around δ 171.0 ppm, and the remaining aromatic carbons appeared between δ 104.0, and 148.0 ppm.
The NMR of 7b showed the methylene protons (S-CH2-) at δ 4.30 ppm, and the amino group protons at δ 6.31 ppm. The methylene carbon (S-CH2-) appeared at δ 39.2 ppm, whereas the carbonyl carbon was found at δ 192.8 ppm.
NMR of triazolo-thiadiazines 8a–b showed -SCH2- protons as singlets around δ 4.50 ppm, whereas the respective carbon was observed at δ 22.9 ppm. The thiadiazine quaternary carbon resulted from condensation appeared at δ 156.0 ppm in 8a and δ 154.8 ppm in 8b. The two triazole carbons in 8a–b were assigned around δ 142.0, and 146.5 ppm. The indole NH proton was identified around δ 12.00 ppm.
2.1.2. X-Ray Diffraction Analysis
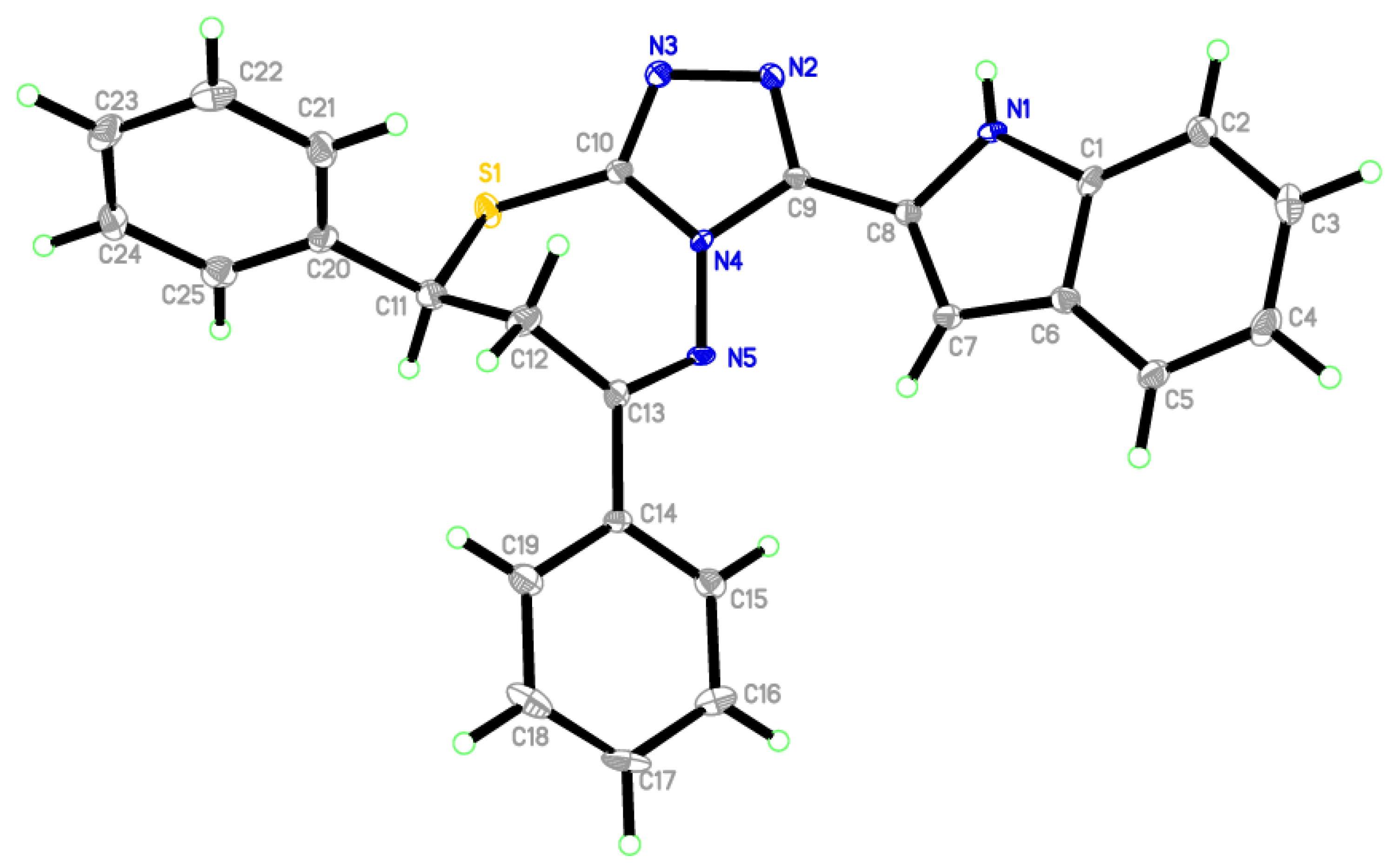
Compound 4a fortunately was obtained in a single crystal, which was solved using X-ray single crystal diffraction [20,21]. Table S1 lists the crystallographic data collection and structure refinement features. The later compound crystalized in a space group, a = 10.1159 (11) Å, b = 10.6507 (11) Å, c = 10.9680 (12) Å, α = 95.737 (3)°, β = 116.290 (3)°, γ = 100.618 (3)°, V = 1018.99 (19) Å3 and triclinic crystal system with P−1. Table S2 describes some bond angles and selected interatomic distances. The unit cell of the titled compound contained one molecule which appeared in the unit cell, showing that all bond angles and bond lengths of the benzene rings were in the ordinary framework. The indole ring (C1-C8/N1) formed a dihedral angle of 15.35° with the triazole ring (C9/N2/N3/C10C4)). The structural feature of 4a as depicted in Figure 2 shows an imine functionality, and the C13=N5 bond is [1.296 (5) (15) Å]. In the molecular packing (Figure S1), molecules were connected via strong classical intermolecular hydrogen bonds N1—H1N1···N2i (Table S3).
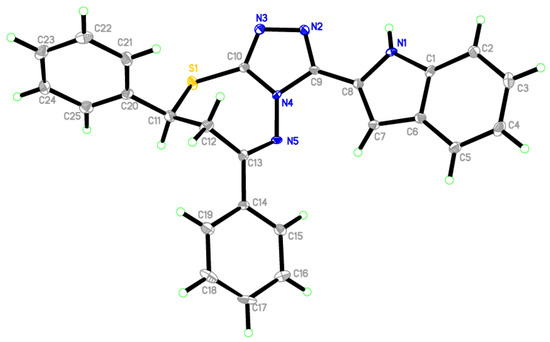
Figure 2.
ORTEP of 4a.
2.2. Anticancer Activity
The cytotoxic activity of the synthesized indolo-triazolo-thiadiazepines was tested in vitro against two cancer cell lines namely human liver cancer (HEPG-2), and breast cancer (MCF-7). The hits showed IC50 between 12.9 to 44.6 µg/mL and 14.7 to 48.7 µg/mL for the tested cancer cell lines respectively, compared to doxorubicin (IC50 4.0 µg/mL) (Table 1). The triazolo-thiadiazepine ring notably improved the binding properties and subsequent antiproliferative activity, as observed from the low activity of compounds 8a and 8b compared to that of compounds such as 4b and 4c, which possessed the triazolo-thiadiazepine nucleus. Compounds having para-substituted phenyl with an electron withdrawing group, such as 4c, 4e, and 4b were shown to have better activity than the ortho 4g or meta 4f or para 4d with electron donating analogues. Interestingly, compounds bearing a heterocyclic substituent showed slightly better activity against HEPG-2 than their phenyl counterparts (Compounds 4i, and 4j compared to compounds 4c, and 4d), an observation that was not noted against the MCF-7 cell line, which could be explained by the difference in compound uptake by the two cancer cell types. The chemaxon-calculated LogP was found to be approximately 4.65 for the heterocyclic derivatives, and 5.62 for the phenyl derivatives, with no big difference in the pKa compounds. The possible difference in LogP compounds could have impacted the penetration through the cancer cells and favored the HEPG-2 cells in the case of the heterocyclic derivatives.

Table 1.
IC50 results for indolyl-triazolothiadiazepine hybrids tested on HEPG2 and MCF-7 cancer cell lines.
2.3. Molecular Modeling
We have previously reported the design and synthesis of indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogues as potent anticancer agents [17]. Herein, we are presenting a lead optimization through ring variation, ring expansion, and functional group extension techniques for EGFR inhibition. Ring expansion and variation with a triazolo-thiadiazepine ring was performed to increase the flexibility of the molecule and allow better interaction of the important binding groups with the enzyme sub pockets. The triazolo-thiadiazepine ring allowed better positioning of the substituents in the hydrophobic sub pocket and the cleft region. The triazolo-thiadiazepine ring had the advantage of being a spider-like scaffold, which enables the exploration and optimization of the substituents around the scaffold for optimum enzyme binding. It also allows for pharmacokinetic optimization and preclinical development.
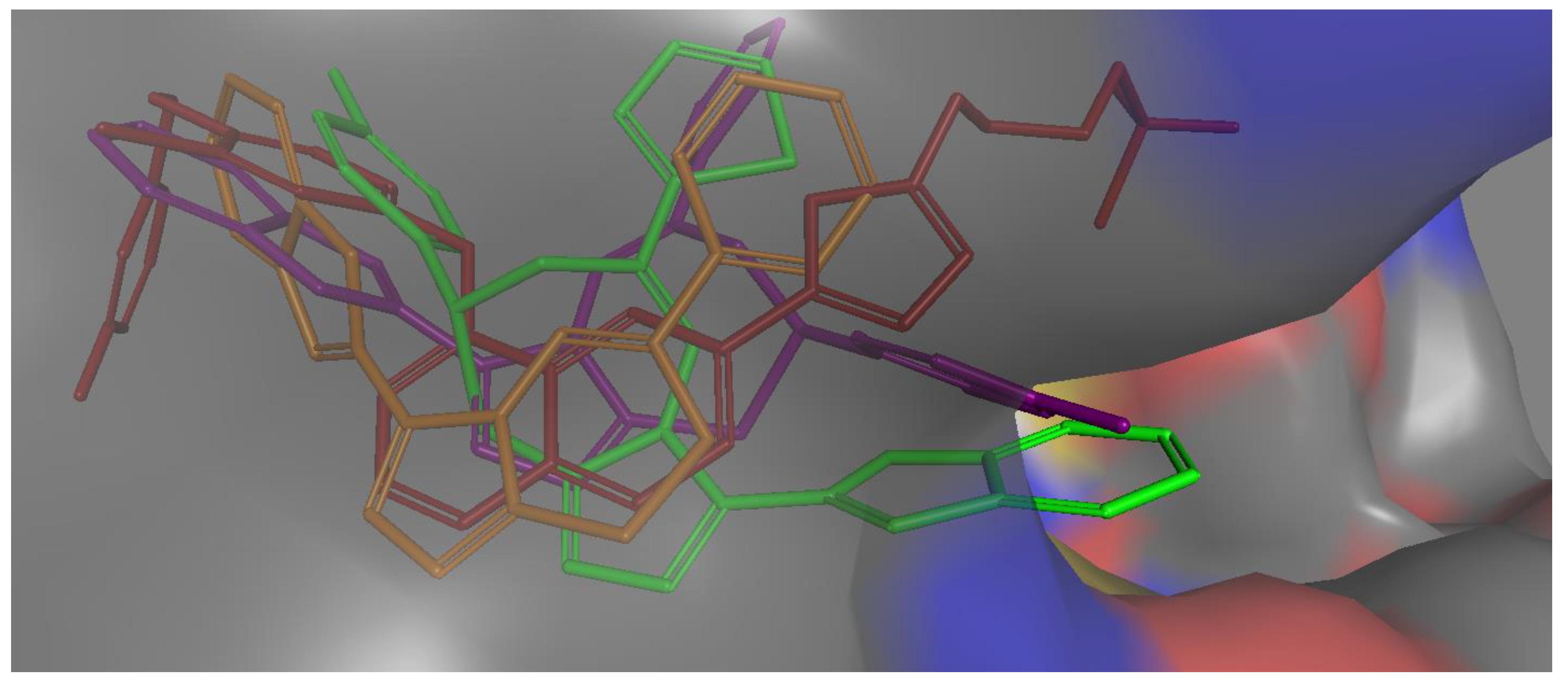
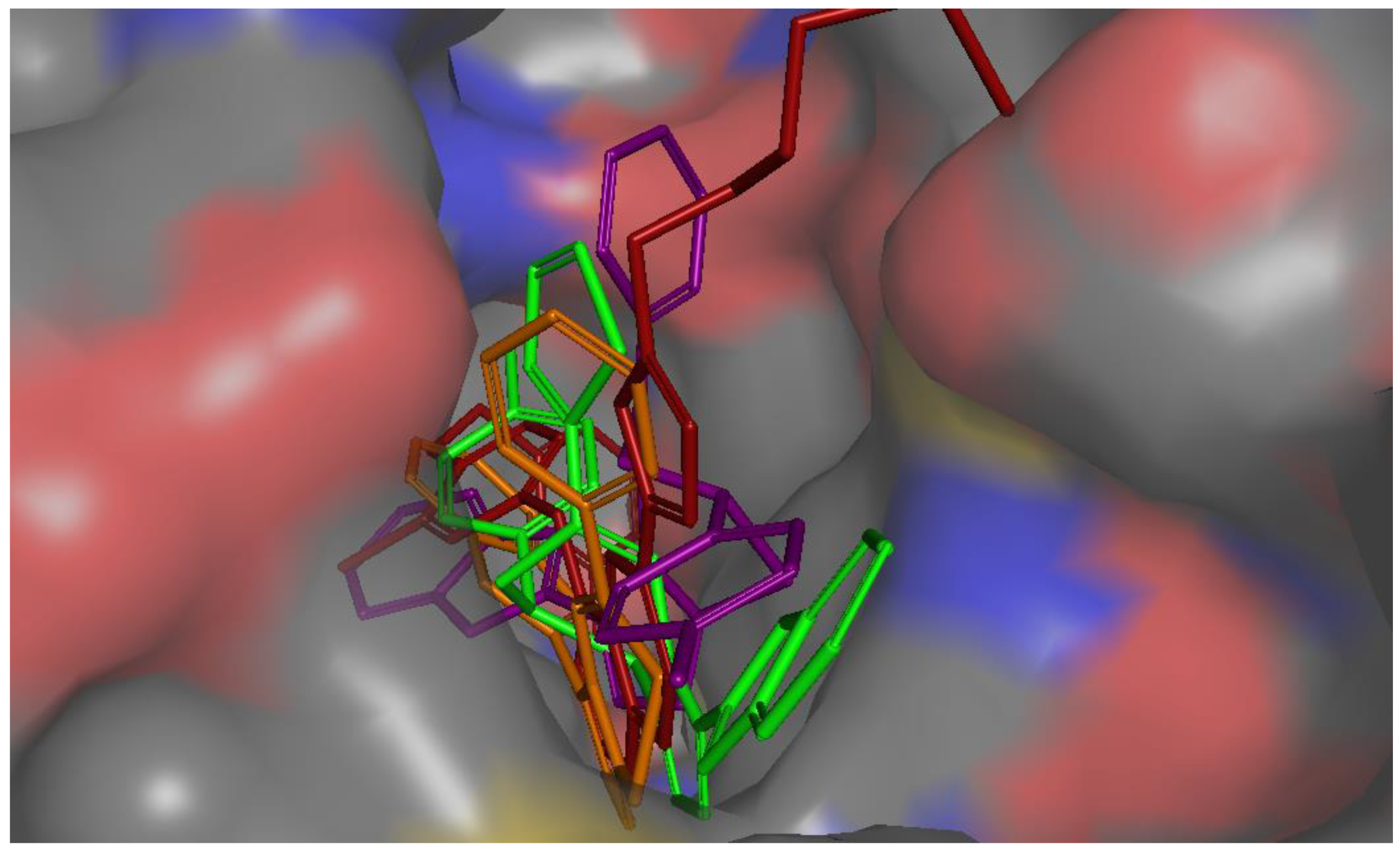
All active compounds showed poses that are comparable to the co-crystallized inhibitor and occupied the enzyme’s three main binding pockets (Figure 3 and Figure 4). The triazolo-thiadiazepine ring acts as an anchor that positions the compounds tightly in the active site through hydrogen bonding with key residue Met 793. The bicyclic, slightly flexible system also allowed for better interaction of the aryl and heteroaryl substituents, with the residues in the cleft and hydrophobic regions (Figure 3 and Figure 4). The para-halo substituted derivatives demonstrated better interaction through dipole–dipole bonds, while the meta-substituted, and ortho-substituted compounds were not able to form such an interaction. This partially explained the lower activity of the ortho-, and meta-substituted derivatives, as well as the para-methyl substituted derivatives. The binding scores were slightly better in the case of the phenyl derivatives than for the heterocyclic ones; however, this was not conclusive, and the difference in activity could not be explained based on the binding energy calculation.
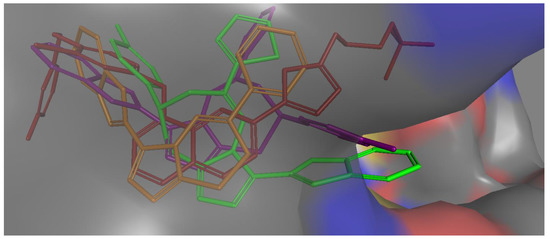
Figure 3.
Molecular surface representation of compound 4b purple, compound 4j green, compound 8a brown, and co-crystallized ligand (red) in EGFR active site (front view).
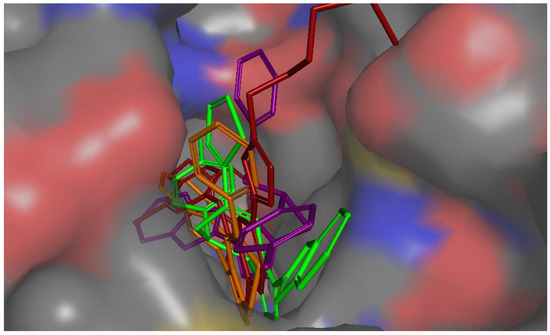
Figure 4.
Molecular surface representation of compound 4b purple, compound 4j green, compound 8a brown, and co-crystallized ligand (red) in EGFR active site (access channel view).
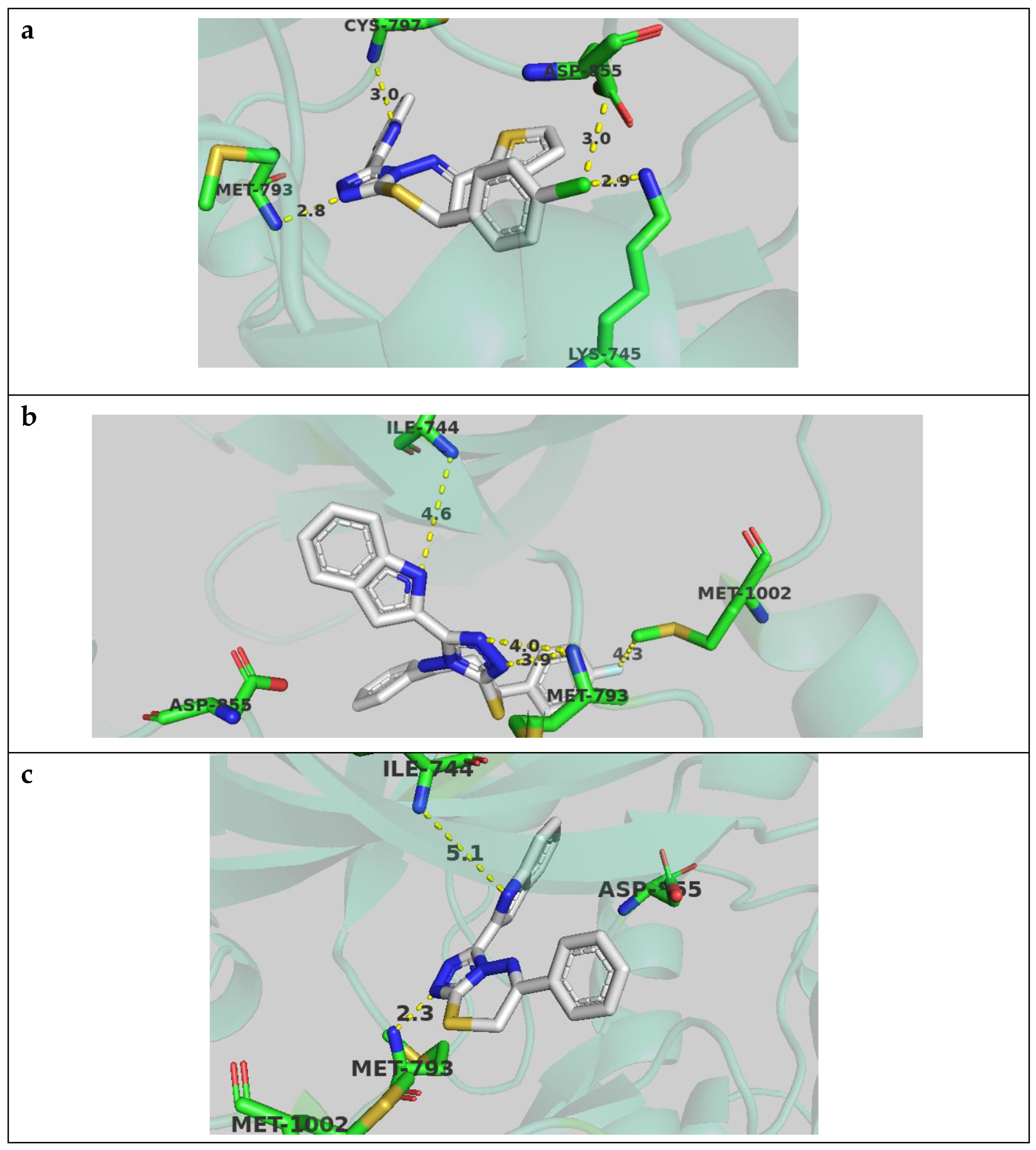
Compound 4j docked well in the active site and showed an important hydrogen bond with the key Met 793 residue, with the triazole anchor at a distance of 2.8 angstrom. The p-chlorophenyl ring occupied the hydrophobic pocket and formed a dipole–dipole interaction with Lys 745 side chain. The indole ring was positioned well in the cleft region and formed a hydrogen bond with Cys 797 (Figure 5a).
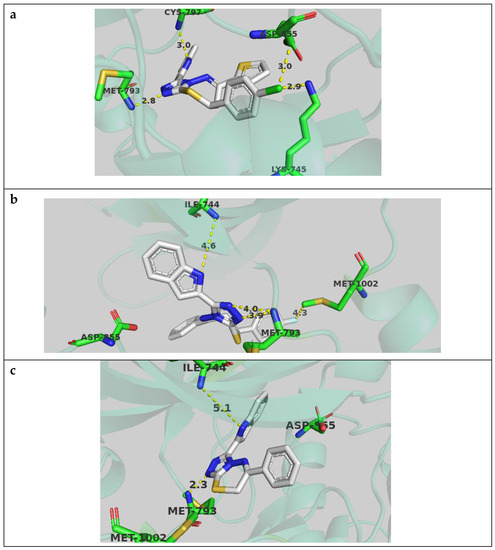
Figure 5.
Compound 4j (a), Compound 4b (b), and compound 8a (c) docked in EGFR active site and performing interactions with key residues. Distances are represented as yellow dotted lines and are in angstrom.
Compound 4b showed a similar pose at the active site but with the indole ring occupying the hydrophobic pocket, and exhibited potential hydrogen bonding with Ile 744 with the possible induced fit observed in biological systems. The p-fluorophenyl interacted with residues in the cleft region, such as Met 1002 (Figure 5b).
Compound 8a had only one phenyl substituent in the thiadiazepine ring and showed comparable poses to other compounds, but with less interaction. The indole ring occupied the hydrophobic region and the triazole ring coordinated to Met 793, but lacked the interaction with the cleft region (Figure 5c).
In conclusion, the triazolo-thiadiazepine ring represents a good scaffold for EGFR inhibition, yielded active compounds that occupied all enzyme sub pockets, and has good potential for further optimization and development.
3. Materials and Methods
3.1. Experiments: Chemistry
Melt-temp apparatus (SMP10) used for melting point (m.p.) determination in open capillaries and uncorrected. All reaction progress was monitored using TLC (eluent: n-hexane /ethyl acetate 4:6 for compounds 4a–k; and CH2Cl2/MeOH 9:1 for the compounds 7b, 8a and 8b) (TLC plates purchased from Merck, type: 60 F254/0.25 mm). The distillation technique was used for solvent purification if necessary. NMR (1H, 13C, and 2D) were recorded in DMSO-d6 using Brüker AC 300-600 spectrometers. Thermofinnigan MAT 95XP spectrometers used for molecular mass determination including HRMS spectral data determination.
3.2. Synthesis of Chalcones
Chalcones were synthesized by stirring the appropriate aldehydes and active hydrogen ketones in ethanol, with a few drops of aq. NaOH (50%). The target chalcones were precipitated and subsequently underwent filtration and recrystallization from EtOH to give pure compounds [18]. 4-Amino-1,2,4-triazole-3-thione derivative 1 was prepared according to the procedures [22].
3.3. Synthesis of Thiadiazepine 4a–k
The 4-amino-1,2,4-triazole-3-thione derivative 1 (1.0 mmol) and the suitable chalcones 2a–k (1.1 mmol) were added to a previously dehydrated PTSA (3.0 mmol) in toluene and heated under reflux for 30 min. The organic layer was washed with water and aqueous solution of NaHCO3, followed by filtration, drying, and recrystallization from DMF/EtOH.
(E)-7,8-Dihydro-3-(1H-indol-2-yl)-6,8-diphenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4a Yellow crystalline materials, m.p. 240–242 °C; 1H NMR (300 MHz) δ 3.45 (dd, 1 H, Jgem 13.7, Jtrans 13.0 Hz, CHHThiadiazepin), 3.66 (dd, 1 H, Jgem 13.7, Jcis 4.7 Hz, CHHThiadiazepin), 5.33 (dd, 1 H, J 12.6, J 4.7 Hz, CH7Thiadiazepin), 7.00–7.70 (m, 13 H, H-3Indol, H-4Indol, H-5Indol, H-6Indol, H-7Indol, 8 HPh), 8.23 (d, 2 H, J 7.1 Hz, 2 HPh), 12.07 (br. s, 1H, NHIndol); 13C NMR (125 MHz) δ 36.6, 53.5, 104, 112.0, 119.8, 121.1, 123.3, 123.6, 126.6 127.6, 128.0, 128.1, 128.6, 129.2, 132.5, 133.8, 136.9, 142.3, 143.3, 147.5, 171.7; HRMS (FAB +ve) calcd for C25H20N5S (M + 1): 422.1439. Found: 422.1391.
(E)-8-(4-Fluorophenyl)-3-(1H-indol-2-yl)-6-phenyl-7,8-dihydro-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4b. White solid product, m.p. 272–273 °C; 1H NMR (300 MHz) δ 3.47 (dd, 1 H, Jgem 13.7, Jtrans 13.1 Hz, CHHThiadiazepin), 3.67 (dd, 1 H, Jgem 13.7, Jcis 4.8 Hz, CHHThiadiazepin), 5.37 (dd, 1 H, J 12.5, J 4.8 Hz, CH7Thiadiazepin), 7.00–7.70 (m, 12 H, H-3Indol, H-4Indol, H-5Indol, H-6Indol, H-7Indol, 7 HPh), 8.24 (d, 2 H, J 7.2 Hz, 2 HPh), 12.07 (br. s, 1 H, NHIndol); 13C NMR (75 MHz) δ 36.5, 52.7, 104.0, 112.0, 115.3, 115.5, 119.8, 121.1, 123.3, 123.6, 127.6, 128.1, 128.7, 128.8, 129.2, 132.5, 133.7, 136.9, 138.7, 143.2, 147.5, 171.7; HRMS (FAB+) calcd for C25H19N5SF (M + 1): 440.1345. Found: 440.1350.
(E)-8-(4-Chlorophenyl)-7,8-Dihydro-3-(1H-indol-2-yl)-6-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4c. White solid product, m.p. 271–272 °C; 1H NMR (300 MHz) δ 3.46 (dd, 1 H, Jgem 13.7, Jtrans 13.2 Hz, CHHThiadiazepin), 3.66 (dd, 1 H, Jgem 13.7, Jcis 4.7 Hz, CHHThiadiazepin), 5.35 (dd, 1 H, J 12.6, J 4.7 Hz, CH7Thiadiazepin), 7.00–7.73 (m, 13 H, H-3Indol, H-4Indol, H-5Indol, H-6Indol, H-7Indol, 8 HPh), 8.24 (d, 2 H, J 7.1 Hz, 2 HPh), 12.07 (br. s, 1H, NHIndol); 13C NMR (75 MHz) δ 36.3, 52.7, 104.1, 112.0, 119.8, 121.1, 123.4, 123.6, 127.6, 128.1, 128.6, 129.3, 132.5, 132.6, 132.7, 136.9, 141.5, 143.1, 147.6, 171.7; HRMS (EI) calcd for C25H18N5SCl (M): 455.0971. Found: 455.0960.
(E)-8-(p-Tosyl)-7,8-dihydro-3-(1H-indol-2-yl)-6-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4d. Yellowish white needles, m.p. 263–264 °C; 1H NMR (400 MHz) δ 2.31 (s, 3 H, CH3), 3.44 (dd, 1 H, Jgem 13.6, Jtrans 12.8 Hz, CHHThiadiazepin), 3.64 (dd, 1 H, Jgem 13.6, Jcis 4.8 Hz, CHHThiadiazepin), 5.31 (dd, 1 H, J 12.6, J 5.2 Hz, CH7Thiadiazepin), 7.03–7.07 (m, 2 H, H-3Indol, H-5Indol), 7.19–7.23 (m, 3 H, H-6Indol, 2 HPh), 7.41 (d, 2 H, J 8.0 Hz, 2 HPh), 7.51 (d, 1 H, J6,7 8.0 Hz, H-7Indol), 7.64–7.74 (m, 4 H, H-4Indol, 3 HPh), 8.23 (d, 2 H, J 8.0 Hz, 2 HPh), 12.07 (br. s, 1 H, NHIndol); 13C NMR (100 MHz) δ 20.7, 36.7, 53.4, 104.0, 112.0, 119.8, 121.1, 123.3, 123.6, 126.5, 127.6, 128.0, 129.1, 129.3, 132.5, 133.8, 136.9, 137.5, 139.5, 143.4, 147.5, 171.7; HRMS (EI) calcd for C26H21N5S (M): 435.1518. Found: 435.1529.
(E)-8-(4-Bromophenyl)-7,8-dihydro-3-(1H-indol-2-yl)-6-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4e. Yellow crystalline materials, m.p. 271–272 °C; 1H NMR (300 MHz) δ 3.49 (dd, 1 H, Jgem 13.8, Jtrans 13.5 Hz, CHHThiadiazepin), 3.65 (dd, 1 H, Jgem 13.8, Jcis 4.5 Hz, CHHThiadiazepin), 5.35 (dd, 1 H, J 12.6, J 4.5 Hz, CH7Thiadiazepin), 7.00–7.04 (m, 2 H, H-3Indol, H-5Indol), 7.19 (dd, 1 H, J5,6 7.2, J6,7 8.4 Hz, H-6Indol), 7.46–7.49 (m, 3 H, H-7Indol, 2 HPh), 7.58–7.71 (m, 6 H, H-4Indol, 5 HPh), 8.24 (dd, 1 H, J 7.2 Hz, 2 HPh), 12.06 (br. s, 1H, NHIndol); 13C NMR (100 MHz) δ 36.2, 52.8, 104.1, 112.0, 119.8, 121.1, 121.2, 123.4, 123.7, 127.6, 12.1, 129.9, 129.3, 131.5, 132.6, 133.7, 136.9, 141.9, 143.2, 147.6, 171.7; CHN-microanalysis calcd for C25H18N5BrS: 60.00% C, 3.63% H, 14.00% N, 6.41% S. Found: 59.29% C, 4.13% H, 14.80% N, 6.69% S.
(E)-8-(3-Bromophenyl)-7,8-dihydro-3-(1H-indol-2-yl)-6-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4f. Yellow crystalline materials, m.p. 263–265 °C; 1H NMR (300 MHz) δ 3.54 (dd, 1 H, Jgem 13.8, Jtrans 13.5 Hz, CHHThiadiazepin), 3.69 (dd, 1 H, Jgem 13.8, Jcis 4.8 Hz, CHHThiadiazepin), 5.32 (dd, 1 H, J 12.6, J 4.8 Hz, CH7Thiadiazepin), 7.00–7.05 (m, 2 H, H-3Indol, H-5Indol), 7.19 (dd, 1 H, J5,6 7.2, J6,7 8.4 Hz, H-6Indol), 7.36 (dd, 1 H, J ≈ 7.8 Hz, HPh), 7.49 (d, 1 H, J6,7 8.4 Hz, H-7Indol), 7.53 (d, 2 H, J 7.8 Hz, 2 HPh), 7.62–7.76 (m, 5 H, H-4Indol, 4 HPh), 8.26 (d, 2 H, J 7.2 Hz, 2 HPh), 12.07 (br. s, 1H, NHIndol); 13C NMR (100 MHz) δ 35.9, 52.7, 104.0, 112.0, 119.8, 121.1, 121.8, 123.3, 123.6, 125.8, 127.6, 128.1, 129.3, 129.5, 130.8, 130.9, 132.6, 133.7, 136.9, 143.0, 145.0, 147.6, 171.6; CHN-microanalysis calcd for C25H18N5BrS: 60.00% C, 3.63% H, 14.00% N, 6.41% S. Found: 59.93% C, 3.87% H, 14.02% N, 6.25% S.
(E)-7,8-Dihydro-3-(1H-indol-2-yl)-8-(2-nitrophenyl)-6-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4g. Yellow crystalline materials, m.p. 270–272 °C; 1H NMR (300 MHz) δ 3.61 (dd, 1 H, Jgem 13.8, Jtrans 13.5 Hz, CHHThiadiazepin), 3.76 (dd, 1 H, Jgem 13.8, Jcis 4.5 Hz, CHHThiadiazepin), 5.60 (dd, 1 H, J 12.9, J 4.7 Hz, CH7Thiadiazepin), 7.01–7.07 (m, 2 H, H-3Indol, H-5Indol), 7.20 (dd, 1 H, J5,6 7.2, J6,7 8.1 Hz, H-6Indol), 7.49 (d, 1 H, J6,7 8.1 Hz, H-7Indol), 7.59–7.73 (m, 5 H, H-4Indol, 4 HPh), 7.83 (dd, 1 H, J 7.8, J 7.5 Hz, HPh), 7.99 (d, 1 H, J 7.8 Hz, HPh), 8.06 (d, 1 H, J 8.1 Hz, HPh), 8.32 (d, 2 H, J 7.2 Hz, 2 HPh), 12.09 (br. s, 1H, NHIndol); 13C NMR (100 MHz) δ 35.3, 48.7, 104.1, 112.0, 119.8, 121.8, 123.4, 123.6, 124.7, 127.6, 128.1, 128.5, 129.3, 132.8, 133.6, 134.4, 136.9, 137.3, 143.7, 146.7, 147.6, 171.4; HRMS (EI) calcd for C25H18N5O2S (M+): 466.1212. Found: 466.1233.
(E)-8-(4-Chlorophenyl)-6-(furan-2-yl)-7,8-Dihydro-3-(1H-indol-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4h. Yellowish white solid product, m.p. 275–276 °C; 1H NMR (400 MHz) δ 3.38–3.44 (m, 1 H, CHHThiadiazepin), 3.51 (dd, 1 H, Jgem 14.0, Jcis 5.2 Hz, CHHThiadiazepin), 5.35 (dd, 1 H, J 12.0, J 5.2 Hz, H-7Thiadiazepin), 6.85–6.86 (m, 1 H, H-4Furyl), 7.033 (dd, 1 H, J4,5 8.0, J5,6 7.6 Hz, H-5Indol), 7.08 (s, 1 H, H-3Indol), 7.19 (dd, 1 H, J5,6 7.6, J6,7 ≈ 8.0 Hz, H-6Indol), 7.44–7.53 (m, 5 H, H-7Indol, 4 HPh), 7.62 (d, 1 H, J4,5 8.0 Hz, H-4Indol), 7.71 (d, 1 H, J 3.6 Hz, H-3Furyl), 8.16 (s, H, H-5Furyl), 12.01 (br. s, 1H, NHIndol); 13C NMR (100 MHz) δ 35.9, 52.8, 104.1, 112.0, 113.3, 119.5, 119.9, 121.1, 123.4, 123.6, 127.6, 128.6, 128.7, 132.7, 136.9, 141.2, 143.2, 147.5, 148.3, 148.7, 161.3; HRMS (EI) calcd for C23H16N5OSCl (M+): 445.0764. Found: 445.0764.
(E)-6-(Furan-2-yl)-8-7,8-dihydro-3-(1H-indol-2-yl)-8-p-tolyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4i. Brown solid product, m.p. 256–257 °C; 1H NMR (400 MHz) δ 2.29 (s, 3 H, CH3), 3.33–3.50 (m, 2 H, H-6Thiadiazepin, H-6′Thiadiazepin,), 5.20 (dd, 1 H, J 12.0, J 5.2 Hz, H-7Thiadiazepin), 6.84-6.85 (m, 1 H, H-4Furyl), 7.03 (dd, 1 H, J4,5 8.0, J5,6 7.6 Hz, H-5Indol), 7.09 (s, 1 H, H-3Indol), 7.16–7.21 (m, 3 H, H-6Indol, 2 HPh), 7.35 (d, 2 H, J 8.0 Hz, 2 HPh), 7.49 (d, 1 H, J6,7 8.4 Hz, H-7Indol), 7.62 (d, 1 H, J4,5 8.0 Hz, H-4Indol), 7.66 (d, 1 H, J 3.2 Hz, H-3Furyl), 8.15 (s, 1 H, H-5Furyl), 12.01 (br. s, 1H, NHIndol); 13C NMR (100 MHz) δ 20.7, 36.3, 53.5, 104.1, 112.0, 113.3, 119.2, 119.9, 121.1, 123.4, 123.6, 126.6, 127.6, 129.2, 136.9, 137.6, 139.3, 143.5, 147.5, 148.4, 148.6, 161.4; HRMS (EI) calcd for C24H19N5OS (M+): 425.1310. Found: 425.1327.
(E)-8-(4-Chlorophenyl)-7,8-Dihydro-3-(1H-indol-2-yl)-6-(thiophen-2-yl)- [1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine 4j. Yellow powder product, m.p. 280–281 °C; 1H NMR (300 MHz) δ 3.48 (dd, 1 H, Jgem 13.8, Jtrans 12.6 Hz, CHHThiadiazepin), 3.70 (dd, 1 H, Jgem 13.5, Jcis 5.1 Hz, CHHThiadiazepin), 5.35 (dd, 1 H, J 12.0, J 4.8 Hz, H-7Thiadiazepin), 7.01-7.55 (m, 9 H, 1 H-4Thiophen, H-3Indol, H-5Indol, H-6Indol, H-7Indol, 4 HPh), 7.61 (d, 1 H, J4,5 7.8 Hz, H-4Indol), 8.04 (d, 1 H, J 4.8 Hz, H-3Thiophen), 8.10 (d, 1 H, J 3.3 Hz, H-5Thiophen), 12.04 (br. s, 1H, NHIndol); HRMS (EI) calcd for C23H16N5S2Cl (M+): 461.0536. Found: 461.0559.
(E)-8-7,8-Dihydro-3-(1H-indol-2-yl)-6-(thiophen-2-yl)-8-p-tolyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine4k. White solid, m.p. 277–278 °C; 1H NMR (400 MHz) δ 2.13 (s, 3 H, CH3), 3.44 (dd, 1 H, Jgem 13.2, Jtrans 12.8 Hz, 6Thiadiazepin,), 3.65 (dd, 1 H, Jgem 13.8, Jcis 4.8 Hz, 6′Thiadiazepin,)), 5.20 (dd, 1 H, J 12.0, J 4.8 Hz, H-7Thiadiazepin), 7.01-7.33 (m, 6 H, H-3Indol, H-5Indol, H-6Indol, 2 HPh, HThiophen), 7.38 (d, 2 H, J 8.0 Hz, 2 HPh), 7.48 (d, 1 H, J6,7 8.4 Hz, H-7Indol), 7.61 (d, 1 H, J4,5 7.6 Hz, H-4Indol), 8.02-8.06 (m, 2 H, H-3Thiophen, H-5Thiophen), 12.04 (br. s, 1H, NHIndol); 13C NMR (100 MHz) δ 20.7, 36.9, 53.4, 104.0, 112.0, 119.9, 121.1, 123.4, 123.7, 126.7, 128.9, 129.1, 134.1, 134.1, 136.9, 137.6, 138.5, 139.3, 143.6, 147.3, 166.8; HRMS (EI) calcd for C24H19N5S2 (M+): 441.1082. Found: 441.1078.
3.4. Procedure for 8a,b
A mixture of 4-amino-1,2,4-triazole-3-thione derivative 1 (1.0 mmol) and the relevant phenacyl bromide (1.1 mmol) was refluxed for 4 h in 10 mL ethanol and left to cool to room temperature. The formed ppt was collected by filtration, dried and recrystallized from DMF/EtOH to give 8a, and 7b respectively.
3.5. Cyclization of 7b
A mixture of 7b (1.0 mmol) and dehydrated PTSA (1.0 mmol) was refluxed in ethanol for 20 min and then left to cool, then the ppt was filtered, washed with water and aqueous sodium hydrogen carbonate, dried, and recrystallized from DMF/EtOH to give 8b.
4-Amino-3-(3-bromoacetophenonylsulfanyl)-5-(1H-indol-2-yl)-1,2,4-triazole7b. Yellow crystals, m.p. 267–268 °C; 1H NMR (300 MHz) δ δ 4.93 (SCH2), 6.31 (s, 2 H, NH2), 7.03 (dd, 1 H, J4,5 8.1, J5,6 7.2 Hz, H-5Indol), 7.16 (dd, 1H, J5,6 7.2, J6,7 8.1 Hz, H-6Indol), 7.29 (s, 1 H, H-3Indol), 7.45 (d, 1 H, J6,7 8.1 Hz, H-7Indol), 7.54 (dd, 1 H, J 7.8 Hz, HPh), 7.61 (d, 1H, J4,5 7.8 Hz, H-4Indol), 7.87–7.90 (m, 1 H, HPh), 8.04 (d, 1 H, J 7.8 Hz, HPh), 8.19 (s, 1 H, HPh), 11.70 (br. s, 1 H, NHIndol); 13C NMR (75 MHz) δ 39.2, 102.4, 111.9, 119.6, 120.8, 122.2, 122.9, 123.9, 127.4, 127.6, 131.0, 131.0, 136.2, 136.5, 137.6, 149.4, 152.6, 192.8; LRMS (EI) m/z (Int. %): 63.1 (12.4), 82.9 (33.5), 84.9 (26.8), 142.0 (100), 143.1 (21.0), 182.9 (23.5), 185.0 (14.8), 227.1 (19.1), 228.1 (25.6), 244.1 (14.28), 409.0 (70), 410.0 (21.7), 411.0 (71), 412.0 (18.9), 427.1 (4), 429.1 (4.63); HRMS (EI) calcd for C18H12N5BrS (M-H2O): 408.9984. Found: 408.9997.
7H-3-(1H-Indol-2-yl)-6-phenyl-1,2,4-triazolo[3,4-b][1,3,4-thiadiazine8a. Brownish yellow crystalline materials, m.p. 269–271 °C; 1H NMR (400 MHz) δ 4.49 (s, 2 H, CH2Thiadiazin), 7.05 (dd, 1 H, J4,5 7.6, J5,6 7.3 Hz, H-5Indol), 7.20 (dd, 1H, J5,6 7.3, J6,7 8.1 Hz, H-6Indol), 7.25 (d, 1 H, J 1.1 Hz, H-3Indol), 7.48 (d, 1 H, J6,7 8.1 Hz, H-7Indol), 7.61–7.69 (m, 4 H, H-4Indol, 3 HPh), 8.10–8.13 (m, 2 H, 2 HPh), 11.97 (br. s, 1 H, NHIndol); 13C NMR (100 MHz) δ 22.9, 103.8, 111.9, 119.7, 121.2, 123.3123.3, 127.6, 127.7, 129.2, 132.0, 133.5, 136.9, 142.1, 146.6, 156.0; HRMS (EI) calcd for C18H13N5S (M+): 331.0892. Found: 331.0888.
6-(3-Bromophenyl)-3-(1H-indol-2-yl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine8b. Yellow crystals, m.p. 252–253 °C; 1H NMR (600 MHz) δ 4.48 (s, 2 H, CH2Thiadiazin), 7.05 (dd, 1 H, J4,5 7.8, J5,6 7.2 Hz, H-5Indol), 7.19-7.21 (m, 2 H, H-3Indol, H-6Indol), 7.48 (d, 1 H, J6,7 7.8 Hz, H-7Indol), 7.60 (dd, 1 H, J 7.8, J 8.4 Hz, HPh), 7.67 (d, 1 H, J4,5 7.8 Hz, H-4Indol), 7.86 (d, 1 H, J 8.4 Hz, HPh), 8.13 (d, 1 H, J 7.8 Hz, HPh), 8.26 (s, 1 H, HPh), 11.99 (s, 1 H, NHIndol); 13C NMR (DMSO-d6, 150 MHz) δ 23.0, 103.9, 112.0, 119.8, 121.2, 122.4, 123.1, 123.4, 126.6, 127.6, 130.3, 131.4, 134.6, 135.8, 136.9, 142.0, 146.7, 154.8; HRMS (ESI+) calcd for C18H13BrN5S (M + 1): 410.00750. Found: 410.0069.
4. Conclusions
PTSA were found to be a useful catalyst for the synthesis of indolo-triazolo-thiadiazepine-targeted molecules in high yields. The obtained compounds were fully characterized, and the X-ray crystal structure for 4a was resolved. The triazolo-thiadiazepine scaffold represents a very promising scaffold, being spider-like, and is suitable for the parallel synthesis and subsequent development of highly selective EGFR inhibitors, as well as for selective multi-targeting to improve efficacy and reduce toxicity. We successfully prepared compounds demonstrating good antiproliferative activity, compared to that of the standard drug doxorubicin, including compounds 4b, and 4j. The molecular modeling studies demonstrated that the scaffold acts as an anchor binding the key residue Met793 and allowing the substituents to interact with the substrate access channel and the hydrophobic sub pocket. This work represents a good starting point to utilize triazolo-thiadiazepine as a promising scaffold for the optimization and preclinical development of EGFR inhibitors.
Supplementary Materials
NMR spectrum, X-ray crystallographic analysis, cytotoxicity assay, and molecular docking study associated with this article can be found in the online version.
Author Contributions
Conceptualization, A.T.A.B.; Data curation, M.S.G.; Formal analysis, H.A.G. and A.B.; Funding acquisition, A.B.; Methodology, A.T.A.B.; Software, H.A.G.; Supervision, A.T.A.B. and E.S.H.E.A.; Validation, M.S.G.; Visualization, E.S.H.E.A.; Writing—original draft, A.T.A.B.; A.B.; Writing—review & editing, A.B.
Funding
The authors would like to extend their sincere appreciation to Researchers Supporting Project Number (RSP-2019/64), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- El Ashry, E.S.H.; El Tamany, E.S.H.; Abdel Fattah, M.E.; Boraei, A.T.A.; Abd El-Nabi, H.M. Regioselective synthesis, characterization and antimicrobial evaluation of S-glycosides and S, N-diglycosides of 1,2-dihydro-5-(1H-indol-2-yl)-1,2,4-triazole-3-thione. Eur. J. Med. Chem. 2013, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Salgin, G.U.; Gokhamaa, K.N.; Gostal, O.; Koysal, Y.; Kilici, E.; Isik, S.; Aktay, G.; Ozalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Maddila, S.; Pagadala, R.; Jonnalagadda, S.B. 1,2,4-Triazoles: A review of synthetic approaches and the biological activity. Lett. Org. Chem. 2013, 10, 693–714. [Google Scholar] [CrossRef]
- Boraei, A.A.; Ashour, H.K.; El Tamany, E.S.H.; Abdelmoaty, N.; El-Falouji, A.I.; Gomaa, M.S. Design and synthesis of new phthalazine-based derivatives as potential EGFR inhibitors for the treatment of hepatocellular carcinoma. Bioorg. Chem. 2019, 85, 293–307. [Google Scholar] [CrossRef]
- Bagal, S.K.; Brown, A.D.; Cox, P.J.; Omoto, K.; Owen, R.M.; Pryde, D.C.; Sidders, B.; Skerratt, S.E.; Stevens, E.B.; Storer, R.I.; et al. Ion channels as therapeutic targets: A drug discovery perspective. J. Med. Chem. 2012, 56, 593–624. [Google Scholar] [CrossRef]
- Yamamoto, H.; Asai, H. Effects of (−)-cis-2,3-dihydro-3-(4-methylpiperazinylmethyl)-2-phenyl-1,5-benzothiazepin-4-(5H)-one hydrochloride (BTM-1086) on ulceration, gastric secretion and mucosal blood flow in experimental animals. Chem. Pharm. Bull. 1986, 34, 3844–3853. [Google Scholar] [CrossRef]
- Saha, D.; Jain, G.; Sharma, A. Benzothiazepines: Chemistry of a privileged scaffold. RSC Adv. 2015, 5, 70619–70639. [Google Scholar] [CrossRef]
- Ye, N.; Neumeyer, J.L.; Baldessarini, R.J.; Zhen, X.; Zhang, A. Recent progress in development of dopamine receptor subtype-selective agents: Potential therapeutics for neurological and psychiatric disorders. Chem. Rev. 2013, 113, 123–178. [Google Scholar] [CrossRef]
- Sekhar, B.C. Fused 1, 5-benzothiazepines from o-aminothiophenol and its derivatives as versatile synthons. Acta Chim. Slov. 2014, 61, 651–680. [Google Scholar]
- Danneberg, P.; Weber, K.H. Chemical structure and biological activity of the diazepines. Br. J. Clin. Pharmacol. 1983, 16, 231S–244S. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; El Sayed, O.A.; El-Shamy, H.A. Synthesis and antimicrobial evaluation of novel oxa (thia) diazolylquinolines and oxa (thia) diazepino [7, 6-b]quinolones. Arch. Pharm. 1993, 326, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Ammar, Y.A.; Ghorab, M.M.; El-Sharief, A.M.S.; Mohamed, S.I. Naproxen in heterocyclic chemistry: Novel syntheses of triazoles, triazolothiadiazines, triazolothiadiazoles, and triazolothiadiazepine bearing an asymmetric carbon atom and radiostability of the biologically active compounds. Heteroat. Chem. 2002, 13, 199–206. [Google Scholar] [CrossRef]
- Kiefer, L.; Gorojankina, T.; Dauban, P.; Faure, H.; Ruat, M.; Dodd, R.H. Design and synthesis of cyclic sulfonamides and sulfamates as new calcium sensing receptor agonists. Bioorg. Med. Chem. Lett. 2010, 20, 7483–7487. [Google Scholar] [CrossRef]
- Zeng, Y.; Zeng, H.; Zhang, H.; Geng, L.; Zhao, X.; Cheng, J. Synthesis of novel β-lactam-fused 1,5-benzothiazepine derivatives bearing quinoline moiety. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1–7. [Google Scholar] [CrossRef]
- Van Snick, W.; Aibuldinov, Y.K.; Dehaen, W. An efficient synthetic route towards novel thienobenzothiazoles, thienobenzothiazepines, and thienobenzothiazines. Tetrahedron 2013, 69, 4176–4184. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Gomaa, M.S.; El Sayed, E.S.H.; Duerkop, A. Design, selective alkylation and X-ray crystal structure determination of dihydro-indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogue as potent anticancer agents. Eur. J. Med. Chem. 2017, 125, 360–371. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Al Majid, A.M.; Al-Othman, Z.A. Highly enantioselective Friedel–Crafts alkylation of indoles with α, β-unsaturated ketones with simple Cu(II)–oxazoline–imidazoline catalysts. Tetrahedron 2013, 69, 5185–5192. [Google Scholar] [CrossRef]
- Sunil, D.; Isloor, A.M.; Shetty, P.; Pai, K.S.R.; Balaji, S. Triazolo-thiadiazepine as a potent apoptotic DNA degradation agent and MetAP2 inhibitor against human breast adenocarcinoma cells. Chem. Biol. Interface 2015, 5, 63–75. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.A. A new direct synthetic access to 4-Amino-2-N-(glycosyl/propyl)-1,2,4-triazole-3-thiones via hydrazinolysis of 3-N-((acylated gylcosyl)/allyl)-1,3,4-oxadiazole-2-thiones. Arkivoc 2016, 3, 71–81. [Google Scholar]
Sample Availability: Samples of the compounds 4a–k and 8a,b are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).