Synthesis and Electrochemistry of New Furylpyrazolino[60]fullerene Derivatives by Efficient Microwave Radiation
Abstract
:1. Introduction
2. Results
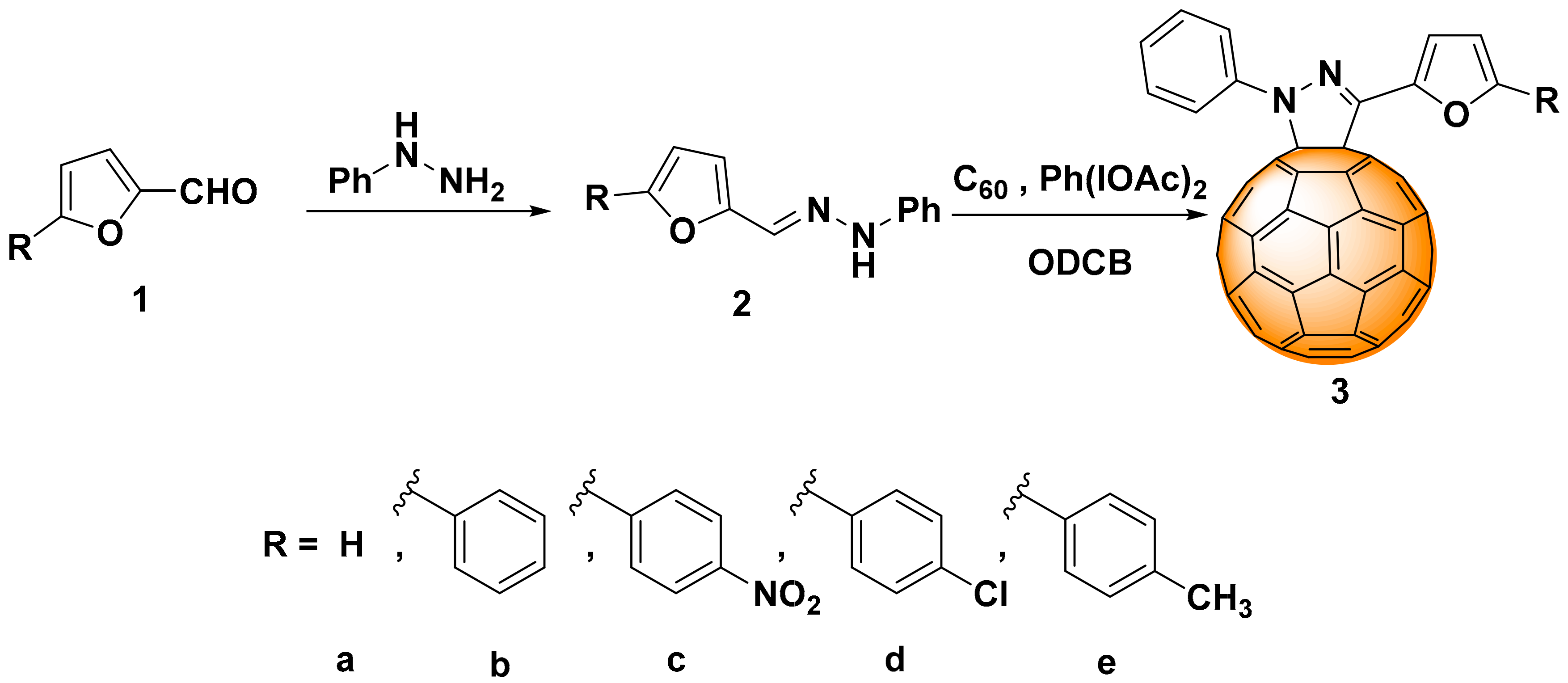
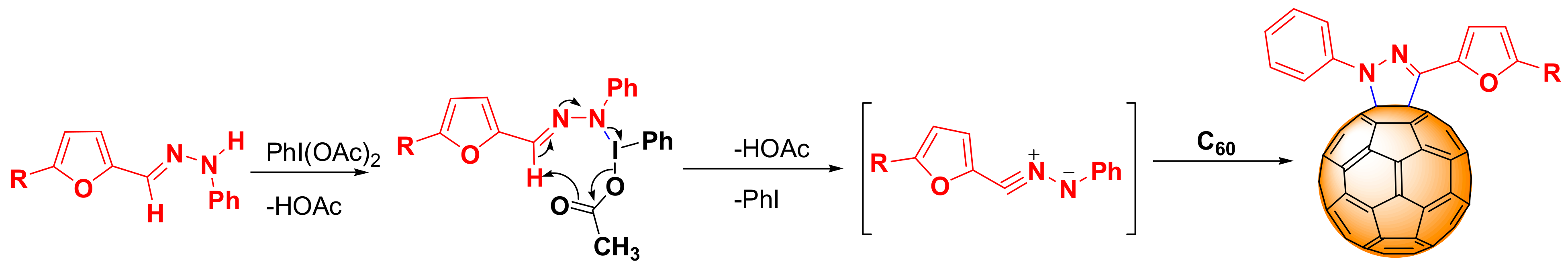
2.1. Synthesis
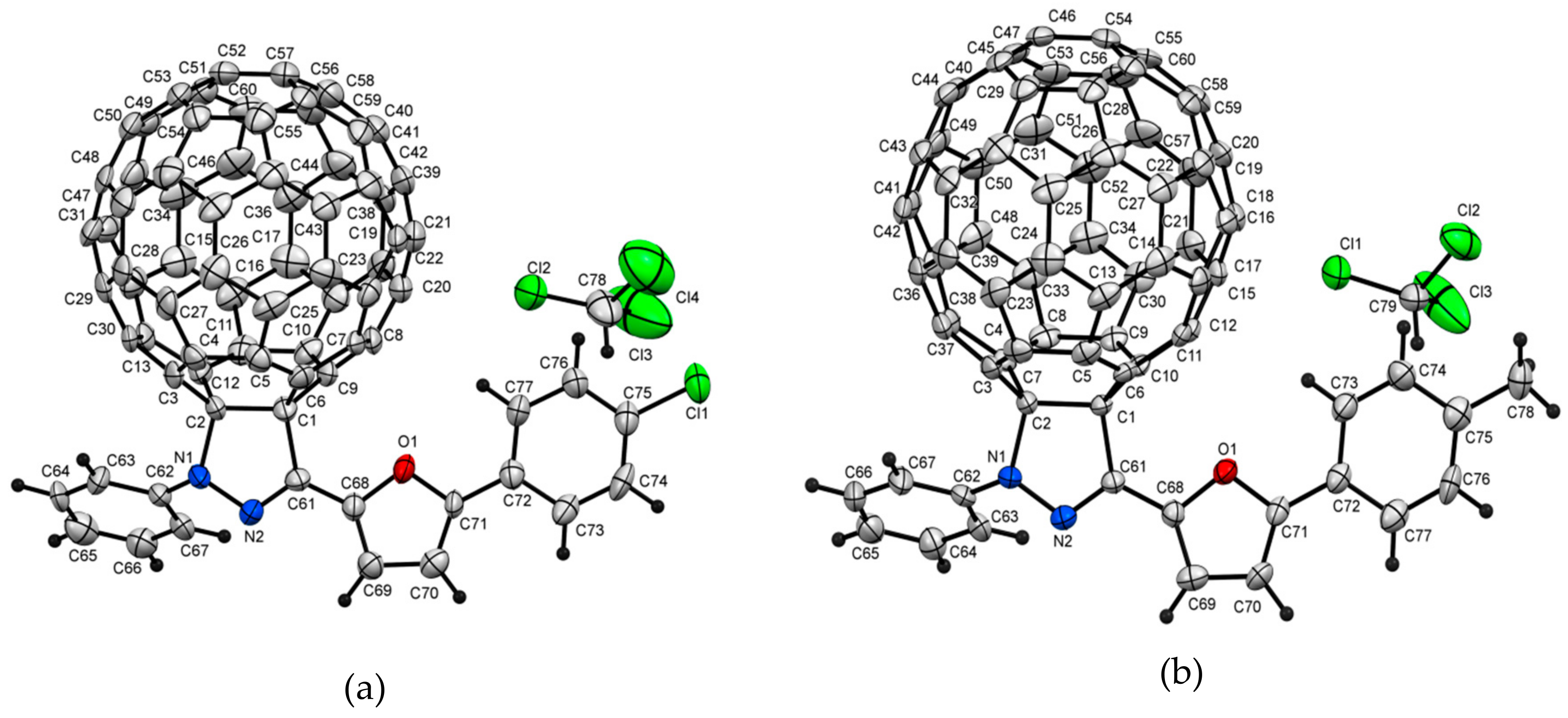
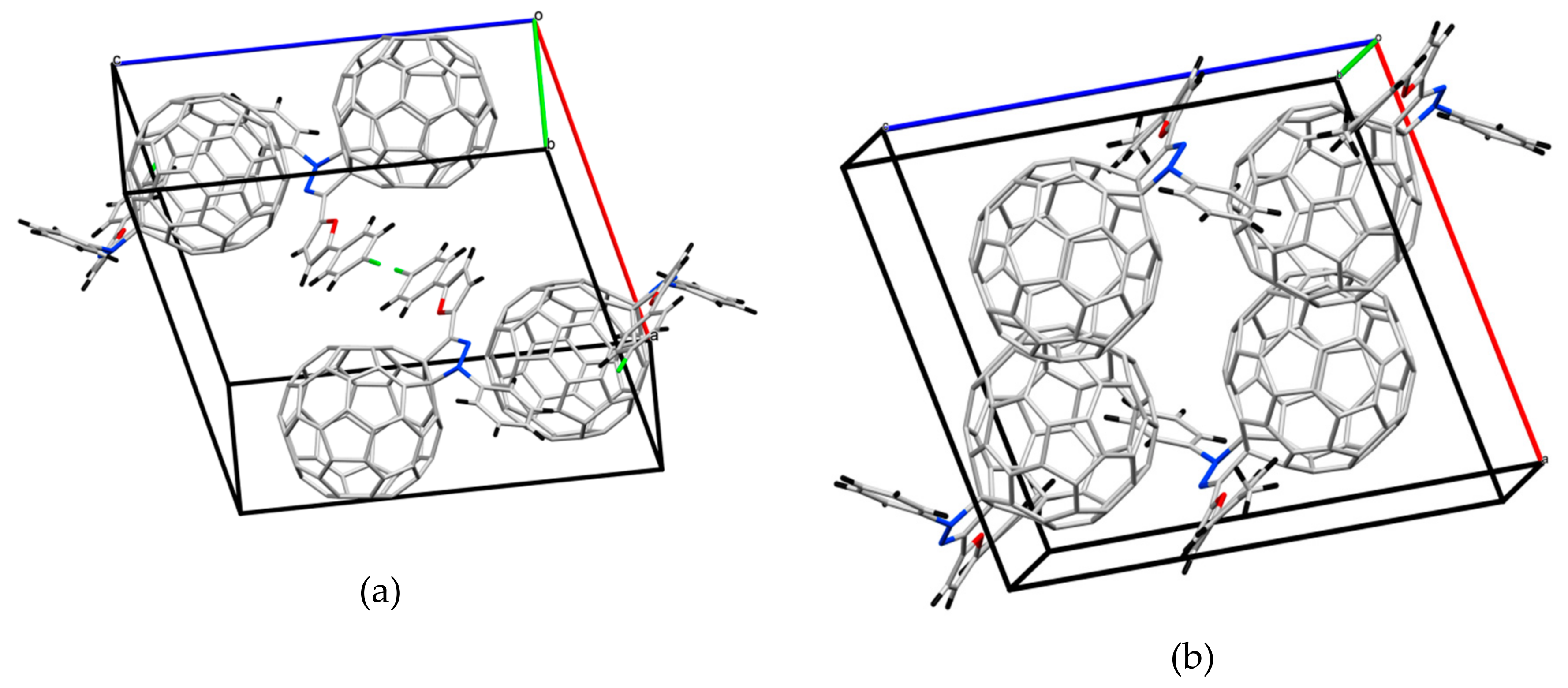
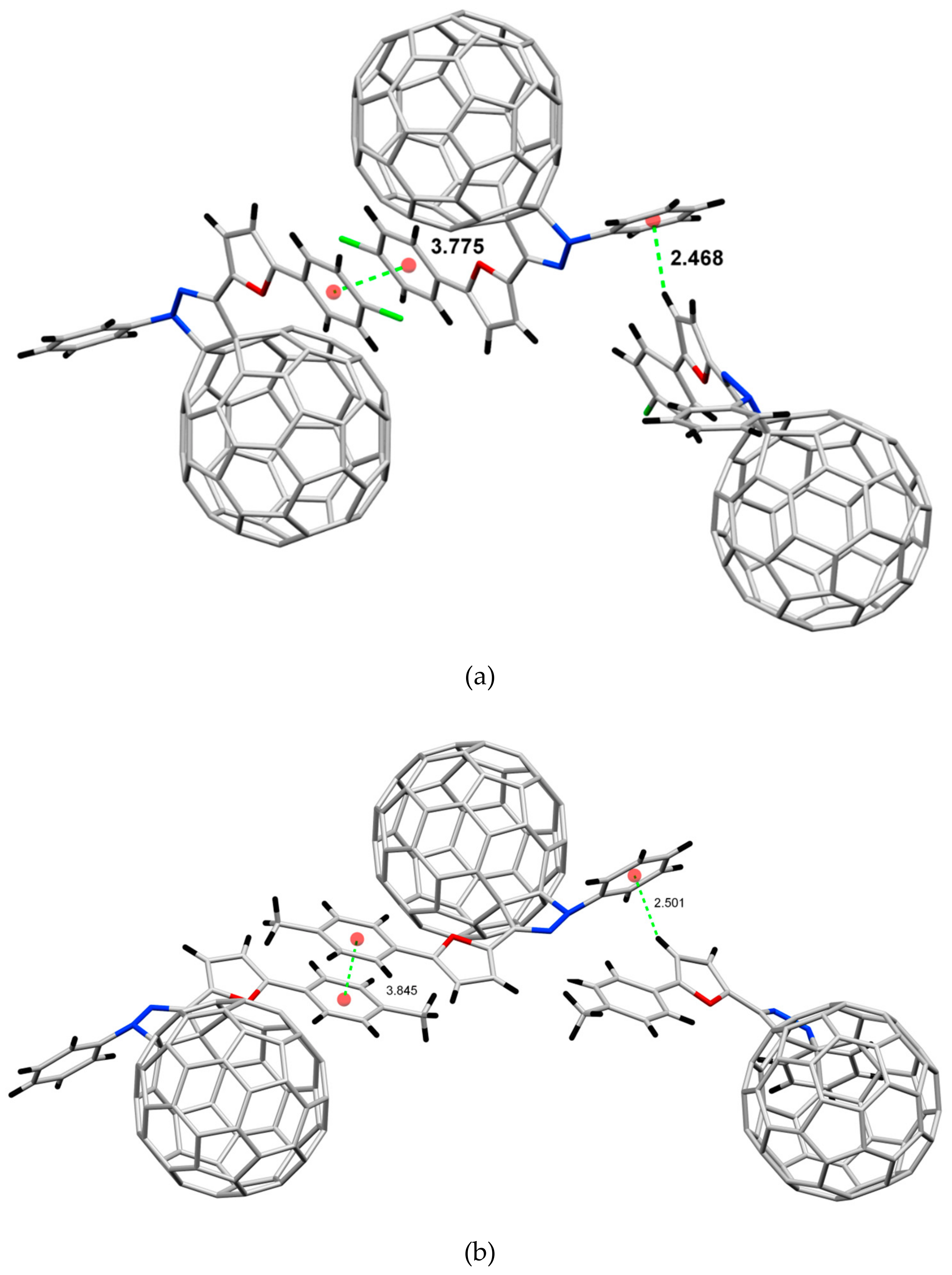
2.2. Crystal Structure
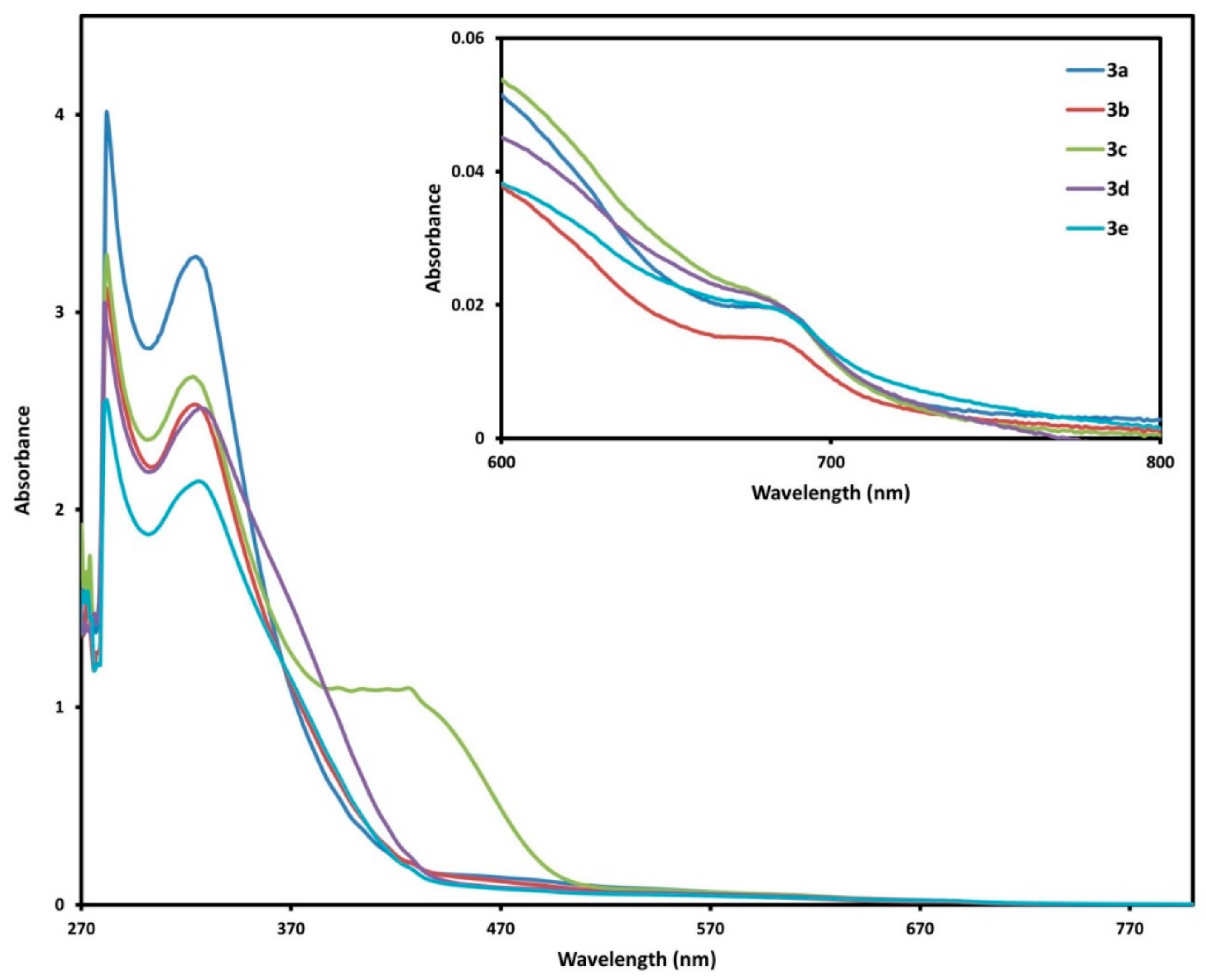
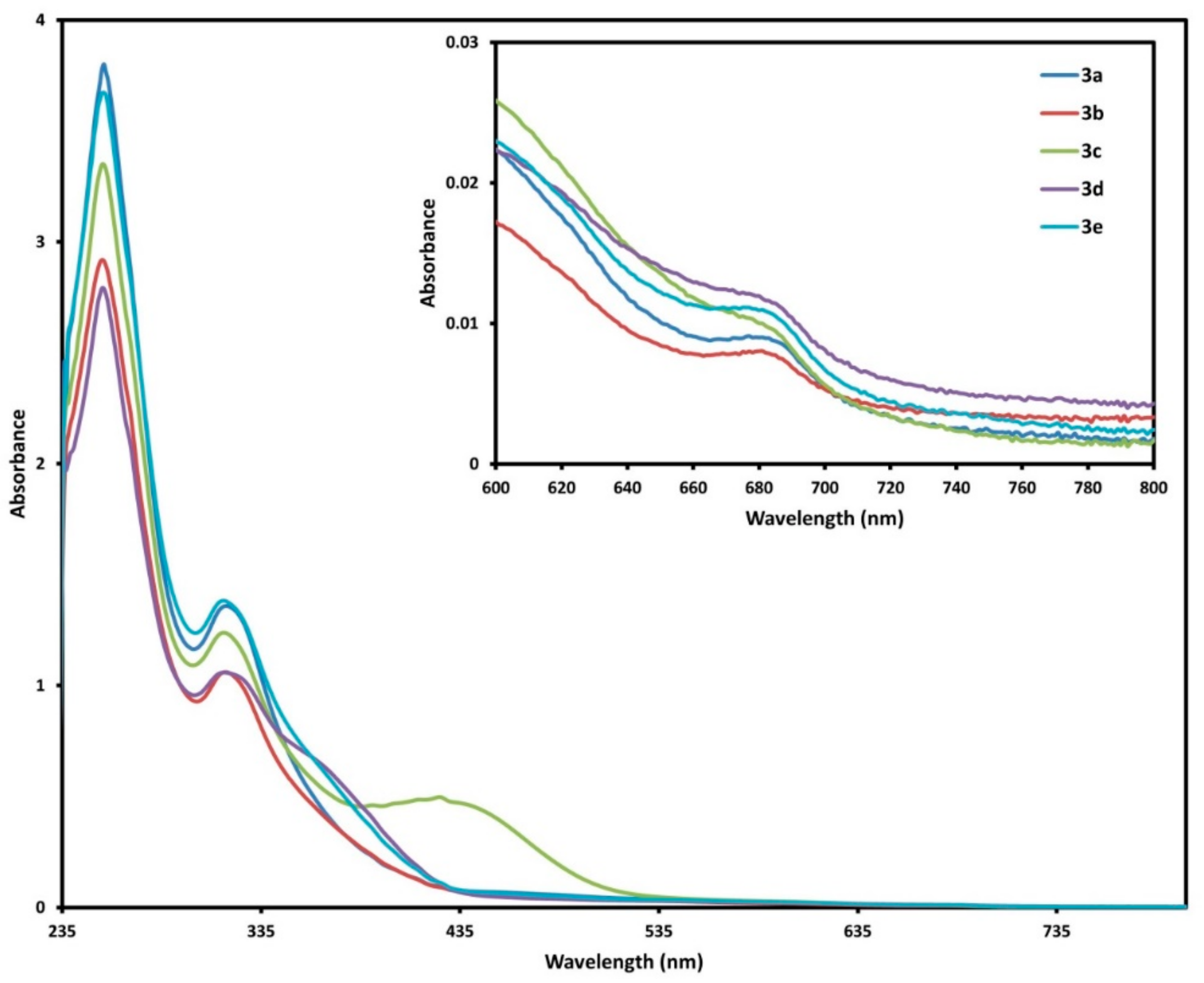
2.3. UV-Vis Spectra
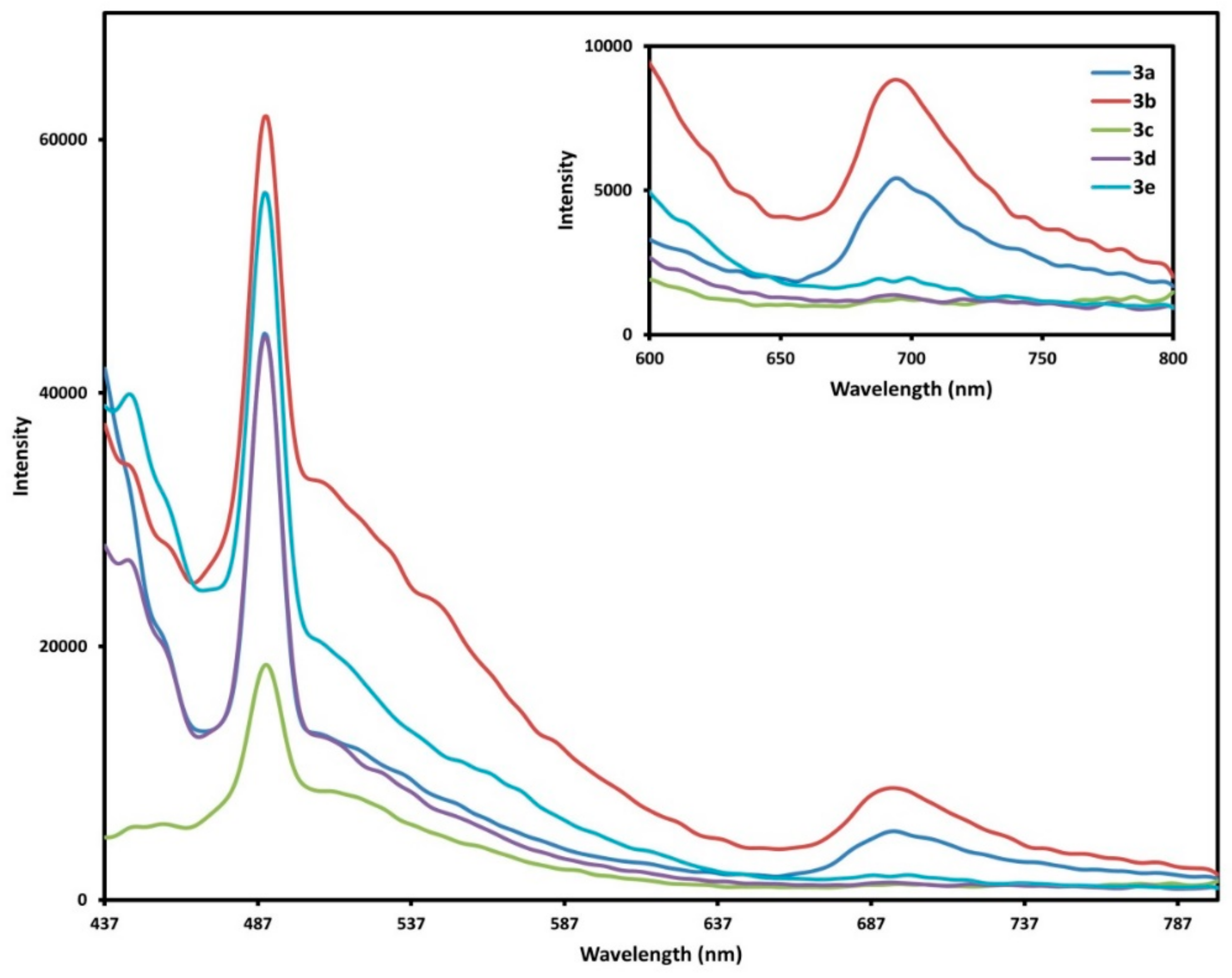
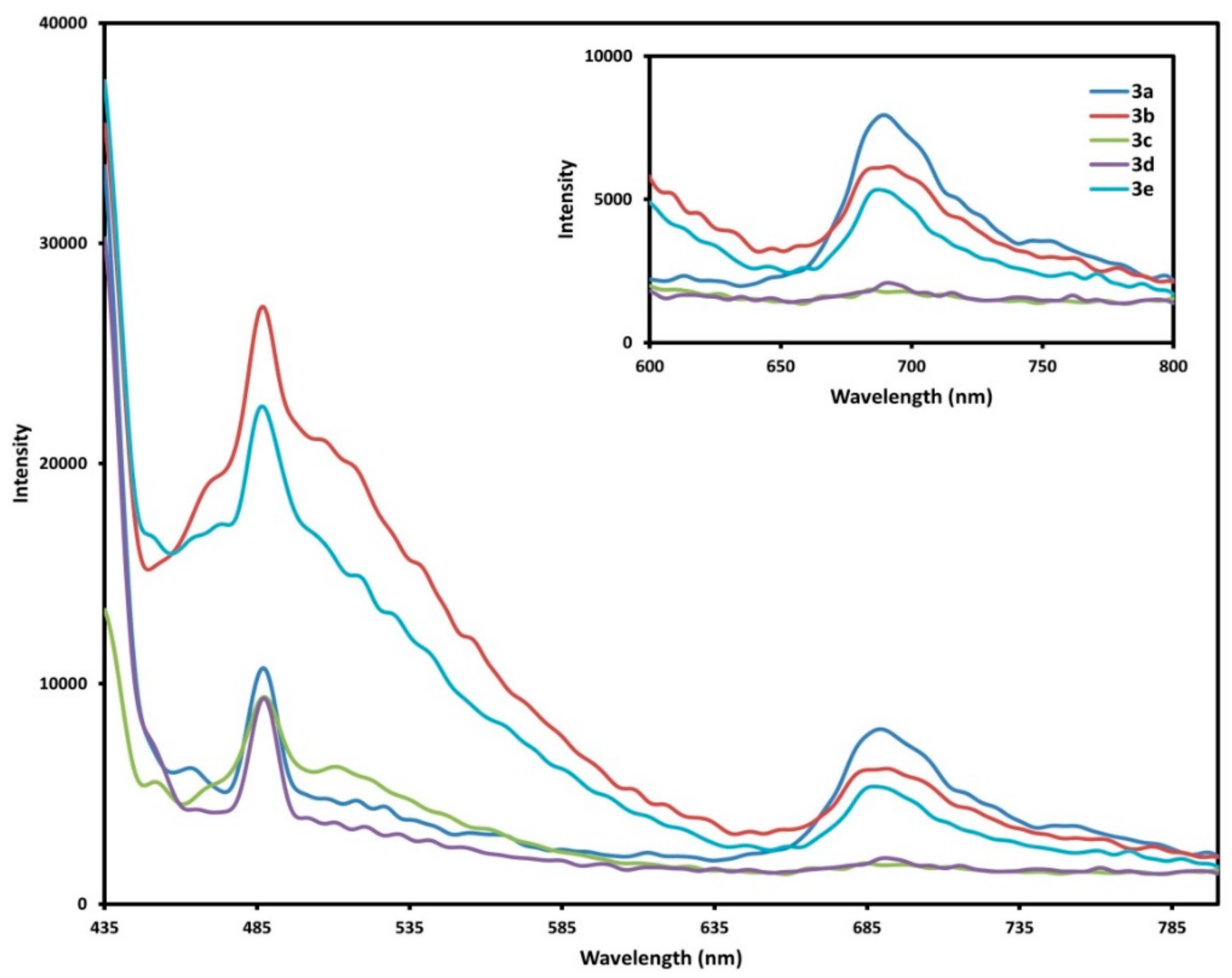
2.4. Fluorescence Spectra
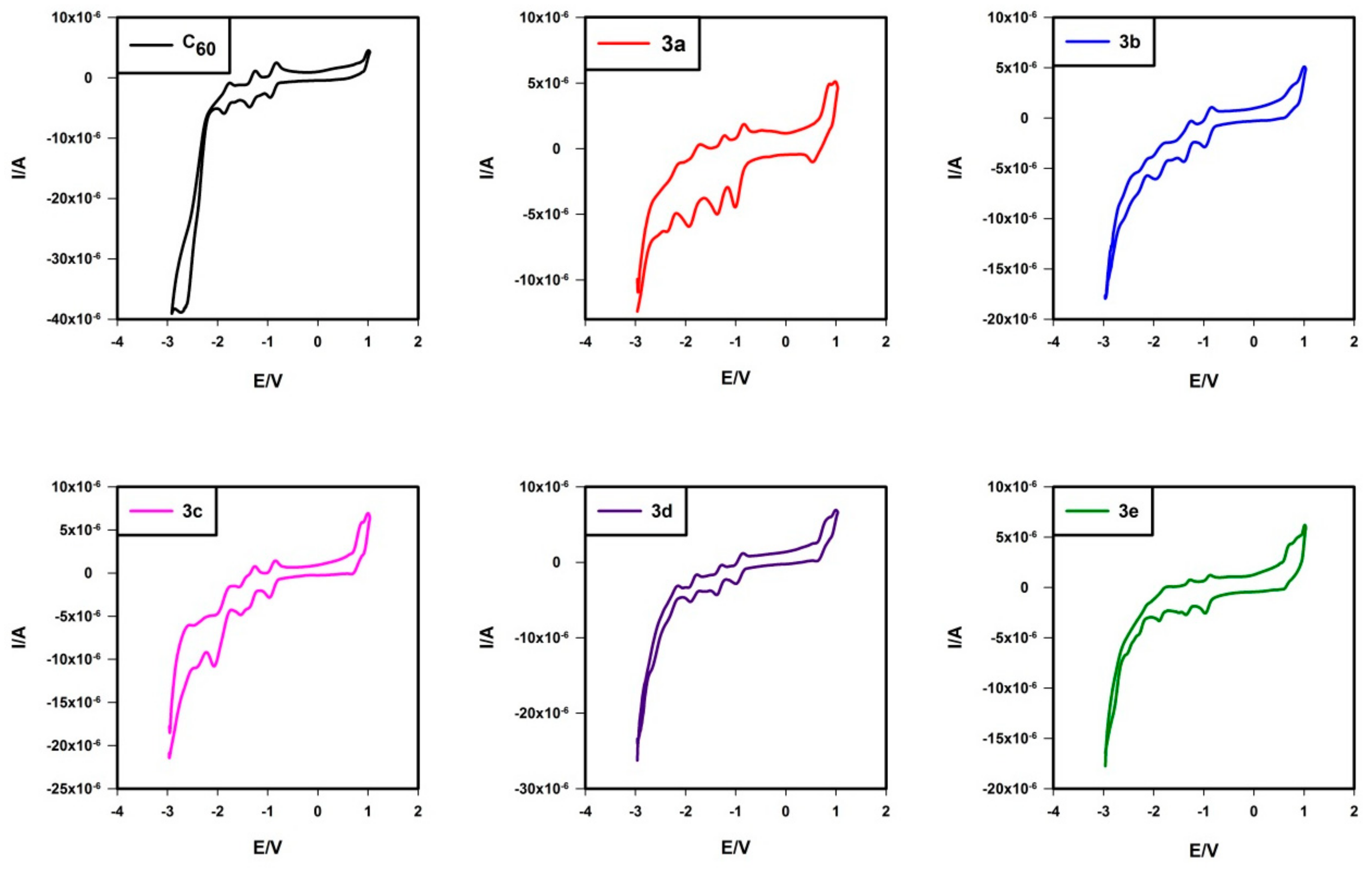
2.5. Electrochemistry
3. Materials and Methods
3.1. General Methods
3.2. Synthesis
3.2.1. General Synthesis Procedure of Substituted Hydrazones (2a–e)
3.2.2. General Synthesis Procedure of Cycloadducts (3a–e)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Kräutler, B. The Chemistry of the Fullerenes. Angew. Chem. 1995, 107, 1654. [Google Scholar] [CrossRef]
- Sariciftci, N.S. Role of buckminsterfullerene, C60, in organic photoelectric devices. Prog. Quantum Electron. 1995, 19, 131–159. [Google Scholar] [CrossRef]
- Prato, M. [60] Fullerene chemistry for materials science applications. J. Mater. Chem. 1997, 7, 1097–1109. [Google Scholar] [CrossRef]
- Diederich, F.; Kessinger, R. Templated regioselective and stereoselective synthesis in fullerene chemistry. Acc. Chem. Res. 1999, 32, 537–545. [Google Scholar] [CrossRef]
- Shanbogh, P.P.; Sundaram, N.G. Fullerenes revisited. Resonance 2015, 20, 123–1353. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Montellano López, A.; Mateo-Alonso, A.; Prato, M. Materials chemistry of fullerene C60 derivatives. J. Mater. Chem. 2011, 21, 1305–1318. [Google Scholar] [CrossRef]
- Haddon, R.C. Chemistry of the fullerenes: The manifestation of strain in a class of continuous aromatic molecules. Science 1993, 261, 1545–1550. [Google Scholar] [CrossRef]
- Haddon, R.C. Electronic structure, conductivity and superconductivity of alkali metal doped C60. Acc. Chem. Res. 1992, 25, 127–133. [Google Scholar] [CrossRef]
- Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2005. [Google Scholar]
- Martín, N. New challenges in fullerene chemistry. Chem. Commun. 2006, 37, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Tzirakis, M.D.; Orfanopoulos, M. Radical reactions of fullerenes: From synthetic organic chemistry to materials science and biology. Chem. Rev. 2013, 113, 5262–5321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-E.; Li, F.; Wang, G.-W. Mechanochemistry of fullerenes and related materials. Chem. Soc. Rev. 2013, 42, 7535–7570. [Google Scholar] [CrossRef] [PubMed]
- Maroto, E.E.; de Cózar, A.; Filippone, S.; Martín-Domenech, Á.; Suarez, M.; Cossío, F.P.; Martín, N. Hierarchical Selectivity in Fullerenes: Site-, Regio-, Diastereo-, and Enantiocontrol of the 1, 3-Dipolar Cycloaddition to C70. Angew. Chem. Int. Ed. 2011, 50, 6060–6064. [Google Scholar] [CrossRef]
- Okumura, M.; Mikawa, M.; Yokawa, T.; Kanazawa, Y.; Kato, H.; Shinohara, H. Evaluation of water-soluble metallofullerenes as MRI contrast agents. Acad. Radiol. 2002, 9 (Suppl. 2), S495–S497. [Google Scholar] [CrossRef]
- Xing, G.; Yuan, H.; He, R.; Gao, X.; Jing, L.; Zhao, F.; Chai, Z.; Zhao, Y. The strong MRI relaxivity of paramagnetic nanoparticles. J. Phys. Chem. B 2008, 112, 6288–6291. [Google Scholar] [CrossRef]
- Meng, H.; Xing, G.; Sun, B.; Zhao, F.; Lei, H.; Li, W.; Song, Y.; Chen, Z.; Yuan, H.; Wang, X.; et al. Potent angiogenesis inhibition by the particulate form of fullerene derivatives. Acs Nano 2010, 4, 2773–2783. [Google Scholar] [CrossRef]
- Piotrowski, P.; Jakubow, K.; Kowalewska, B.; Kaim, A. Dioxygen insensitive C70/AuNPs hybrid system for rapid and quantitative glucose biosensing. Rsc Adv. 2017, 7, 45634–45640. [Google Scholar] [CrossRef] [Green Version]
- Gross, S.; Gilead, A.; Scherz, A.; Neeman, M.; Salomon, Y. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat. Med. 2003, 9, 1327–1331. [Google Scholar] [CrossRef]
- Delgado, J.L.; Martín, N.; de la Cruz, P.; Langa, F. Pyrazolinofullerenes: A less known type of highly versatile fullerene derivatives. Chem. Soc. Rev. 2011, 40, 5232–5241. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.L.; de la Cruz, P.; López-Arza, V.; Langa, F.; Gan, Z.; Araki, Y.; Ito, O. Synthesis and photoinduced intermolecular electronic acceptor ability of pyrazolo [60] fullerenes vs. tetrathiafulvalene. Bull. Chem. Soc. Jpn. 2005, 78, 1500–1507. [Google Scholar] [CrossRef]
- Delgado, J.L.; de la Cruz, P.; López-Arza, V.; Langa, F.; Kimball, D.B.; Haley, M.M.; Araki, Y.; Ito, O. The isoindazole nucleus as a donor in fullerene-based dyads. Evidence for electron transfer. J. Org. Chem. 2004, 69, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Langa, F.; de la Cruz, P.; Espíldora, E.; de la Hoz, A.; Bourdelande, J.L.; Sánchez, L.; Martín, N. C60-based triads with improved electron-acceptor properties: Pyrazolylpyrazolino [60] fullerenes. J. Org. Chem. 2001, 66, 5033–5041. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; El-Khouly, M.E.; de la Cruz, P.; Araki, Y.; Ito, O.; Langa, F. Comparison between the Photophysical Properties of Pyrazolo-and Isoxazolo [60] fullerenes with Dual Donors (Ferrocene, Aniline and Alkoxyphenyl). Eur. J. Org. Chem. 2007, 2007, 2175–2185. [Google Scholar] [CrossRef]
- Wang, X.; Perzon, E.; Delgado, J.L.; de la Cruz, P.; Zhang, F.; Langa, F.; Andersson, M.; Inganäs, O. Infrared photocurrent spectral response from plastic solar cell with low-band-gap polyfluorene and fullerene derivative. Appl. Phys. Lett. 2004, 85, 5081–5083. [Google Scholar] [CrossRef]
- Wang, X.; Perzon, E.; Oswald, F.; Langa, F.; Admassie, S.; Andersson, M.R.; Inganäs, O. Enhanced photocurrent spectral response in low-bandgap polyfluorene and C70-derivative-based solar cells. Adv. Funct. Mater. 2005, 15, 1665–1670. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Gisselbrecht, J.-P.; Gross, M.; Krasnikov, V.; Tsamouras, D.; Hadziioannou, G.; Gómez-Escalonilla, M.J.; Langa, F.; Eckert, J.-F.; et al. Photoinduced processes in fullerenopyrrolidine and fullerenopyrazoline derivatives substituted with an oligophenylenevinylene moiety. J. Mater. Chem. 2002, 12, 2077–2087. [Google Scholar] [CrossRef] [Green Version]
- Langa, F.; Gomez-Escalonilla, M.J.; Rueff, J.-M.; Figueira Duarte, T.M.; Nierengarten, J.-F.; Palermo, V.; Samorì, P.; Rio, Y.; Accorsi, G.; Armaroli, N. Pyrazolino [60] fullerene–Oligophenylenevinylene Dumbbell-Shaped Arrays: Synthesis, Electrochemistry, Photophysics, and Self-Assembly on Surfaces. Chem. – A Eur. J. 2005, 11, 4405–4415. [Google Scholar] [CrossRef]
- Yang, H.-T.; Ruan, X.-J.; Miao, C.-B.; Sun, X.-Q. An efficient one-step synthesis of fulleroisoxazolines and fulleropyrazolines mediated by (diacetoxyiodo) benzene. Tetrahedron Lett. 2010, 51, 6056–6059. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Masoomi, R. Microwave-assisted synthesis of fulleropyrazolines/fulleroisoxazolines mediated by (diacetoxyiodo) benzene: A rapid and green procedure. Rsc Adv. 2014, 4, 2954–2960. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Q.; Lou, J.-C.; Lu, T.-T.; Zhang, Y.-M.; Wei, T.-B. Colorimetric probes designed to provide high sensitivity and single selectivity for CN− in aqueous solution. New J. Chem. 2015, 39, 7206–7210. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Lin, Q.; Wei, T.-B.; Qin, X.-P.; Li, Y. A novel smart organogel which could allow a two channel anion response by proton controlled reversible sol–gel transition and color changes. Chem. Commun. 2009, 45, 6074–6076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shi, B.; Zhang, Y.; Lin, Q.; Yao, H.; You, X.; Wei, T. A selective fluorogenic chemodosimeter for Hg2+ based on the dimerization of desulfurized product. Tetrahedron 2013, 69, 10292–10298. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortis, A.; Moreno, A.; Langa, F. Cycloadditions under microwave irradiation conditions: Methods and applications. Eur. J. Org. Chem. 2000, 2000, 3659–3673. [Google Scholar] [CrossRef]
- de la Cruz, P.; de la Hoz, A.; Langa, F.; Illescas, B.; Martin, N. Cycloadditions to [60] fullerene using microwave irradiation: A convenient and expeditious procedure. Tetrahedron 1997, 53, 2599–2608. [Google Scholar] [CrossRef]
- Langa, F.; de la Cruz, P.; Espíldora, E.; García, J.J.; Pérez, M.C.; de la Hoz, A. Fullerene chemistry under microwave irradiation. Carbon 2000, 38, 1641–1646. [Google Scholar] [CrossRef]
- Langa, F.; de la Cruz, P. Microwave irradiation: An important tool to functionalize fullerenes and carbon nanotubes. Comb. Chem. High Throughput Screen. 2007, 10, 766–782. [Google Scholar] [CrossRef]
- Racanè, L.; Tralić-Kulenović, V.; Boykin, W.D.; Karminski-Zamola, G. Synthesis of New Cyano-Substituted bis-Benzothiazolyl Arylfurans and Arylthiophenes. Molecules 2003, 8, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Malinowski, S. Reaction of Diazo Compounds with Unsaturated Compounds II. Arylation of furfural. Pol. J. Chem 1953, 27, 54–61. [Google Scholar]
- Langa, F.; de la Cruz, P.; Delgado, J.L.; Gómez-Escalonilla, M.J.; González-Cortés, A.; de la Hoz, A.; López-Arza, V. The importance of the linking bridge in donor–C60 electroactive dyads. New J. Chem. 2002, 26, 76–80. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J. Non-Covalent Interactions in the Synthesis and Design of New Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Nakamura, Y.; Minowa, T.; Tobita, S.; Shizuka, H.; Nishimura, J. Synthesis and electronic properties of C60–o-quinodimethane adducts. J. Chem. Soc. Perkin Trans. 2 1995. [Google Scholar] [CrossRef]
- Oswald, F.; Chopin, S.; de la Cruz, P.; Orduna, J.; Garín, J.; Sandanayaka, A.S.D.; Araki, Y.; Ito, O.; Delgado, J.L.; Cousseau, J.; et al. Through-space communication in a TTF–C60–TTF triad. New J. Chem. 2007, 31, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Safaei-Ghomi, J.; Masoomi, R. Grinding-induced synthesis of heterocyclic fullerene derivatives under solvent-free conditions. Chem. Heterocycl. Compd. 2015, 51, 39–43. [Google Scholar] [CrossRef]
Sample Availability: Samples of the synthesized compounds are available from the corresponding authors. |
| Method A a | Method B b | ||||
|---|---|---|---|---|---|
| Entry | Product | Yield c (%) | Recovered C60 % | Yield c (%) | Recovered C60 % |
| 2a | 3a | 15 | 44 | 20 | 30 |
| 2b | 3b | 15 | 50 | 25 | 35 |
| 2c | 3c | 19 | 40 | 30 | 30 |
| 2d | 3d | 20 | 42 | 34 | 33 |
| 2e | 3e | 19 | 43 | 25 | 34 |
| 3a | −1.048 | −1.457 | −1.911 | −2.374 | 0.807 b |
| 3b | −1.055 | −1.451 | −1.993 (−2.106) | −2.416 (−2.581) | 0.755 b |
| 3c | −1.093 | −1.410 (−1.616) | −1.885 | −2.419 | 0.839 b |
| 3d | −1.054 | −1.432 | −1.949 | −2.336 | 0.788 b |
| 3e | −1.087 | −1.477 | −1.961 | −2.316 | 0.710 b |
| C60 | −1.058 | −1.458 | −1.959 | −2.472 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Matar, H.M.; BinSabt, M.H.; Shalaby, M.A. Synthesis and Electrochemistry of New Furylpyrazolino[60]fullerene Derivatives by Efficient Microwave Radiation. Molecules 2019, 24, 4435. https://doi.org/10.3390/molecules24244435
Al-Matar HM, BinSabt MH, Shalaby MA. Synthesis and Electrochemistry of New Furylpyrazolino[60]fullerene Derivatives by Efficient Microwave Radiation. Molecules. 2019; 24(24):4435. https://doi.org/10.3390/molecules24244435
Chicago/Turabian StyleAl-Matar, Hamad M., Mohammad H. BinSabt, and Mona A. Shalaby. 2019. "Synthesis and Electrochemistry of New Furylpyrazolino[60]fullerene Derivatives by Efficient Microwave Radiation" Molecules 24, no. 24: 4435. https://doi.org/10.3390/molecules24244435





