2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities †
Abstract
1. Introduction
2. Results and Discussion
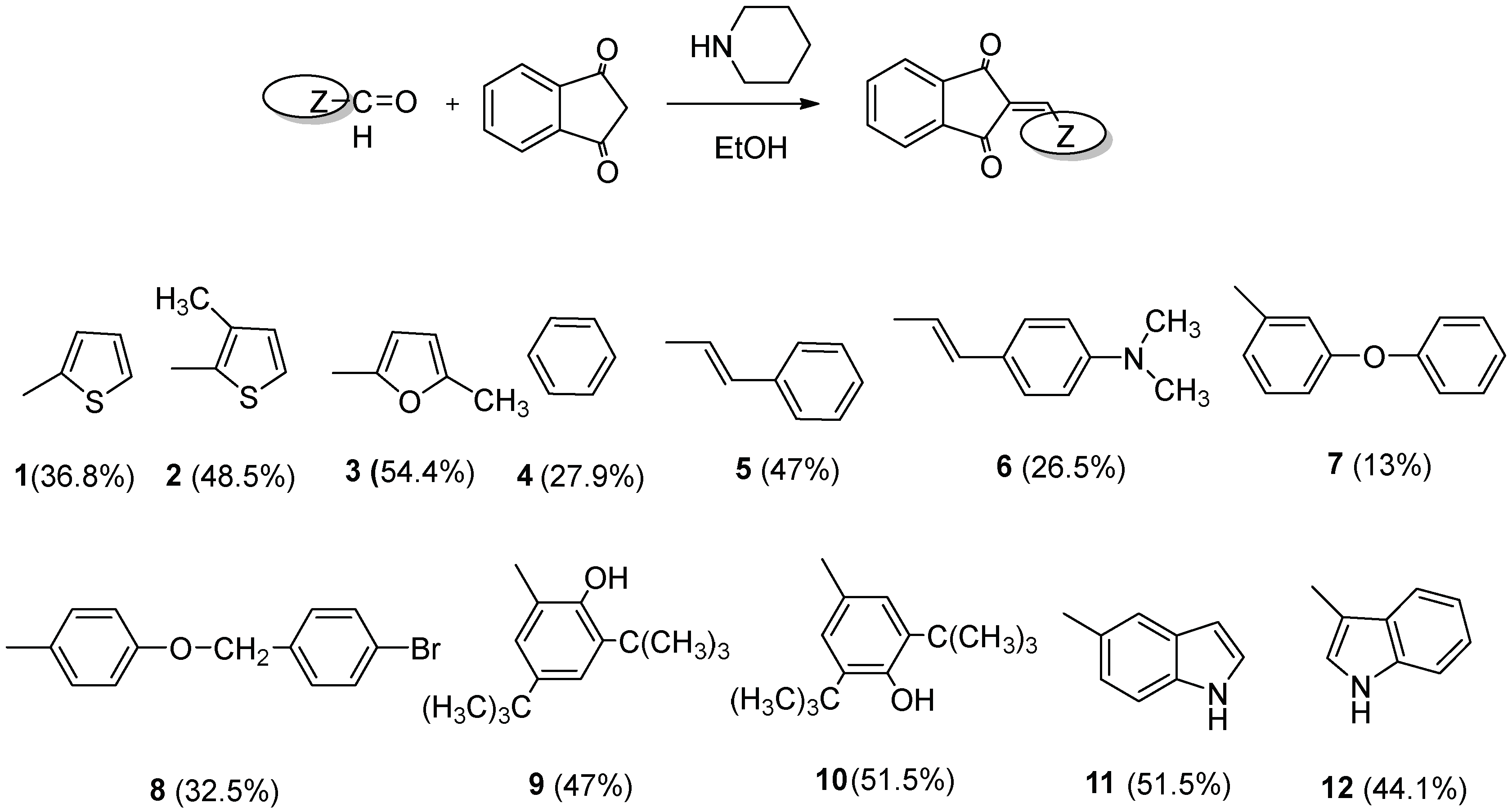
2.1. Chemistry
2.2. Physicochemical Studies
2.2.1. Experimental Determination of Lipophilicity as RM Values
2.2.2. In Silico Determination of Lipophilicity Values as MilogP
2.2.3. Molecular Properties Prediction-Lipinski “Rule of Five”
2.3. Biological Evaluation
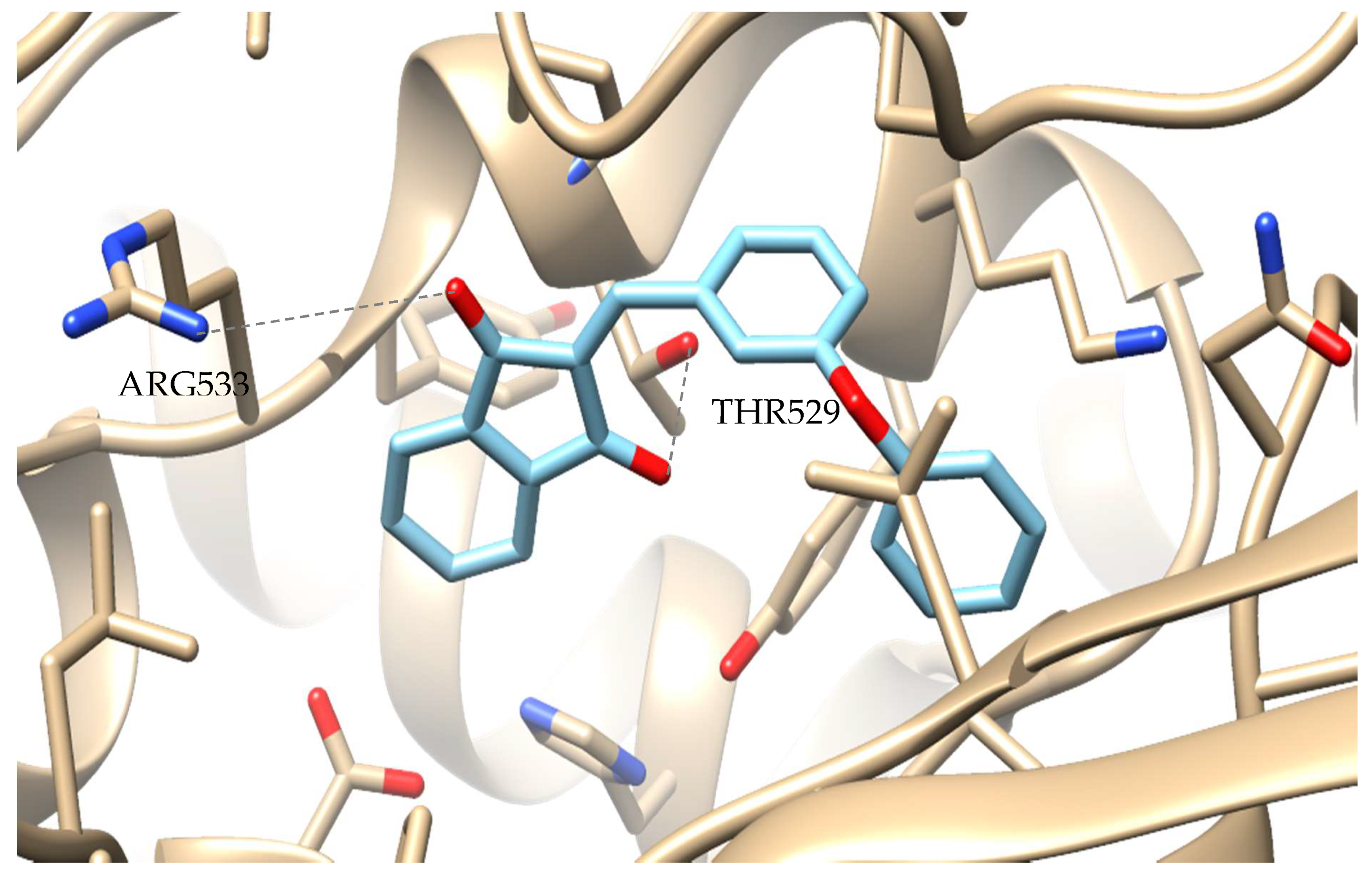
2.4. Computational Studies—Docking Simulation Soybean Lipoxygenase
Molecular Modeling of the Synthesized Derivatives in Soybean LOX
3. Experimental Section
3.1. Materials and Instruments
3.2. Chemistry General Procedure
3.2.1. (Z)-2-(thiophen-3-ylmethylene)-1H-indene-1,3(2H)-dione (1)
3.2.2. (Z)-2-((3-methylthiophen-2-yl)methylene)-1H-indene-1,3(2H)-dione (2)
3.2.3. (Z)-2-((5-methylfuran-2-yl)methylene)-1H-indene-1,3(2H)-dione (3)
3.2.4. 2-benzylidene-1H-indene-1,3(2H)-dione (4)
3.2.5. (Z)-2-(3-phenylallylidene)-1H-indene-1,3(2H)-dione (5)
3.2.6. (Z)-2-(3-(4-(dimethylamino)phenyl)allylidene)-1H-indene-1,3(2H)-dione (6)
3.2.7. (Z)-2-(3-phenoxybenzylidene)-1H-indene-1,3(2H)-dione (7)
3.2.8. (Z)-2-(4-((4-bromobenzyl)oxy)benzylidene)-1H-indene-1,3(2H)-dione (8)
3.2.9. (Z)-2-(3,5-di-tert-butyl-2-hydroxybenzylidene)-1H-indene-1,3(2H)-dione (9)
3.2.10. (Z)-2-(3,5-di-tert-butyl-4-hydroxybenzylidene)-1H-indene-1,3(2H)-dione (10)
3.2.11. (Z)-2-((1H-indol-5-yl)methylene)-1H-indene-1,3(2H)-dione (11)
3.2.12. (Z)-2-((1H-indol-3-yl)methylene)-1H-indene-1,3(2H)-dione (12)
3.3. Physicochemical Studies
3.3.1. Molecular Properties Prediction-Lipinski “Rule of Five”
3.3.2. Determination of RMValues
3.4. Biological Assays
3.4.1. Biological In Vitro Assays
Determination of the Reducing Activity of the Stable Radical 1,1-Diphenyl-picrylhydrazyl (DPPH)
ABTS+•—Decolorization Assay for Antioxidant Activity
Inhibition of Linoleic Acid Peroxidation
Measurement of Superoxide Anion Radical Scavenging Activity
Soybean Lipoxygenase Inhibition Study in Vitro
In Vitro Inhibition of Trypsin Induced Proteolysis
In Vitro Inhibition of Thrombin
3.4.2. Biological in Vivo Assays
Inhibition of the Carrageenin-Induced Edema
3.5. Computational Methods. Molecular Docking Studies on Soybean Lipoxygenase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Calder, P.C. Long-chain fatty acids and inflammation. P. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Smith, W. Eicosanoid nomenclature. Prostaglandins 1989, 38, 125–133. [Google Scholar] [CrossRef]
- Stables, M.J.; Gilroy, D.W. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011, 50, 35–51. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and Cardiovascular Disease: From Pathogenesis to Therapeutic Target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Popović, M.; Smiljanić, K.; Dobutović, B.; Syrovets, T.; Simmet, T.; Isenović, E.R. Thrombin and vascular inflammation. Mol. Cell. Biochem. 2012, 359, 301–313. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution Phase of Inflammation: Novel Endogenous Anti-Inflammatory and Proresolving Lipid Mediators and Pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef]
- Nawroth, P.P.; Stern, D.M. A pathway of coagulation on endothelial cells. J. Cell. Biochem. 1985, 28, 253–264. [Google Scholar] [CrossRef]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes/Metab. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.Y.; Jung, Y.J.; Surh, Y.J.; Lee, S.S.; Park, K.K. Antioxidative and antitumor promoting effects of [6]-paradol and its homologs. Mutat. Res. 2001, 496, 199–206. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girao da Cruz, M.T.; Cordeiro, M.N.; Milhazes, N.; Borges, F.; Marques, M.P. Phenolic acid derivatives with potential anticancer properties--a structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.A.; da Cruz, T.G.; Andrade, J.L.; Milhazes, N.; Borges, F.; Marques, M.P. Anticancer activity of phenolic acids of natural or synthetic origin: A structure-activity study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef]
- Silva, F.A.; Borges, F.; Ferreira, M.A. Effects of phenolic propyl esters on the oxidative stability of refined sunflower oil. J. Agric. Food Chem. 2001, 49, 3936–3941. [Google Scholar] [CrossRef]
- Niki, E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? Febs Lett. 2012, 586, 3767–3770. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Liargkova, T.; Hadjipavlou-Litina, D.J.; Koukoulitsa, C.; Voulgari, E.; Avgoustakis, C. Simple chalcones and bis-chalcones ethers as possible pleiotropic agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 302–313. [Google Scholar] [CrossRef]
- Liargkova, T.; Eleftheriadis, N.; Dekker, F.; Voulgari, E.; Avgoustakis, C.; Sagnou, M.; Mavroidi, B.; Pelecanou, M.; Hadjipavlou-Litina, D. Small Multitarget Molecules Incorporating the Enone Moiety. Molecules 2019, 24, 199. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Nasir Abbas Bukhari, S.; Jasamai, M.; Jantan, I. Synthesis and Biological Evaluation of Chalcone Derivatives (Mini Review). Mini Rev. Med. Chem. 2012, 12, 1394–1403. [Google Scholar]
- Gupta, D.; Jaina, D.K.; Trivedi, P. Recent advances in chalcones as antiinfective agents. Int. J. Chem. Sci. 2010, 8, 649–654. [Google Scholar]
- Rahman, M.A. Chalcone: A Valuable Insight into the Recent Advances and Potential Pharmacological Activities. Chem. Sci. J. 2011, 2011, CSJ-29. [Google Scholar] [CrossRef]
- Sinha, S.; Medhi, B.; Sehga, R. Chalcones as an Emerging Lead Molecule for Antimalarial Therapy: A Review. J. Mod. Med. Chem. 2013, 1, 64–77. [Google Scholar]
- Robert, P.; Marcin, K. Indandione and Its Derivatives - Chemical Compounds with High Biological Potential. Mini-Rev. Med. Chem. 2018, 18, 1321–1330. [Google Scholar]
- Hachiro, S.; Yoshiharu, Y.; Youichi, I.; Yoshiyuki, K. Donepezil Hydrochloride (E2020) and Other Acetylcholinesterase Inhibitors. Cur. Med. Chem. 2000, 7, 303–339. [Google Scholar]
- Leoni, L.M.; Hamel, E.; Genini, D.; Shih, H.; Carrera, C.J.; Cottam, H.B.; Carson, D.A. Indanocine, a microtubule-binding indanone and a selective inducer of apoptosis in multidrug-resistant cancer cells. J. Natl. Cancer Inst. 2000, 92, 217–224. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Kandepu, N.M.; Nazarali, A.J.; Kowalchuk, T.P.; Motaganahalli, N.; Quail, J.W.; Mykytiuk, P.A.; Audette, G.F.; Prasad, L.; Perjési, P.; et al. Conformational and Quantitative Structure−Activity Relationship Study of Cytotoxic 2-Arylidenebenzocycloalkanones. J. Med. Chem. 1999, 42, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Zello, G.A.; Oloo, E.O.; Quail, J.W.; Kraatz, H.-B.; Perjési, P.; Aradi, F.; Takács-Novák, K.; Allen, T.M.; Santos, C.L.; et al. Correlations between Cytotoxicity and Topography of Some 2-Arylidenebenzocycloalkanones Determined by X-ray Crystallography. J. Med. Chem. 2002, 45, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Clercq, E.D.; Balzarini, J.; Dimmock, J.R. Molecular modifications of 2-arylidene-1-indanones leading to increased cytotoxic potencies. J. Enz. Inh. Med. Chem. 2007, 22, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, G.; Nauta, W.T. Effects of anti-inflammatory 2-aryl-1,3-indandiones on oxidative phosphorylation in rat liver mitochondria. Biochem. Pharm. 1975, 24, 815–821. [Google Scholar] [CrossRef]
- Rosini, S.; Trallori, L. Antiinflammatory activity of two phenylindandione derivatives Farmaco Sci. 1976, 31, 403–411. Farmaco Sci. 1976, 31, 403–411. [Google Scholar]
- Rosini, S.; Trallori, L.; Silvestri, S. [Pharmacological study of a series of indandione derivatives proposed as anti-inflammatory agents]. Farm. Sci. 1976, 31, 315–321. [Google Scholar]
- Van der Berg, G.; Bultsma, T.; Nauta, W.T. Inhibition of prostanglandin byosynthesis by 2-aryl-1,3-indandiones. Biochem. Pharm. 1975, 24, 1115–1119. [Google Scholar] [CrossRef]
- Barge, M.; Salunkhe, R. Aqueous extract of Balanites roxburghii fruit: A green dispersant for C-C bond formation. Rsc. Adv. 2014, 4, 31177–31183. [Google Scholar] [CrossRef]
- Hassanein, A.Z.A.E.B. Synthesis and Reaction of Some Indenopyridine and Thieno[2,3-b]Indeno[2,1-e]Pyridine Derivatives. Synth. Commun. 2000, 30, 3883–3895. [Google Scholar]
- Lee, C.-J.; Sheu, C.-N.; Tsai, C.-C.; Wu, Z.-Z.; Lin, W. Direct β-acylation of 2-arylidene-1,3-indandiones with acyl chlorides catalyzed by organophosphanes. Chem. Commun. 2014, 50, 5304–5306. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Velty, A. Activated hydrotalcites as catalysts for the synthesis of chalcones of pharmaceutical interest. J. Catal. 2004, 221, 474–482. [Google Scholar] [CrossRef]
- Lasri, J.; Gajewski, G.; Guedes da Silva, M.F.C.; Kuznetsov, M.L.; Fernandes, R.R.; Pombeiro, A.J.L. Solvent-dependent reactivities of acyclic nitrones with β-diketones: Catalyst-free syntheses of endiones and enones. Tetrahedron 2012, 68, 7019–7027. [Google Scholar] [CrossRef]
- Mondal, A.; Hazra, R.; Grover, J.; Raghu, M.; Ramasastry, S.S.V. Organophosphine-Catalyzed Intramolecular Hydroacylation of Activated Alkynes. Acs Catal. 2018, 8, 2748–2753. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Guo, X.; Cao, L.; Yu, R.; Li, H.; Pan, S. C−H Bond Oxidation Initiated Pummerer- and Knoevenagel-Type Reactions of Benzyl Sulfide and 1,3-Dicarbonyl Compounds. Org. Lett. 2008, 10, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.-J.; Gurubrahamam, R.; Chen, K. Diastereoselective Synthesis of Functionalized Angularly-Fused Tetracycles via an Organocatalytic Quadruple Reaction Sequence. Adv. Synth. Catal. 2017, 359, 1277–1282. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Chagarovskiy, A.O.; Shumsky, A.N.; Krasnobrov, V.D.; Levina, I.I.; Trushkov, I.V. Lewis Acid Triggered Vinylcyclopropane–Cyclopentene Rearrangement. J. Org. Chem. 2018, 83, 543–560. [Google Scholar] [CrossRef]
- Francos, J.; Borge, J.; Díez, J.; García-Garrido, S.E.; Cadierno, V. Easy entry to donor/acceptor butadiene dyes through a MW-assisted InCl3-catalyzed coupling of propargylic alcohols with indan-1,3-dione in water. Catal. Commun. 2015, 63, 10–14. [Google Scholar] [CrossRef]
- Solanke, P.; Pytela, O.; Bureš, F.; Klikar, M. T-shaped D−π−A−(π−A)2 chromophores with two auxiliary electron acceptors. Dye. Pigm. 2019, 162, 755–762. [Google Scholar] [CrossRef]
- Hori, H.; Nagasawa, H.; Ishibashi, M.; Uto, Y.; Hirata, A.; Saijo, K.; Ohkura, K.; Kirk, K.L.; Uehara, Y. TX-1123: An antitumor 2-hydroxyarylidene-4-cyclopentene-1,3-dione as a protein tyrosine kinase inhibitor having low mitochondrial toxicity. Biorg. Med. Chem. 2002, 10, 3257–3265. [Google Scholar] [CrossRef]
- Varache-Béranger, M.; Nuhrich, A.; Amiell, J.; Dufour, P.; Devaux, G. Synthèse et activité anti-inflammatoire de (3,5-di-tert-butyl-4-hydroxybenzylidène) cyclanones et composés apparentés. Eur. J. Med. Chem. 1991, 26, 551–556. [Google Scholar] [CrossRef]
- Bano, B.; Kanwal; Khan, K.M.; Begum, F.; Lodhi, M.A.; Salar, U.; Khalil, R.; Ul-Haq, Z.; Perveen, S. Benzylidine indane-1,3-diones: As novel urease inhibitors; synthesis, in vitro, and in silico studies. Bioorg. Chem. 2018, 81, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Bate-Smith, E.C.; Westall, R.G. Chromatographic behavior and chemical structure in some naturally occurring phenolic substances. Biochim. Biophys. Acta 1950, 4, 427–440. [Google Scholar] [CrossRef]
- Sakuratani, Y.; Kasai, K.; Noguchi, Y.; Yamada, J. Comparison of Predictivities of Log P Calculation Models Based on Experimental Data for 134 Simple Organic Compounds. Qsar. Comb. Sci. 2007, 26, 109–116. [Google Scholar] [CrossRef]
- Canavan, N. FDA and drug companies alike want ADME-tox testing performed earlier and earlier in a drug’s life cycle. Drug Discov. Dev. 2007, 10, 34–36. [Google Scholar]
- Molinspiration Cheminformatics. Available online: www.molinspiration.com (accessed on 20 April 2018).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Rishton, G.M.; LaBonte, K.; Williams, A.J.; Kassam, K.; Kolovanov, E. Computational approaches to the prediction of blood-brain barrier permeability: A comparative analysis of central nervous system drugs versus secretase inhibitors for Alzheimer’s disease. Curr. Opin. Drug Discov. Devel. 2006, 9, 303–313. [Google Scholar]
- Pontiki, E.; Hadjipavlou-Litina, D. Antioxidant and anti-inflammatory activity of aryl-acetic and hydroxamic acids as novel lipoxygenase inhibitors. Med. Chem. 2006, 2, 251–264. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Biorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies. Molecules 2018, 24, 12. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Steczko, J.; Bolin, J.T.; Otwinowski, Z.; Axelrod, B. Crystallographic determination of the active site iron and its ligands in soybean lipoxygenase L-1. Biochemistry 1993, 32, 6320–6323. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Jankun, E.; Amzel, L.M.; Kroa, B.A.; Funk, M.O., Jr. Structure of soybean lipoxygenase L3 and a comparison with its L1 isoenzyme. Proteins 1997, 29, 15–31. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Lipoxygenase inhibitors: A comparative QSAR study review and evaluation of new QSARs. Med. Res. Rev. 2008, 28, 39–117. [Google Scholar] [CrossRef]
- Martin, G.J. Anti-inflammatory effect of trypsin. Ann. N. Y. Acad. Sci. 1957, 68, 70–88. [Google Scholar] [CrossRef]
- Shah, D.; Mital, K. The Role of Trypsin:Chymotrypsin in Tissue Repair. Adv. Ther. 2018, 35, 31–42. [Google Scholar] [CrossRef]
- Ren, P.; Stark, P.Y.; Johnson, R.L.; Bell, R.G. Mechanism of action of anticoagulants: Correlation between the inhibition of prothrombin synthesis and the regeneration of vitamin K1 from vitamin K1 epoxide. J. Pharm. Exp. 1977, 201, 541–546. [Google Scholar]
- Ilić, M.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Ilaš, J.; Kikelj, D. Thrombin inhibitors with lipid peroxidation and lipoxygenase inhibitory activities. Bioorg. Med. Chem. Lett. 2011, 21, 4705–4709. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B.; Denisova, T.; Drozdova, T.; Trepalin, S. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor and Francis: Abingdon-on-Thames, UK, 2005; p. 1024. [Google Scholar]
- Michaelidou, A.; Hadjipavlou-Litina, D.; Matsini, I.; Tsitsogianni, E. Heterocyclic Aryl(Phenyl)Acetic Acid and Aryl Acetohydroxamic Acids as Antiinflammatory -Antioxidant Agents and Inhibitors of Lipoxygenase and Serine Proteases. Med. Chem. 2007, 3, 439–445. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Fiser, A.; Sali, A. Modeller: Generation and refinement of homology-based protein structure models. Methods Enzym. 2003, 374, 461–491. [Google Scholar]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. Bmc Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinf. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the authors. |
| Compd. | milogP a | TPSA b | No Atoms | No O,N c | No OH, NH d | No Violations | No Rotational Bonds e | Volume f | MW g | logBBB h |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.79 | 34.14 | 17 | 2 | 0 | 0 | 1 | 200.71 | 240.28 | 0.255 |
| 2 | 3.16 | 34.14 | 18 | 2 | 0 | 0 | 1 | 217.27 | 254.31 | 0.312 |
| 3 | 2.52 | 47.28 | 18 | 3 | 0 | 0 | 1 | 208.12 | 238.24 | 0.082 |
| 4 | 2.89 | 34.14 | 18 | 2 | 0 | 0 | 1 | 209.99 | 234.25 | 0.270 |
| 5 | 3.64 | 34.14 | 20 | 2 | 0 | 0 | 2 | 237.41 | 260.29 | 0.387 |
| 6 | 3.75 | 37.38 | 23 | 3 | 0 | 0 | 3 | 283.32 | 303.36 | 0.371 |
| 7 | 4.62 | 43.38 | 25 | 3 | 0 | 0 | 3 | 290.39 | 326.35 | 0.446 |
| 8 | 5.35 | 43.38 | 27 | 3 | 0 | 1 | 4 | 325.07 | 419.27 | 0.560 |
| 9 | 5.94 | 54.37 | 27 | 3 | 3 | 1 | 3 | 350.38 | 362.47 | 0.541 |
| 10 | 5.94 | 54.37 | 27 | 3 | 1 | 1 | 3 | 350.38 | 362.47 | 0.541 |
| 11 | 3.09 | 49.93 | 21 | 3 | 1 | 0 | 1 | 238.97 | 273.29 | 0.144 |
| 12 | 3.04 | 49.93 | 21 | 3 | 1 | 0 | 1 | 238.97 | 273.29 | 0.144 |
| Compd. | RM a (±SD) b | EHYDR | E(HOMO) | RA% 50 µM 20 min | RA% 50 µM 60 min | RA% 100 µM 20 min | RA% 100 µM 60 min | RA% 200 µM 20 min | RA% 200 µM 60 min | LOX (% Inhibition 100 µM) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.031 ± 0 | −20.48 | −0.310777 | 2 | 0 | 16 | 16 | 2 | 6 | 32.6 |
| 2 | 0.031 ± 0 | −17.37 | −0.309938 | 2 | 2 | 16 | 16 | 4 | 7 | 45 |
| 3 | 0.014 ± 0 | −7.67 | 0.291110 | 20 | 23 | 37 | 40 | 61 | 75 | 35.7 |
| 4 | −0.615 ± 0.02 | −6.53 | −0.306356 | 5 | 10 | 26 | 32 | 23 | 32 | 35 |
| 5 | 0.158 ± 0 | −6.71 | −0.302600 | 21 | 25 | 44 | 48 | 57 | 72 | 26.4 |
| 6 | −0.527 ± 0 | −9.86 | −0.271983 | 6 | 6 | 22 | 23 | 15 | 18 | 17.7 |
| 7 | −0.196 ± 0.02 | −7.30 | −0.303773 | 27 | 35 | 40 | 50 | 75 | 85 | 67.3 |
| 8 | * | −9.31 | −0.314300 | 4 | 5 | 7 | 7 | 0 | 0 | 3.6 |
| 9 | −1.038 ± 0 | −5.68 | −0.306356 | 4 | 3 | 3 | 1 | 6 | 6 | no |
| 10 | −0.103 ± 0 | −5.71 | −0.314300 | 13 | 17 | 100 | 85 | 71 | 71 | no |
| 11 | −0.196 ± 0.02 | −12.71 | −0.279993 | 2 | 2 | 19 | 19 | 10 | 10 | 38.6 |
| 12 | −0.134 ± 0.02 | −12.72 | −0.279598 | 3 | 3 | 17 | 17 | 4 | 6 | 58.9 |
| NDGA | - | 81 | 83 | 87 | 93 | 94 | 96 | 93 |
| Compd. | AAPH% 100 µΜ | ABTS +. % 100 µΜ | Iptr% 100 µΜ | TH% 100 µΜ | CPE a % |
|---|---|---|---|---|---|
| 1 | 90 | 9 | no | no | 57 ** |
| 2 | 92 | 2 | no | - | 45 * |
| 3 | no | 20 | 17 | 39 | 50 * |
| 4 | no | 19 | 4 | no | 28 * |
| 5 | no | no | no | - | - |
| 6 | 9 | 22 | 29 | 19 | - |
| 7 | 82 | 37 | 33 | no | 41 ** |
| 8 | 79 | 16 | 85 | 100 | 46 * |
| 9 | 96 | no | 98 | 0 | - |
| 10 | 85 | 39 | no | 27 | 42 * |
| 11 | no | 8 | 6 | - | - |
| 12 | 31 | no | no | - | 42 ** |
| Trolox | 93 | 91 | |||
| Indomethacin | 47 | ||||
| Salycilic Acid | 54 | ||||
| Inogatran | 98 |
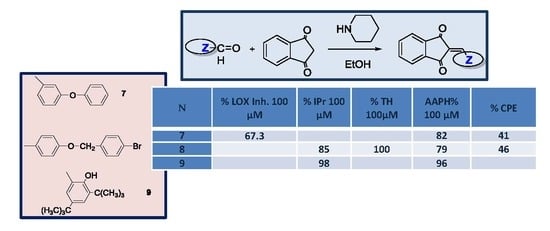
| Compd. | % LOX Inh. 100 μM | % IPr 100 μΜ | % TH 100 μΜ | AAPH% 100 μM | % CPE |
|---|---|---|---|---|---|
| 7 | 67.3 | 82 | 41 | ||
| 8 | 85 | 100 | 79 | 46 | |
| 9 | 98 | 96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouzi, O.; Pontiki, E.; Hadjipavlou-Litina, D. 2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules 2019, 24, 4411. https://doi.org/10.3390/molecules24234411
Kouzi O, Pontiki E, Hadjipavlou-Litina D. 2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules. 2019; 24(23):4411. https://doi.org/10.3390/molecules24234411
Chicago/Turabian StyleKouzi, Olympia, Eleni Pontiki, and Dimitra Hadjipavlou-Litina. 2019. "2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities" Molecules 24, no. 23: 4411. https://doi.org/10.3390/molecules24234411
APA StyleKouzi, O., Pontiki, E., & Hadjipavlou-Litina, D. (2019). 2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules, 24(23), 4411. https://doi.org/10.3390/molecules24234411