Synthesis and Biological Evaluation of BODIPY-PF-543
Abstract
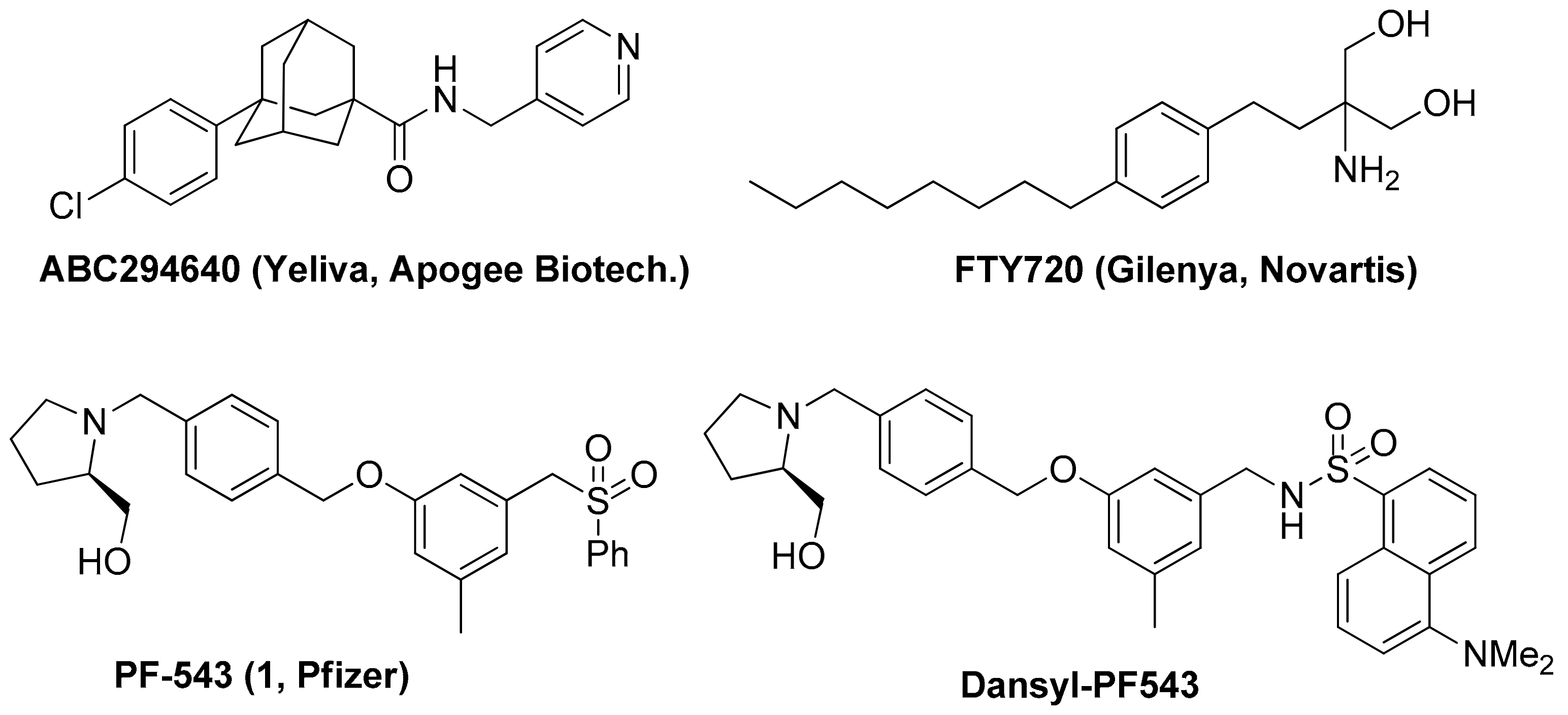
1. Introduction
2. Results and Discussion
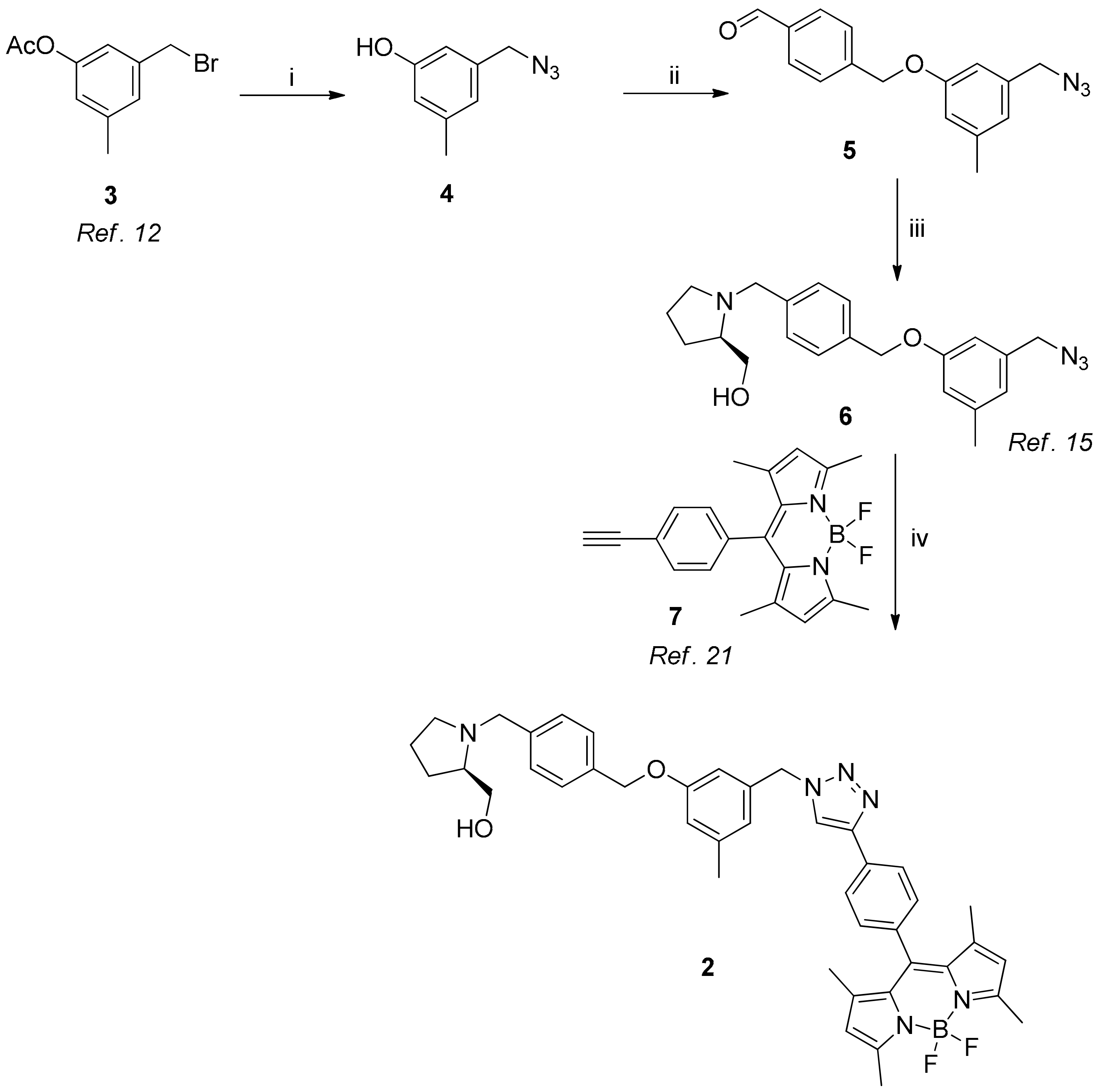
2.1. Chemical Synthesis
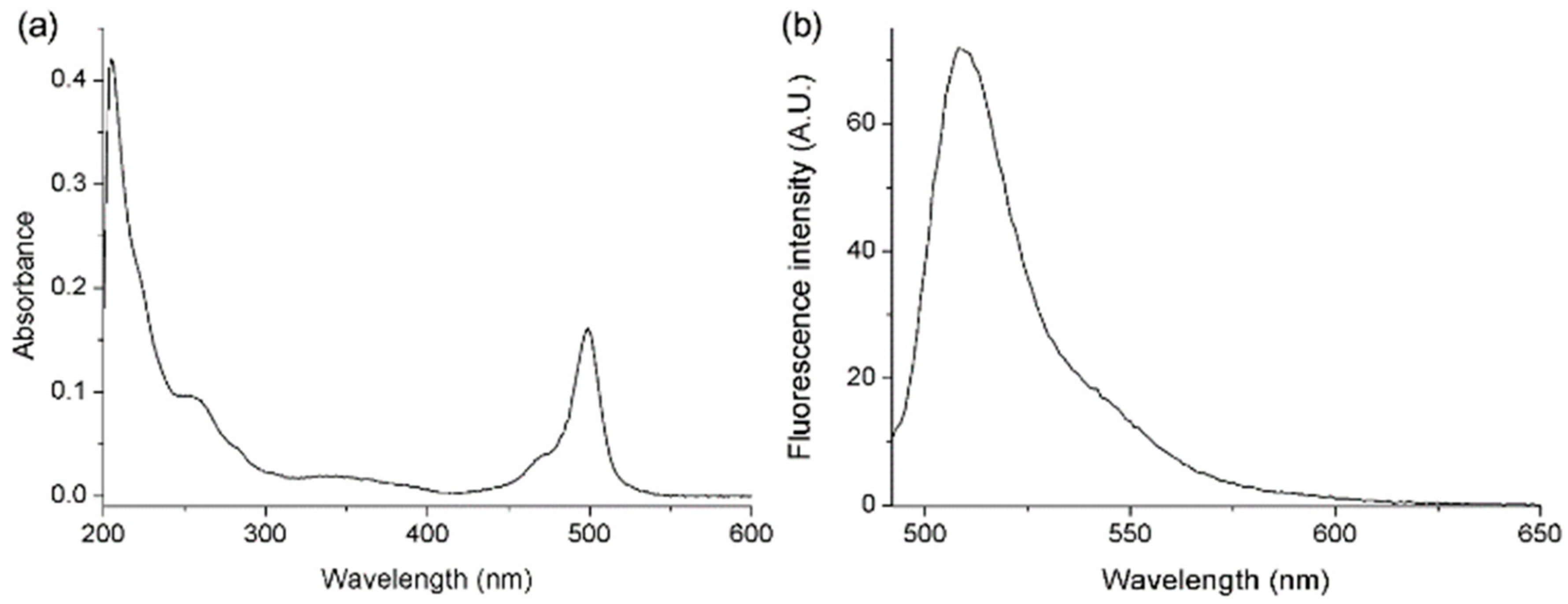
2.2. Absorption and Emission Properties of BODIPY-PF-543
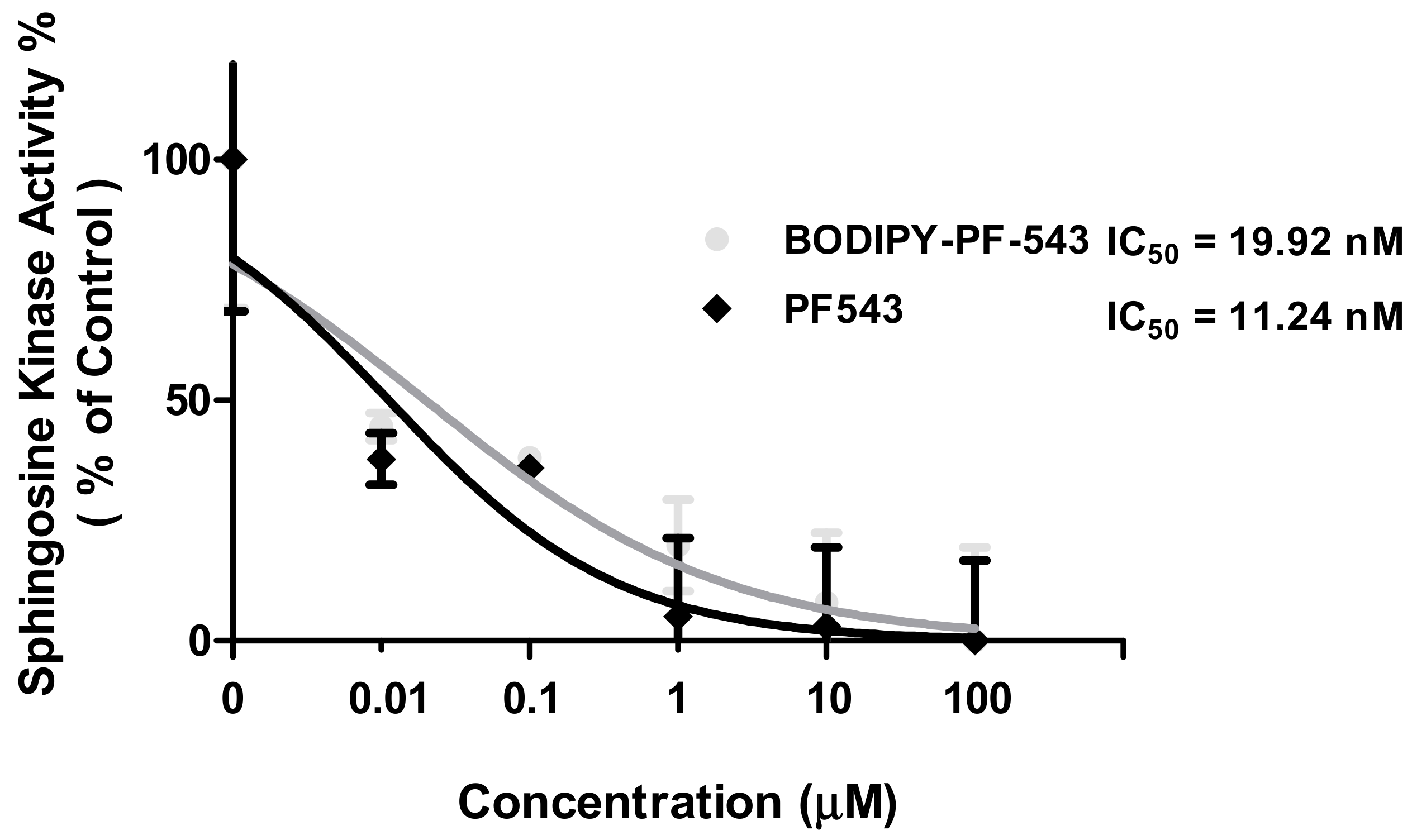
2.3. SK Activity Assay of PF-543 and BODIPY-PF-543
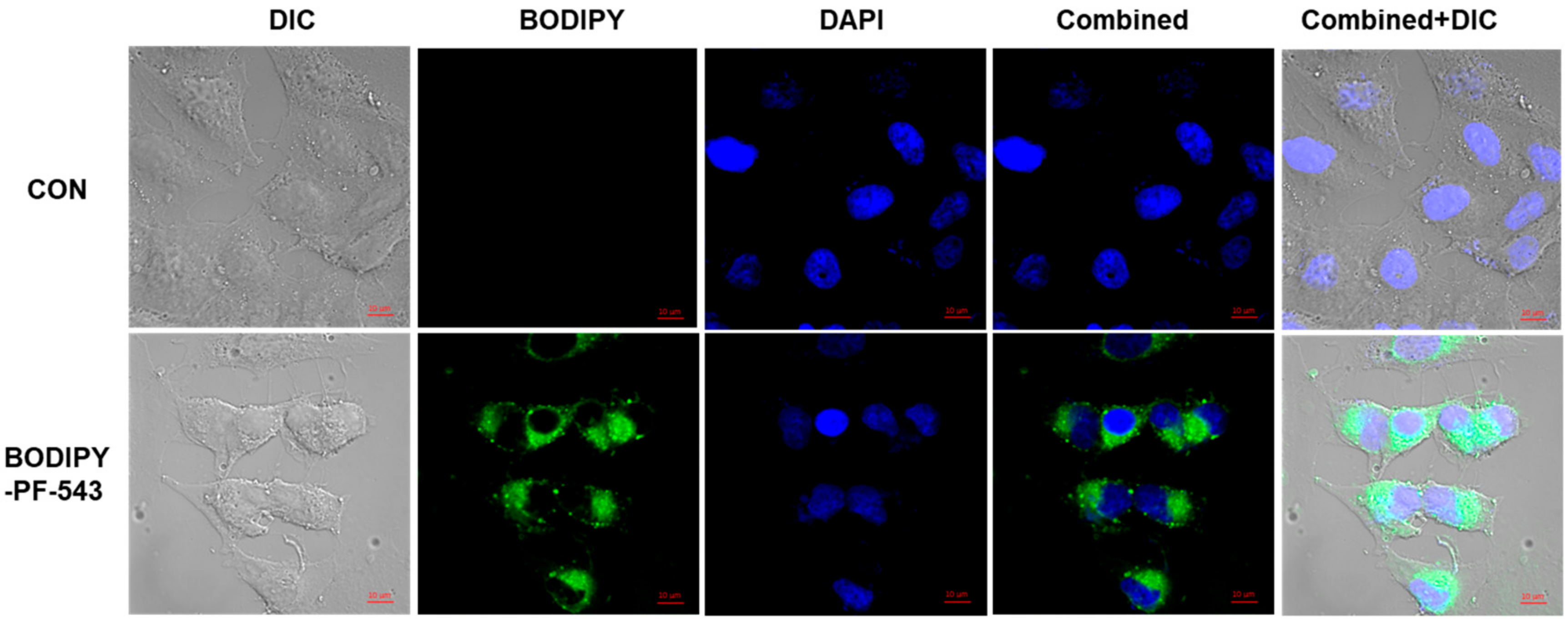
2.4. Confocal Microscopy of BODIPY-PF-543
3. Materials and Methods
3.1. Synthesis in General
3.2. Synthesis
3.3. Absorption and Fluorescence Spectra
3.4. Sphingosine Kinase Activity Assay
3.5. Fluorescence Imaging
3.6. MTT Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Patmanathan, S.N.; Wang, W.; Yap, L.F.; Herr, D.R.; Paterson, I.C. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cell. Signal. 2017, 34, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sanllehi, P.; Abad, J.L.; Casas, J.; Delgado, A. Inhibitors of sphingosine-1-phosphate metabolism (sphingosine kinases and sphingosine-1-phosphate lyase). Chem. Phys. Lipids 2016, 197, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, P.; Kim, S.J.; Upla, P.; Rice, W.J.; Phillips, J.; Timney, B.L.; Pieper, U.; Bonanno, J.B.; Fernandez-Martinez, J.; Hakhverdyan, Z.; et al. Structure, dynamics, evolution, and function of a major scaffold component in the nuclear pore complex. Structure 2013, 21, 560–571. [Google Scholar] [CrossRef]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef]
- Plano, D.; Amin, S.; Sharma, A.K. Importance of sphingosine kinase (SphK) as a target in developing cancer therapeutics and recent developments in the synthesis of novel SphK inhibitors. J. Med. Chem. 2014, 57, 5509–5524. [Google Scholar] [CrossRef]
- White, C.; Alshaker, H.; Cooper, C.; Winkler, M.; Pchejetski, D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget 2016, 7, 23106–23127. [Google Scholar] [CrossRef]
- Cristobal, I.; Madoz-Gurpide, J.; Manso, R.; Gonzalez-Alonso, P.; Rojo, F.; Garcia-Foncillas, J. Potential anti-tumor effects of FTY720 associated with PP2A activation: a brief review. Curr. Med. Res. Opin. 2016, 32, 1137–1141. [Google Scholar] [CrossRef]
- Takasaki, T.; Hagihara, K.; Satoh, R.; Sugiura, R. More than Just an Immunosuppressant: The Emerging Role of FTY720 as a Novel Inducer of ROS and Apoptosis. Oxid. Med. Cell. Longev. 2018, 2018, 4397159. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.D.; Ji, X.J.; Cong, Z.X.; Zhu, J.H.; Zhou, Y. FTY720 for cancer therapy (Review). Oncol. Rep. 2013, 30, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, W.; Ren, L.; Liu, J.; Pang, X.; Chen, X.; Kang, D.; Wang, J.; Du, G. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol. Ther. 2019, 195, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Schnute, M.E.; McReynolds, M.D.; Kasten, T.; Yates, M.; Jerome, G.; Rains, J.W.; Hall, T.; Chrencik, J.; Kraus, M.; Cronin, C.N.; et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem. J. 2012, 444, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Schnute, M.E. Discovery of a Potent and Selective Sphingosine Kinase 1 Inhibitor through the Molecular Combination of Chemotype-Distinct Screening Hits. J. Med. Chem. 2017, 60, 2562–2572. [Google Scholar] [CrossRef]
- Pitman, M.R.; Costabile, M.; Pitson, S.M. Recent advances in the development of sphingosine kinase inhibitors. Cell. Signal. 2016, 28, 1349–1363. [Google Scholar] [CrossRef]
- Park, E.Y.; Lee, T.; Oh, Y.S.; Lee, J.Y.; Shrestha, J.; Hong, S.W.; Jin, Y.J.; Jo, G.; Kim, S.; Hwang, G.T.; et al. Synthesis of dansyl labeled sphingosine kinase 1 inhibitor. Chem. Phys. Lipids 2018, 215, 29–33. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. Engl. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Marfin, Y.S.; Solomonov, A.V.; Timin, A.S.; Rumyantsev, E.V. Recent Advances of Individual BODIPY and BODIPY-Based Functional Materials in Medical Diagnostics and Treatment. Curr. Med. Chem. 2017, 24, 2745–2772. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Worrell, B.L.; Brown, A.M. In Silico Characterization of Structural Distinctions between Isoforms of Human and Mouse Sphingosine Kinases for Accelerating Drug Discovery. J. Chem. Inf. Model 2019, 59, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bittman, R. Synthesis and spectral properties of cholesterol- and FTY720-containing boron dipyrromethene dyes. J. Org. Chem. 2007, 72, 8376–8382. [Google Scholar] [CrossRef] [PubMed]
- Stegemeyer, H. Photoluminescence of Solutions. Von C. A. Parker. Elsevier Publishing Co., Amsterdam-London-New York 1968. 1. Aufl., XVI, 544 S., 188 Abb., 53 Tab., geb. Dfl. 85,–. Angew. Chem. 1969, 81, 1007–1008. [Google Scholar] [CrossRef]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H., Jr.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef]
- Marks, D.L.; Bittman, R.; Pagano, R.E. Use of Bodipy-labeled sphingolipid and cholesterol analogs to examine membrane microdomains in cells. Histochem. Cell Biol. 2008, 130, 819–832. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, J.; Hwang, G.T.; Lee, T.; Kim, S.W.; Oh, Y.S.; Kwon, Y.; Hong, S.W.; Kim, S.; Seop Moon, H.; Baek, D.J.; et al. Synthesis and Biological Evaluation of BODIPY-PF-543. Molecules 2019, 24, 4408. https://doi.org/10.3390/molecules24234408
Shrestha J, Hwang GT, Lee T, Kim SW, Oh YS, Kwon Y, Hong SW, Kim S, Seop Moon H, Baek DJ, et al. Synthesis and Biological Evaluation of BODIPY-PF-543. Molecules. 2019; 24(23):4408. https://doi.org/10.3390/molecules24234408
Chicago/Turabian StyleShrestha, Jitendra, Gil Tae Hwang, Taeho Lee, Seon Woong Kim, Yoon Sin Oh, Yongseok Kwon, Seung Woo Hong, Sanghee Kim, Hong Seop Moon, Dong Jae Baek, and et al. 2019. "Synthesis and Biological Evaluation of BODIPY-PF-543" Molecules 24, no. 23: 4408. https://doi.org/10.3390/molecules24234408
APA StyleShrestha, J., Hwang, G. T., Lee, T., Kim, S. W., Oh, Y. S., Kwon, Y., Hong, S. W., Kim, S., Seop Moon, H., Baek, D. J., & Park, E.-Y. (2019). Synthesis and Biological Evaluation of BODIPY-PF-543. Molecules, 24(23), 4408. https://doi.org/10.3390/molecules24234408





