Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Stability of Isomorphous Substituted SOD
2.1.1. Distribution of Al Atoms in SOD
2.1.2. Structural Stability of Al-Substituted SOD
2.2. Distribution of Zr and Structural Stability of Zr-Substituted SOD
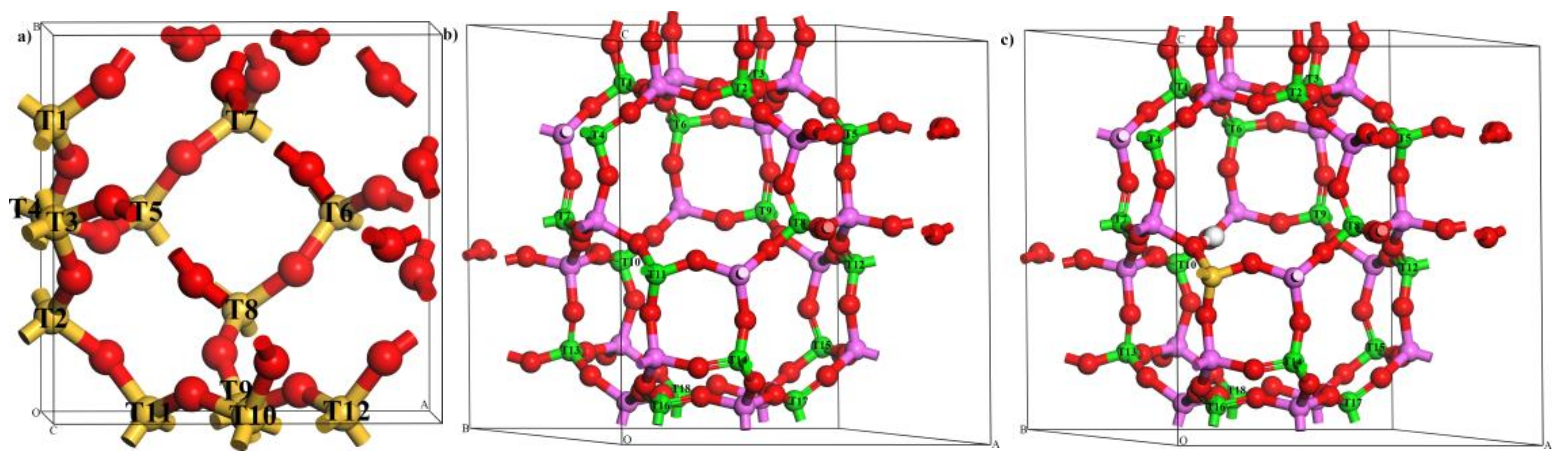
2.2.1. Stable Location of Zr atoms in SOD Framework of Si/Al Equals Eleven
2.2.2. Structural Stability of Zr-Substituted SOD
2.3. Distribution of Zr and Structural Stability of Zr-Substituted AlPO-34 and SAPO-34
2.3.1. Stable Location of Zr Atoms in AlPO-34 Framework
2.3.2. Stable Location of Zr Atoms in SAPO-34 Framework
2.3.3. Structural Stability of Zr-Substituted AlPO-34 and SAPO-34
2.4. Structural Distortion of the Most Stable Zr-Substituted Frameworks
2.4.1. Structural Distortion of the Most Stable Zr-Substituted SOD Framework
2.4.2. Structural Distortion of the Most Stable Zr-Substituted AlPO-34 and SAPO-34 Frameworks
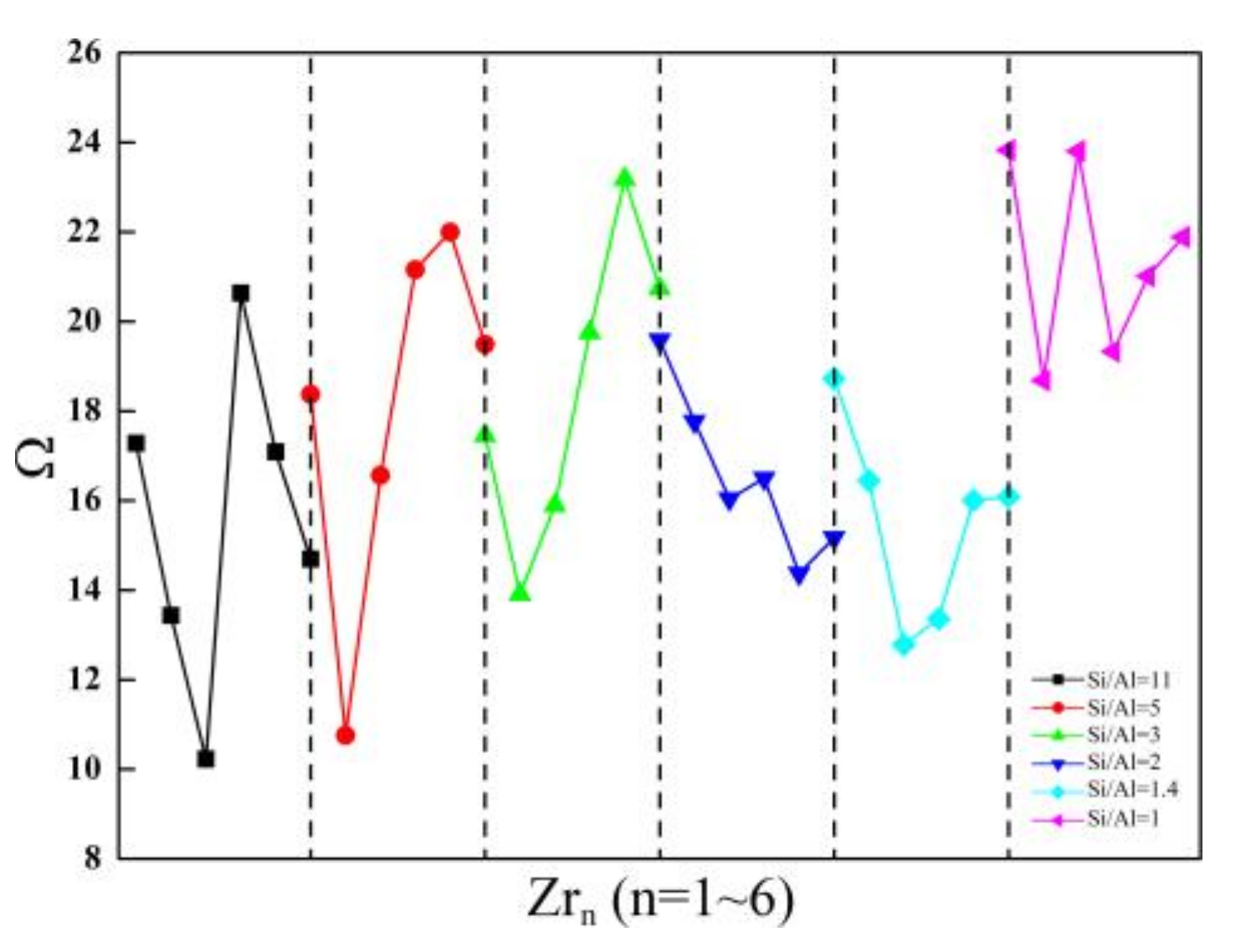
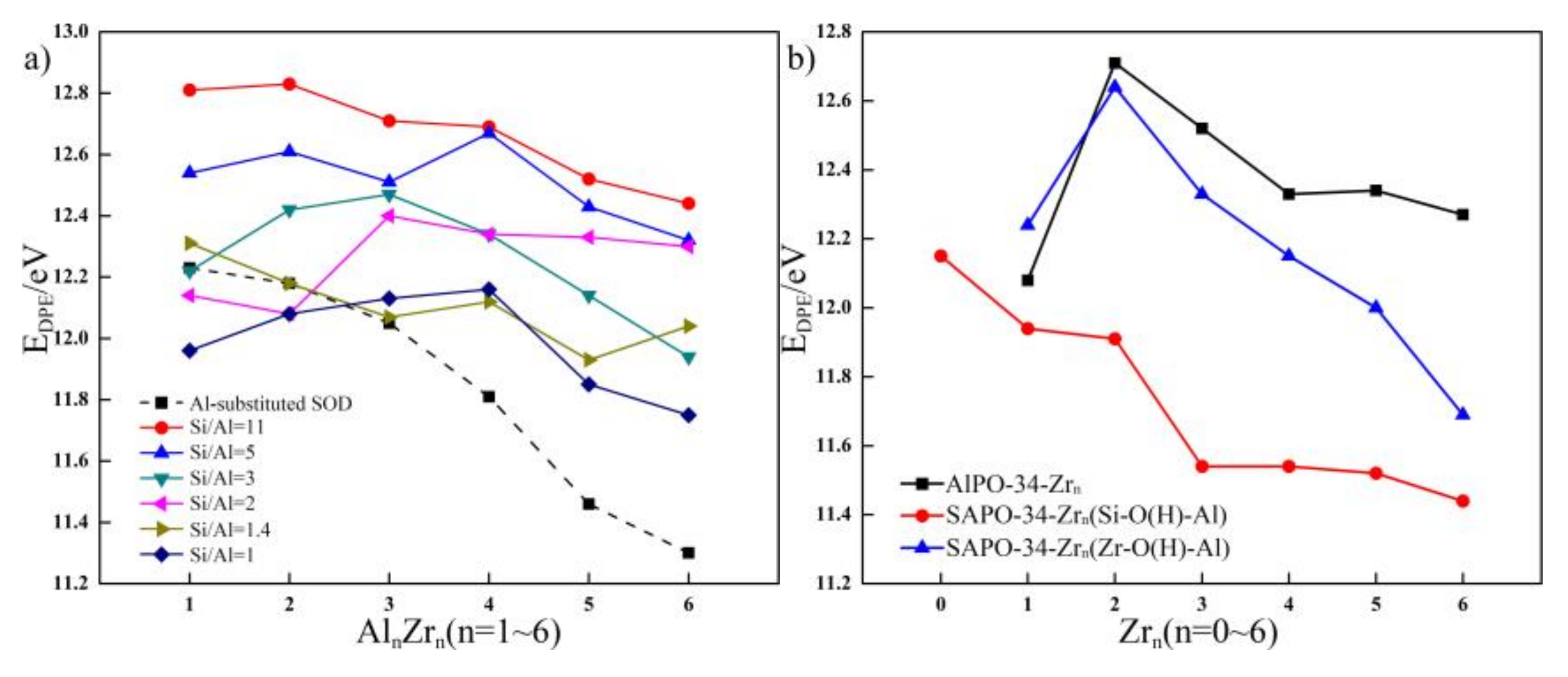
2.5. Brönsted Acidity of the Most Stable Zr-Substituted SOD, AlPO-34, and SAPO-34
2.5.1. Brönsted Acidity of the Zr-Substituted SOD with Si/Al Ratio from Eleven to One
2.5.2. Brönsted Acidity of the Zr-Substituted AlPO-34 and SAPO-34
3. Computational Details
3.1. Model
3.2. Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Structure, Stability, and Lewis Acidity of Mono and Double Ti, Zr, and Sn Framework Substitutions in BEA Zeolites: A Periodic Density Functional Theory Study. J. Phys. Chem. C 2013, 117, 3976–3986. [Google Scholar] [CrossRef]
- Zones, S.I. Translating new materials discoveries in zeolite research to commercial manufacture. Micropor. Mesopor. Mater. 2011, 144, 1–8. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, D.; Xu, L.; Chang, F.; He, Y.; Meng, S.; Su, B.-l.; Liu, Z. Synthesis, characterization and catalytic performance of metal-incorporated SAPO-34 for chloromethane transformation to light olefins. Catal. Today 2008, 131, 262–269. [Google Scholar] [CrossRef]
- Dubois, D.R.; Obrzut, D.L.; Liu, J.; Thundimadathil, J.; Adekkanattu, P.M.; Guin, J.A.; Punnoose, A.; Seehra, M.S. Conversion of methanol to olefins over cobalt-, manganese- and nickel-incorporated SAPO-34 molecular sieves. Fuel Process. Technol. 2003, 83, 203–218. [Google Scholar] [CrossRef]
- Van Niekerk, M.J.; Fletcher, J.C.Q.; O’Connor, C.T. Effect of catalyst modification on the conversion of methanol to light olefins over SAPO-34. Appl. Catal. A 1996, 138, 135–145. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, L.; Feng, G.; Wang, X.; Zhang, R.; Liu, J. Trivalent ions modification for high-silica mordenite: A first principles study. Appl. Surf. Sci. 2018, 433, 627–638. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Lin, M.; Peng, X.; Zhu, B.; Shu, X. Confirmation of the isomorphous substitution by Sn atoms in the framework positions of MFI-typed zeolite. Catal. Today 2018, 316, 193–198. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Jiao, W.; Wang, J.; Fan, W. Recent experimental and theoretical studies on Al siting/acid site distribution in zeolite framework. Curr. Opin. Chem. Eng. 2019, 23, 146–154. [Google Scholar] [CrossRef]
- Losch, P.; Joshi, H.R.; Vozniuk, O.; Grünert, A.; Ochoa-Hernández, C.; Jabraoui, H.; Badawi, M.; Schmidt, W. Proton Mobility, Intrinsic Acid Strength, and Acid Site Location in Zeolites Revealed by Varying Temperature Infrared Spectroscopy and Density Functional Theory Studies. J. Am. Chem. Soc. 2018, 140, 17790–17799. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Xu, R. Needs and trends in rational synthesis of zeolitic materials. Chem. Soc. Rev. 2012, 41, 1729–1741. [Google Scholar] [CrossRef]
- Sedighi, M.; Ghasemi, M.; Sadeqzadeh, M.; Hadi, M. Thorough study of the effect of metal-incorporated SAPO-34 molecular sieves on catalytic performances in MTO process. Powder Technol. 2016, 291, 131–139. [Google Scholar] [CrossRef]
- Sena, F.C.; de Souza, B.F.; de Almeida, N.C.; Cardoso, J.S.; Fernandes, L.D. Influence of framework composition over SAPO-34 and MeAPSO-34 acidity. Appl. Catal. A 2011, 406, 59–62. [Google Scholar] [CrossRef]
- Yuan, S.P.; Wang, J.G.; Li, Y.W.; Jiao, H. Brønsted Acidity of Isomorphously Substituted ZSM-5 by B, Al, Ga, and Fe. Density Functional Investigations. J. Phys. Chem. A 2002, 106, 8167–8172. [Google Scholar] [CrossRef]
- Tielens, F.; Shishido, T.; Dzwigaj, S. What Do Tantalum Framework Sites Look Like in Zeolites? A Combined Theoretical and Experimental Investigation. J. Phys. Chem. C 2010, 114, 9923–9930. [Google Scholar] [CrossRef]
- Ko, Y.S.; Ahn, W.S. Synthesis and characterization of tantalum silicalite molecular sieves with MFI structure. Micropor. Mesopor. Mater. 1999, 30, 283–291. [Google Scholar] [CrossRef]
- Ziolek, M.; Nowak, I.; Kilos, B.; Sobczak, I.; Decyk, P.; Trejda, M.; Volta, J.C. Template synthesis and characterisation of MCM-41 mesoporous molecular sieves containing various transition metal elements—TME (Cu, Fe, Nb, V, Mo). J. Phys. Chem. Solids 2004, 65, 571–581. [Google Scholar] [CrossRef]
- Prakash, A.M.; Kevan, L. Synthesis of Niobium Silicate Molecular Sieves of the MFI Structure: Evidence for Framework Incorporation of the Niobium Ion. J. Am. Chem. Soc. 1998, 120, 13148–13155. [Google Scholar] [CrossRef]
- Wierzchowski, P.T.; Zatorski, L.W. Aldol condensation in gaseous phase by zeolite catalysts. Catal. Lett. 1991, 9, 411–414. [Google Scholar] [CrossRef]
- Rocha, J.; Brandao, P.; Phillippou, A.; Anderson, M.W. Synthesis and characterisation of a novel microporous niobium silicate catalyst. Chem. Commun. 1998, 2687–2688. [Google Scholar] [CrossRef]
- Sobczak, I.; Decyk, P.; Ziolek, M.; Daturi, M.; Lavalley, J.-C.; Kevan, L.; Prakash, A.M. Physicochemical Properties and Catalytic Activity of Cu–NbZSM-5—A Comparative Study with Cu–AlZSM-5. J. Catal. 2002, 207, 101–112. [Google Scholar] [CrossRef]
- Tielens, F. Exploring the reactivity of framework vanadium, niobium, and tantalum sites in zeolitic materials using DFT reactivity descriptors. J. Comput. Chem. 2009, 30, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M. State of the art of Lewis acid-containing zeolites: Lessons from fine chemistry to new biomass transformation processes. Dalton Trans. 2014, 43, 4197–4208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer–Villiger oxidations. Nature 2001, 412, 423. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. PNAS 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [Green Version]
- Sastre, G.; Corma, A. Relation between structure and Lewis acidity of Ti-Beta and TS-1 zeolites: A quantum-chemical study. Chem. Phys. Lett. 1999, 302, 447–453. [Google Scholar] [CrossRef]
- Damin, A.; Bordiga, S.; Zecchina, A.; Lamberti, C. Reactivity of Ti(IV) sites in Ti-zeolites: An embedded cluster approach. J. Chem. Phys. 2002, 117, 226–237. [Google Scholar] [CrossRef]
- Damin, A.; Bonino, F.; Ricchiardi, G.; Bordiga, S.; Zecchina, A.; Lamberti, C. Effect of NH3 Adsorption on the Structural and Vibrational Properties of TS-1. J. Phys. Chem. B 2002, 106, 7524–7526. [Google Scholar] [CrossRef]
- Damin, A.; Bordiga, S.; Zecchina, A.; Doll, K.; Lamberti, C. Ti-chabazite as a model system of Ti(IV) in Ti-zeolites: A periodic approach. J. Chem. Phys. 2003, 118, 10183–10194. [Google Scholar] [CrossRef]
- Shetty, S.; Kulkarni, B.S.; Kanhere, D.G.; Goursot, A.; Pal, S. A Comparative Study of Structural, Acidic and Hydrophilic Properties of Sn−BEA with Ti−BEA Using Periodic Density Functional Theory. J. Phys. Chem. B 2008, 112, 2573–2579. [Google Scholar] [CrossRef]
- Kulkarni, B.S.; Krishnamurty, S.; Pal, S. Probing Lewis acidity and reactivity of Sn- and Ti-beta zeolite using industrially important moieties: A periodic density functional study. J. Mol. Catal. A: Chem. 2010, 329, 36–43. [Google Scholar] [CrossRef]
- Yang, G.; Zhuang, J.; Ma, D.; Lan, X.; Zhou, L.; Liu, X.; Han, X.; Bao, X. A joint experimental–theoretical study on trimethylphosphine adsorption on the Lewis acidic sites present in TS-1 zeolite. J. Mol. Struct. 2008, 882, 24–29. [Google Scholar] [CrossRef]
- Yang , G.; Zhou, L.; Liu, X.; Han, X.; Bao, X. Density Functional Calculations on the Distribution, Acidity, and Catalysis of TiIV and TiIII Ions in MCM-22 Zeolite. Chem. Eur. J. 2011, 17, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Jardillier, N.; Berthomieu, D.; Goursot, A.; Reveles, J.U.; Köster, A.M. Theoretical Study of CuIY Zeolite: Structure and Electronic Properties. J. Phys. Chem. B 2006, 110, 18440–18446. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Goursot, A.; Coq, B.; Delahay, G. Theoretical Study of the Dissociation of N2O in a Transition Metal Ion-Catalyzed Reaction. J. Phys. Chem. B 2004, 108, 8823–8829. [Google Scholar] [CrossRef]
- Löwenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Neurock, M. Perspectives on the first principles elucidation and the design of active sites. J. Catal. 2003, 216, 73–88. [Google Scholar] [CrossRef]
- Kühl, G.H. The coordination of aluminum and silicon in zeolites as studied by x-ray spectrometry. J. Phys. Chem. Solids 1977, 38, 1259–1263. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The Strength of Brønsted Acid Sites in Microporous Aluminosilicates. Acs Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Aghaei, E.; Haghighi, M.; Pazhohniya, Z.; Aghamohammadi, S. One-pot hydrothermal synthesis of nanostructured ZrAPSO-34 powder: Effect of Zr-loading on physicochemical properties and catalytic performance in conversion of methanol to ethylene and propylene. Micropor. Mesopor. Mater. 2016, 226, 331–343. [Google Scholar] [CrossRef]
- Alonso, A.; Valle, B.; Atutxa, A.; Gayubo Ana, G.; Aguayo, A. Development of Alternative Catalysts Based on HZSM-5 Zeolite for the BTO Process. Int. J. Chem. React. Eng. 2007, 5, A61. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 17 March 2017).
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
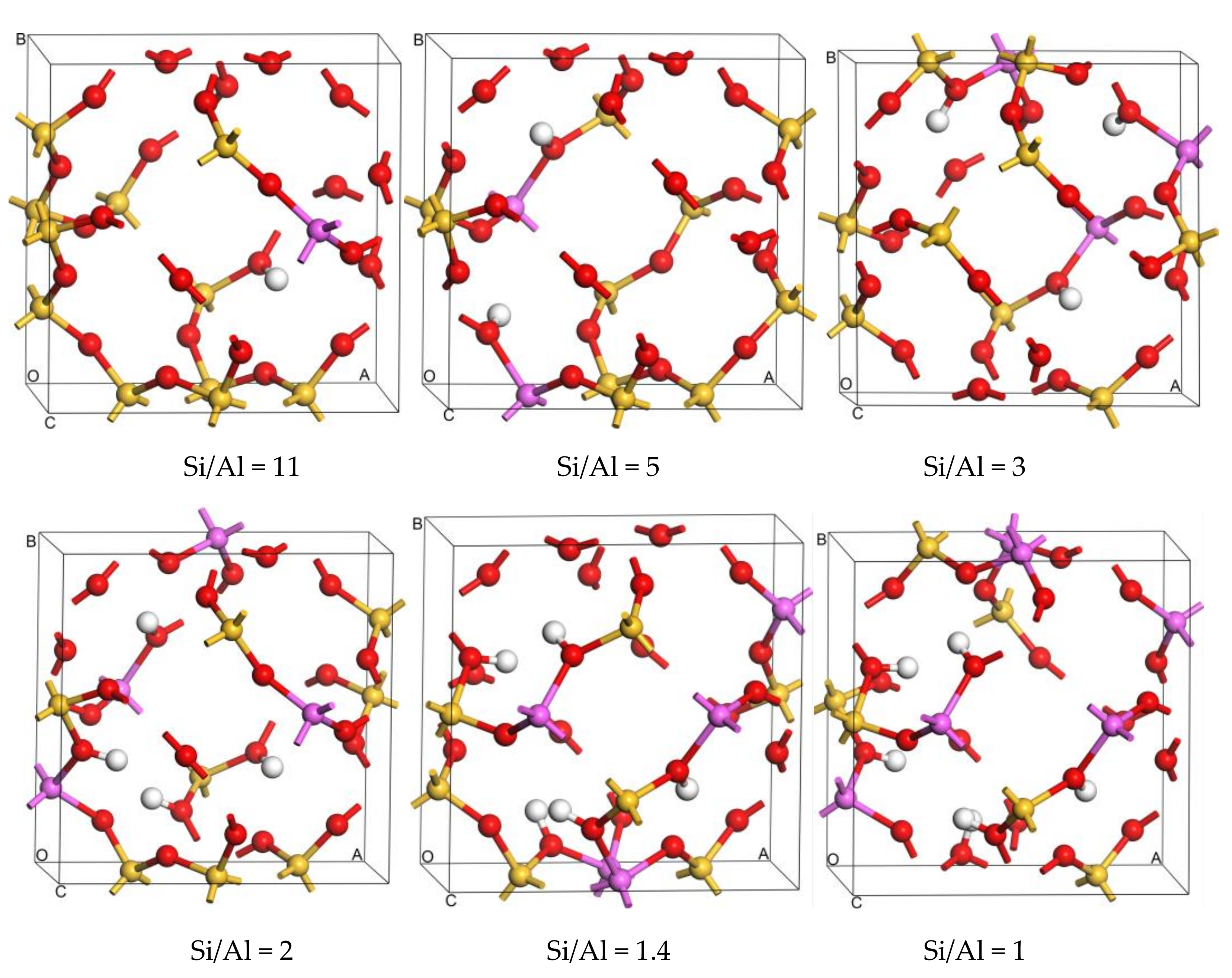
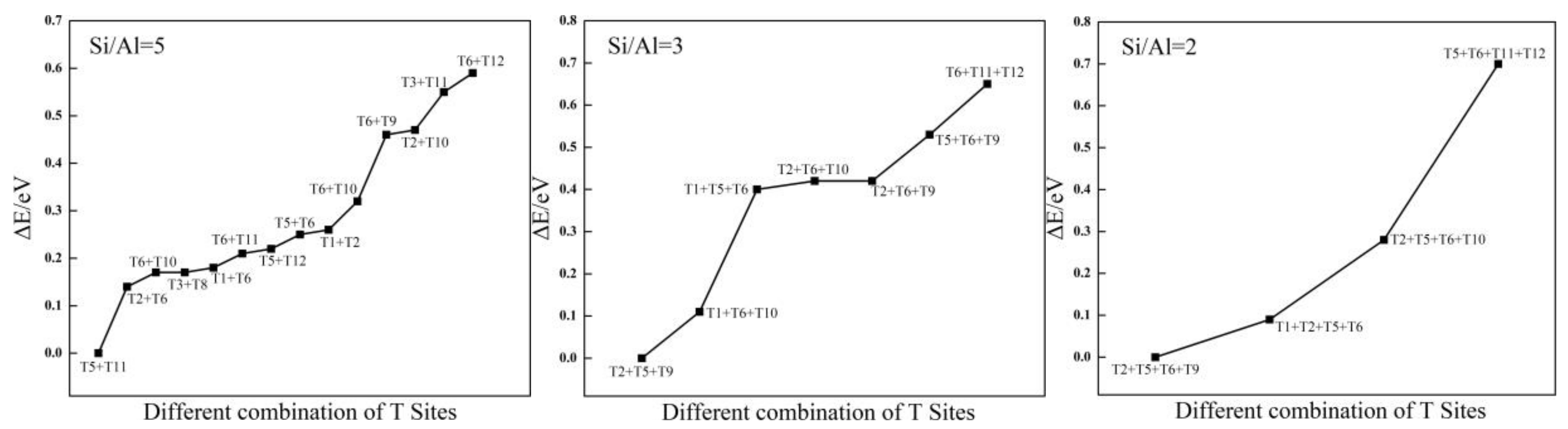
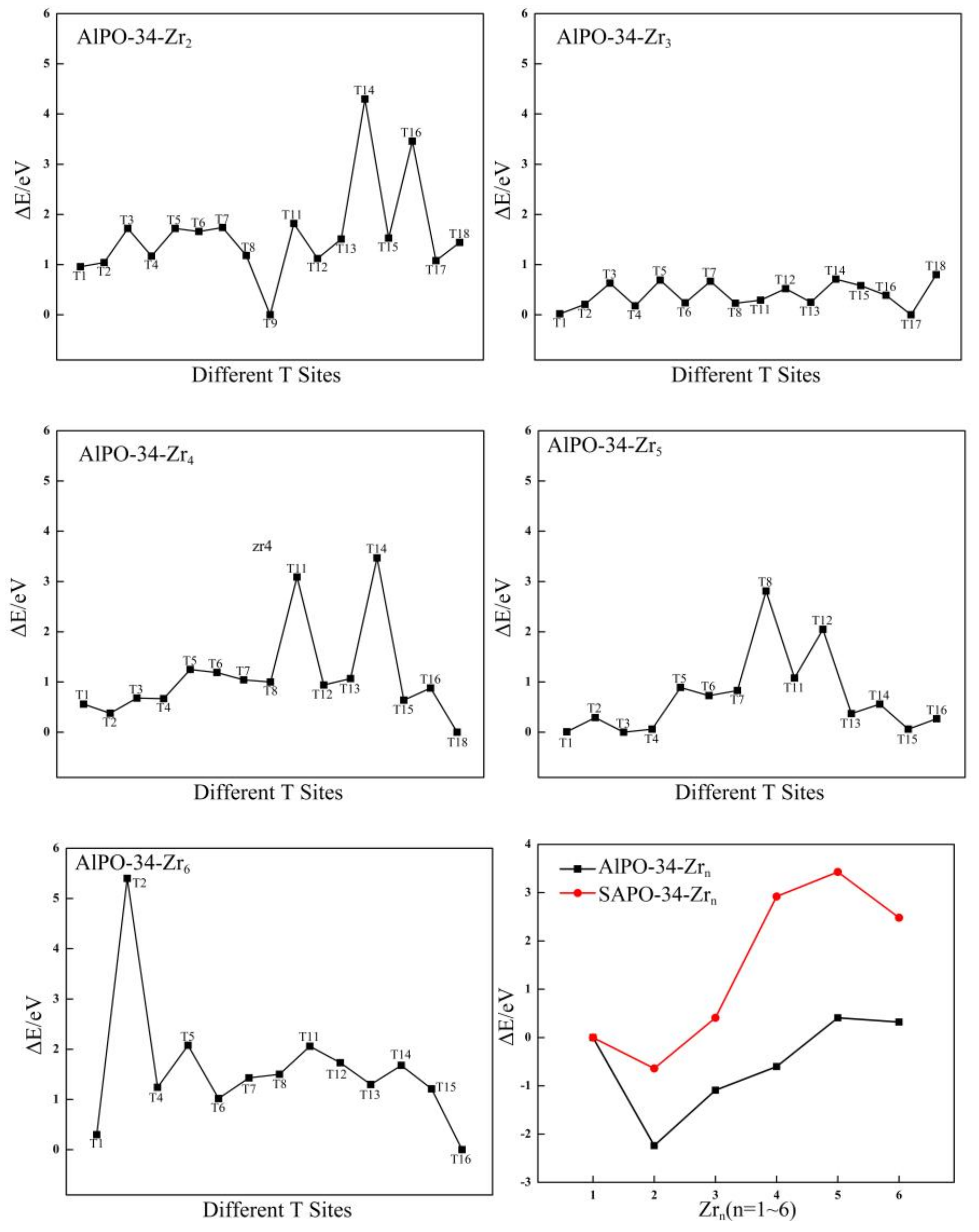
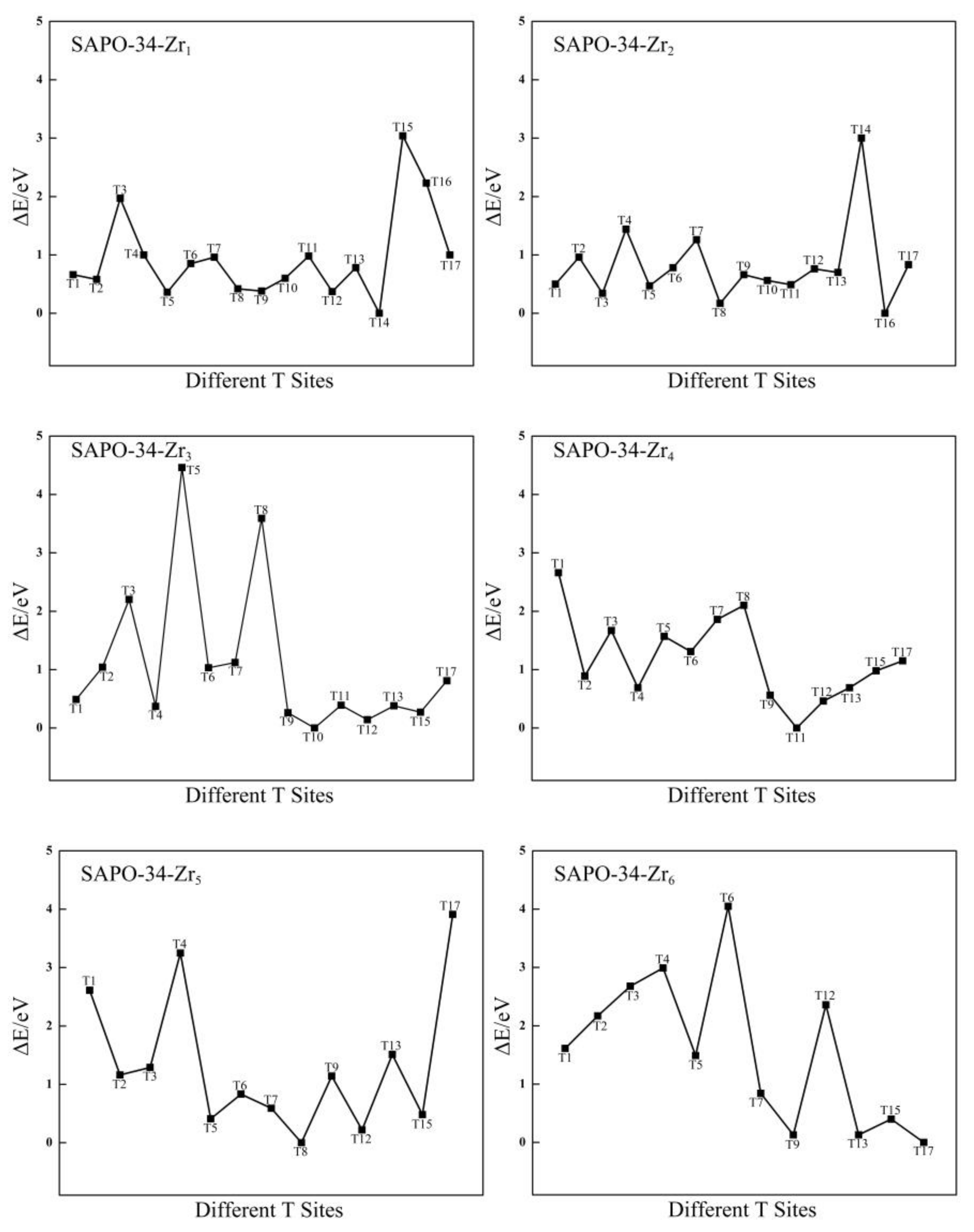
| Framework | T Sites | Eav-sub/eV |
|---|---|---|
| Si/Al = 11 | --- | 0.17 |
| Si/Al = 5 | T5 + T11 | −0.63 |
| Si/Al = 3 | T2 + T5 +T9 | −0.85 |
| Si/Al = 2 | T2 + T5 +T6 + T9 | −0.94 |
| Si/Al = 1.4 | T1 + T2 + T6 + T9 + T10 | −0.92 |
| Si/Al = 1 | --- | −0.96 |
| Zr Content | Si/Al = 11 | Si/Al = 5 | Si/Al = 3 | Si/Al = 2 | Si/Al = 1.4 | Si/Al = 1 |
|---|---|---|---|---|---|---|
| 0 | −9.35 | −9.33 | −9.39 | −9.40 | −9.34 | −9.36 |
| 1 | −9.51 | −9.30 | −9.27 | −9.22 | −9.11 | −9.15 |
| 2 | −9.32 | −9.38 | −9.35 | −9.33 | −9.13 | −9.28 |
| 3 | −9.30 | −9.45 | −9.39 | −9.38 | −9.38 | −9.27 |
| 4 | −9.33 | −9.44 | −9.46 | −9.48 | −9.38 | −9.32 |
| 5 | −9.31 | −9.51 | −9.52 | −9.51 | −9.41 | −9.39 |
| 6 | −9.44 | −9.57 | −9.63 | −9.54 | −9.47 | −9.42 |
| Zr Content | Si/Al = 11 | Si/Al = 5 | Si/Al = 3 | Si/Al = 2 | Si/Al = 1.4 | Si/Al = 1 |
|---|---|---|---|---|---|---|
| Eav-sub | Eav-sub | Eav-sub | Eav-sub | Eav-sub | Eav-sub | |
| 1 | −0.56 | −0.65 | −0.83 | −0.83 | −2.10 | −2.22 |
| 2 | −0.80 | −0.81 | −0.80 | −0.87 | −1.46 | −1.55 |
| 3 | −0.62 | −0.73 | −0.84 | −0.90 | −1.07 | −1.40 |
| 4 | −0.74 | −0.79 | −0.70 | −0.87 | −0.96 | −0.99 |
| 5 | −0.75 | −0.71 | −0.88 | −0.75 | −0.79 | −0.85 |
| 6 | −0.68 | −0.74 | −0.77 | −0.62 | −0.79 | −0.87 |
| Zr Content | AlPO-34 | SAPO-34 |
|---|---|---|
| Eav-sub | Eav-sub | |
| 0 | --- | 2.801 |
| 1 | 3.72 | 0.83 |
| 2 | 0.74 | 0.10 |
| 3 | 0.88 | 0.41 |
| 4 | 0.78 | 0.94 |
| 5 | 0.83 | 0.85 |
| 6 | 0.67 | 0.55 |
| Zr Content | AlPO-34 | SAPO-34 |
|---|---|---|
| 0 | −9.12 | −9.17 |
| 1 | −9.01 | −8.95 |
| 2 | −9.11 | −9.05 |
| 3 | −9.15 | −9.11 |
| 4 | −9.20 | −9.21 |
| 5 | −9.22 | −9.26 |
| 6 | −9.27 | −9.31 |
| Framework | ΘAl/° | ΩSi→Al |
|---|---|---|
| Si/Al = 11 | 6.18 | 5.79 |
| Si/Al = 5 | 5.23 | 4.75 |
| Si/Al = 3 | 4.70 | 4.17 |
| Si/Al = 2 | 5.66 | 5.22 |
| Si/Al = 1.4 | 6.46 | 6.11 |
| Si/Al = 1 | 5.99 | 5.59 |
| Framework | ΘZr/° | ΩP⟶Zr |
| AlPO-34-Zr1 | 3.06 | 1.51 |
| AlPO-34-Zr2 | 12.66 | 9.38 |
| AlPO-34-Zr3 | 11.28 | 8.25 |
| AlPO-34-Zr4 | 13.07 | 9.71 |
| AlPO-34-Zr5 | 14.43 | 10.83 |
| AlPO-34-Zr6 | 13.27 | 9.88 |
| Framework | ΘZr/° | ΩP⟶Zr |
| SAPO-34 | 6.01(ΘSi) | 3.93(ΩP⟶Si) |
| SAPO-34-Zr1 | 5.85 | 3.80 |
| SAPO-34-Zr2 | 13.53 | 10.09 |
| SAPO-34-Zr3 | 13.17 | 9.80 |
| SAPO-34-Zr4 | 12.52 | 9.26 |
| SAPO-34-Zr5 | 12.18 | 8.98 |
| SAPO-34-Zr6 | 13.55 | 10.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xing, B.; Wang, B.; Li, R. Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks. Molecules 2019, 24, 4466. https://doi.org/10.3390/molecules24244466
Li D, Xing B, Wang B, Li R. Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks. Molecules. 2019; 24(24):4466. https://doi.org/10.3390/molecules24244466
Chicago/Turabian StyleLi, Duichun, Bin Xing, Baojun Wang, and Ruifeng Li. 2019. "Theoretical Study of Zirconium Isomorphous Substitution into Zeolite Frameworks" Molecules 24, no. 24: 4466. https://doi.org/10.3390/molecules24244466





