Synthesis and Optoelectronic Characterization of Perylene Diimide-Quinoline Based Small Molecules
Abstract
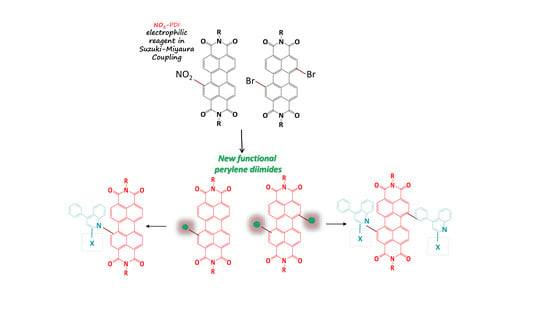
1. Introduction
2. Results and Discussion
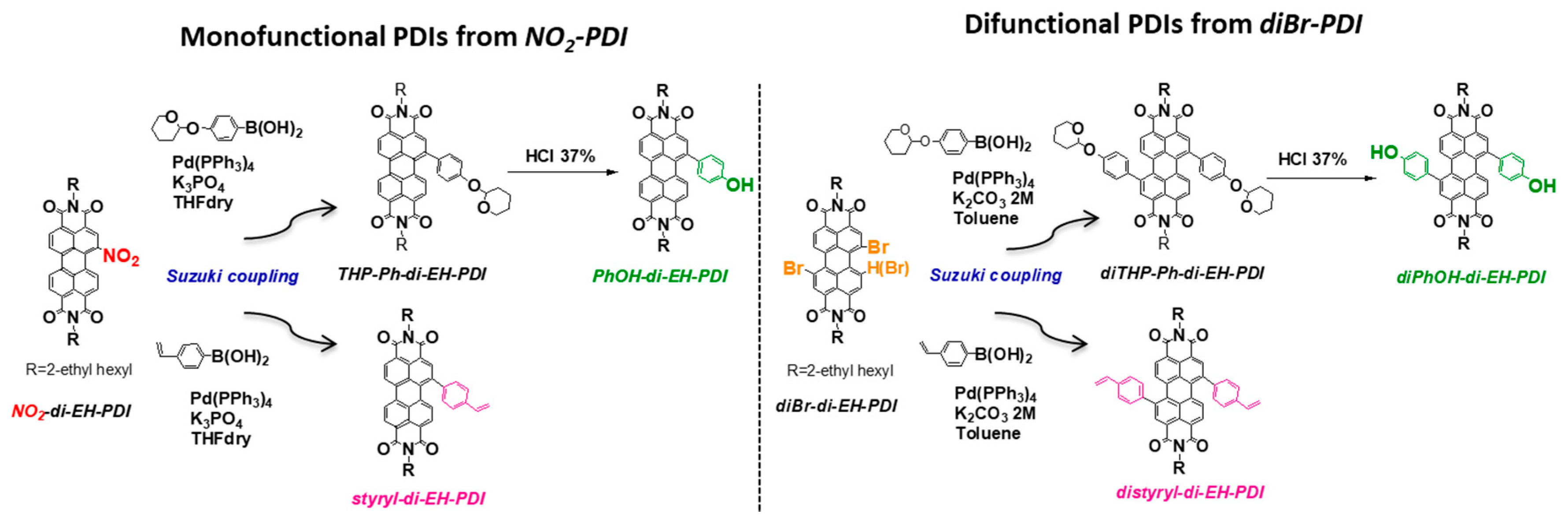
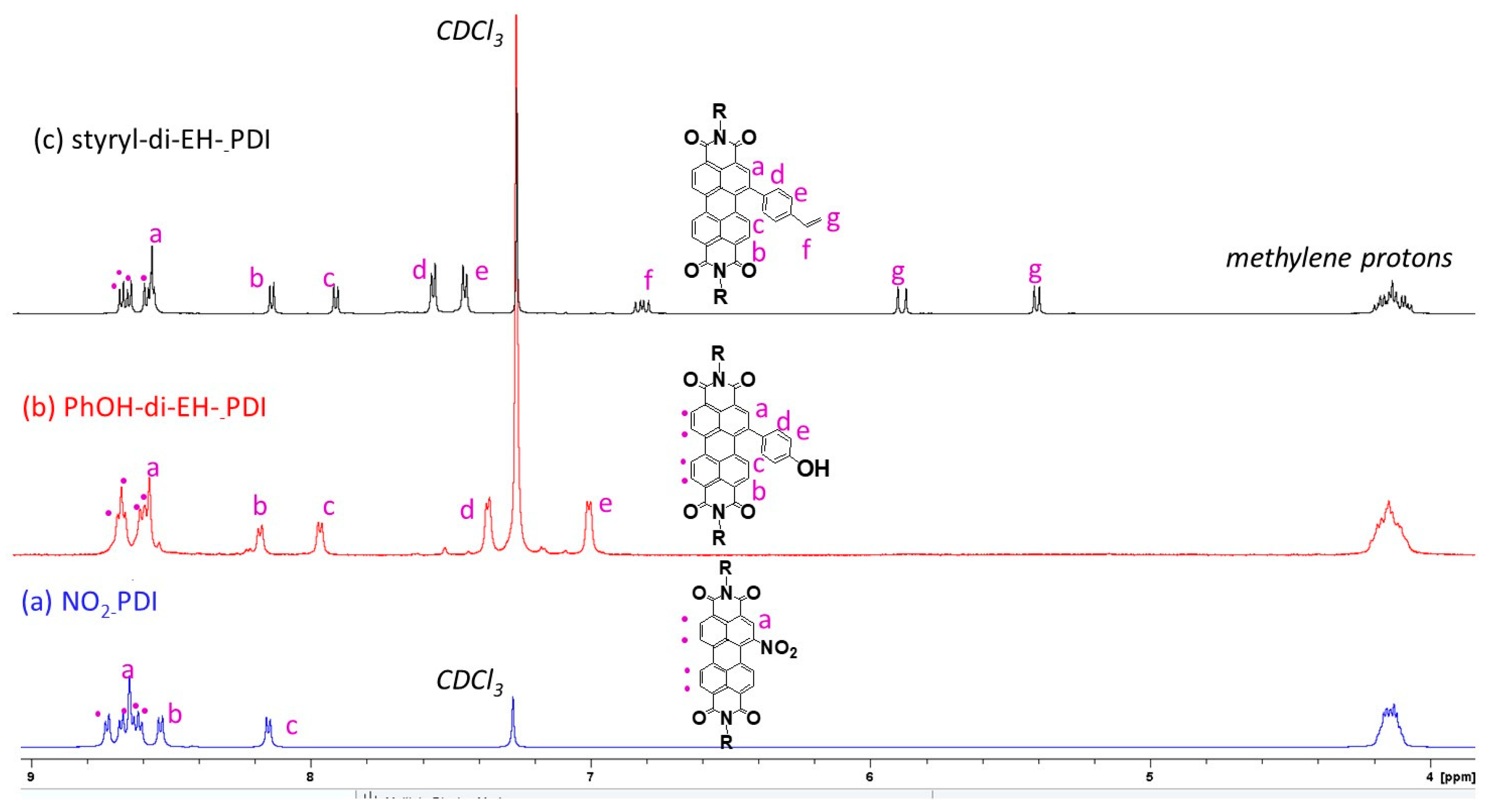
2.1. Synthesis of Mono and Difunctional Perylene Diimide Derivatives
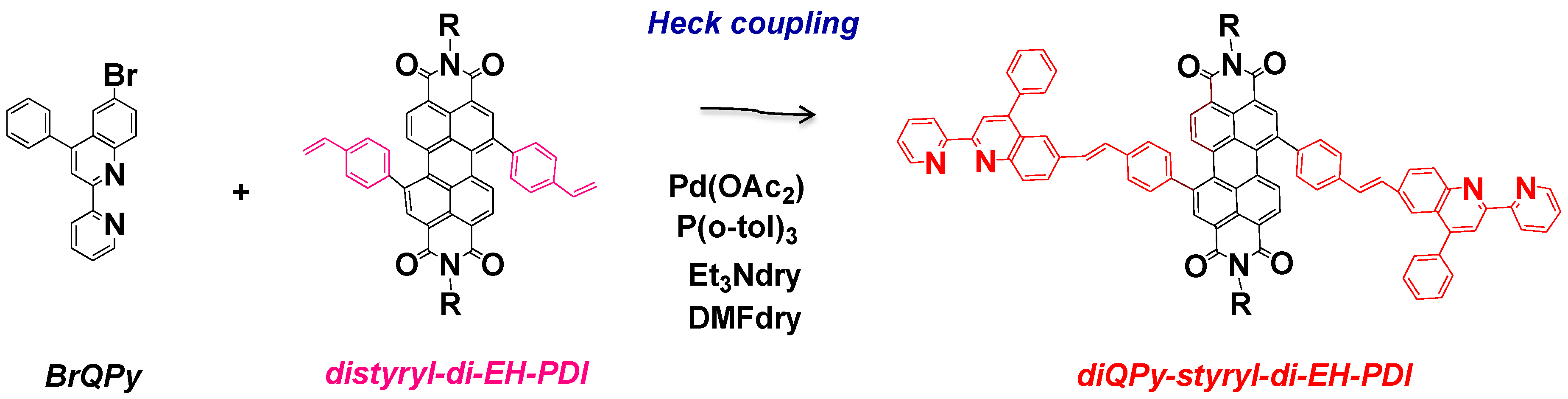
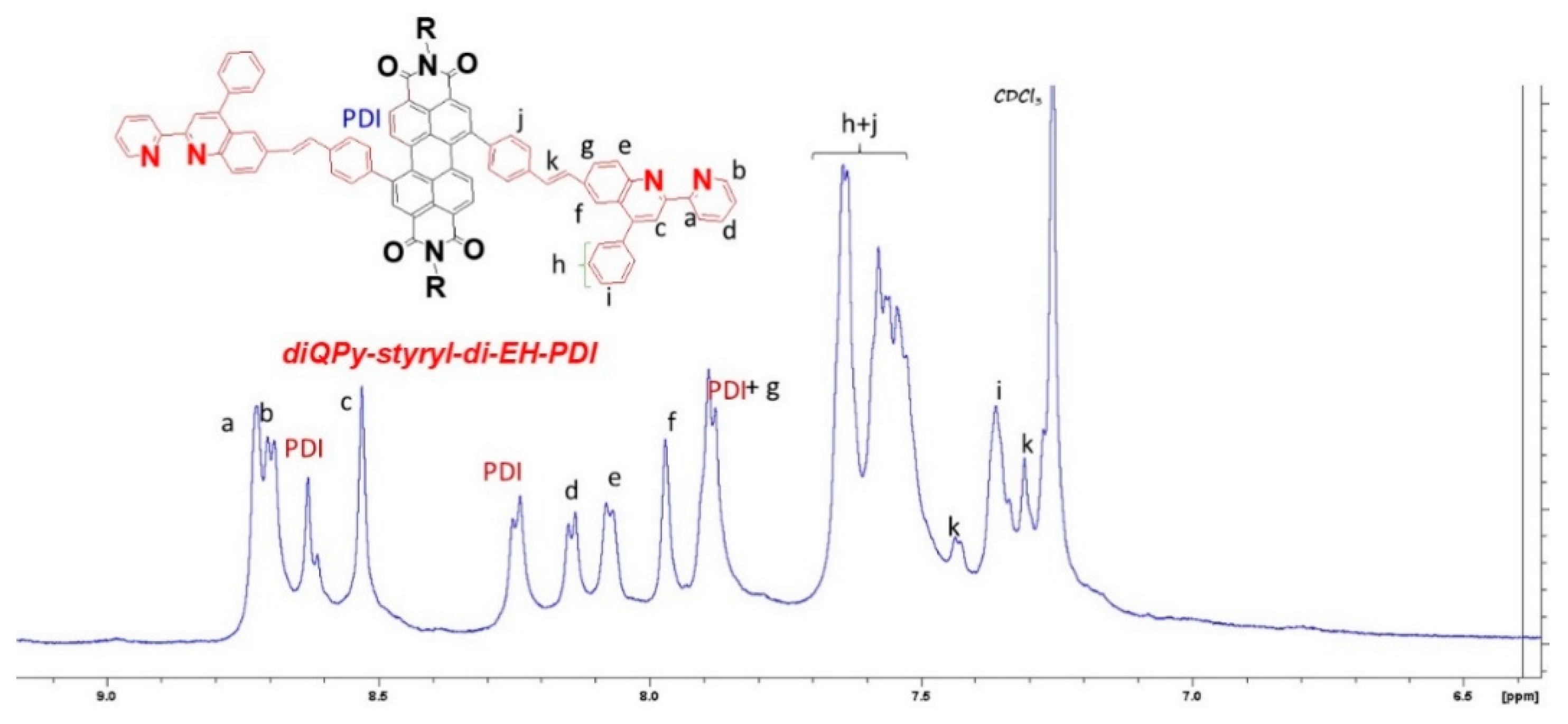
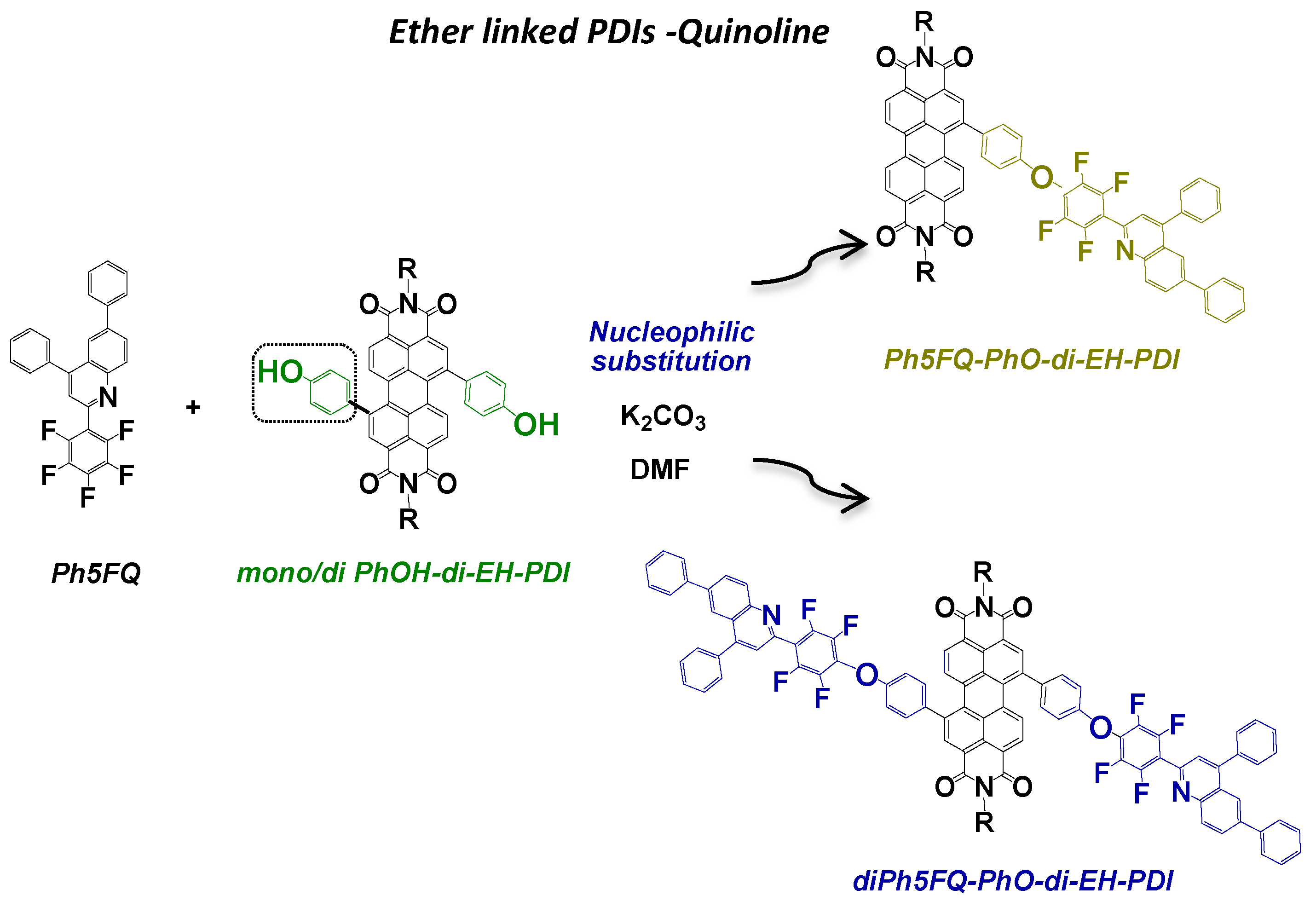
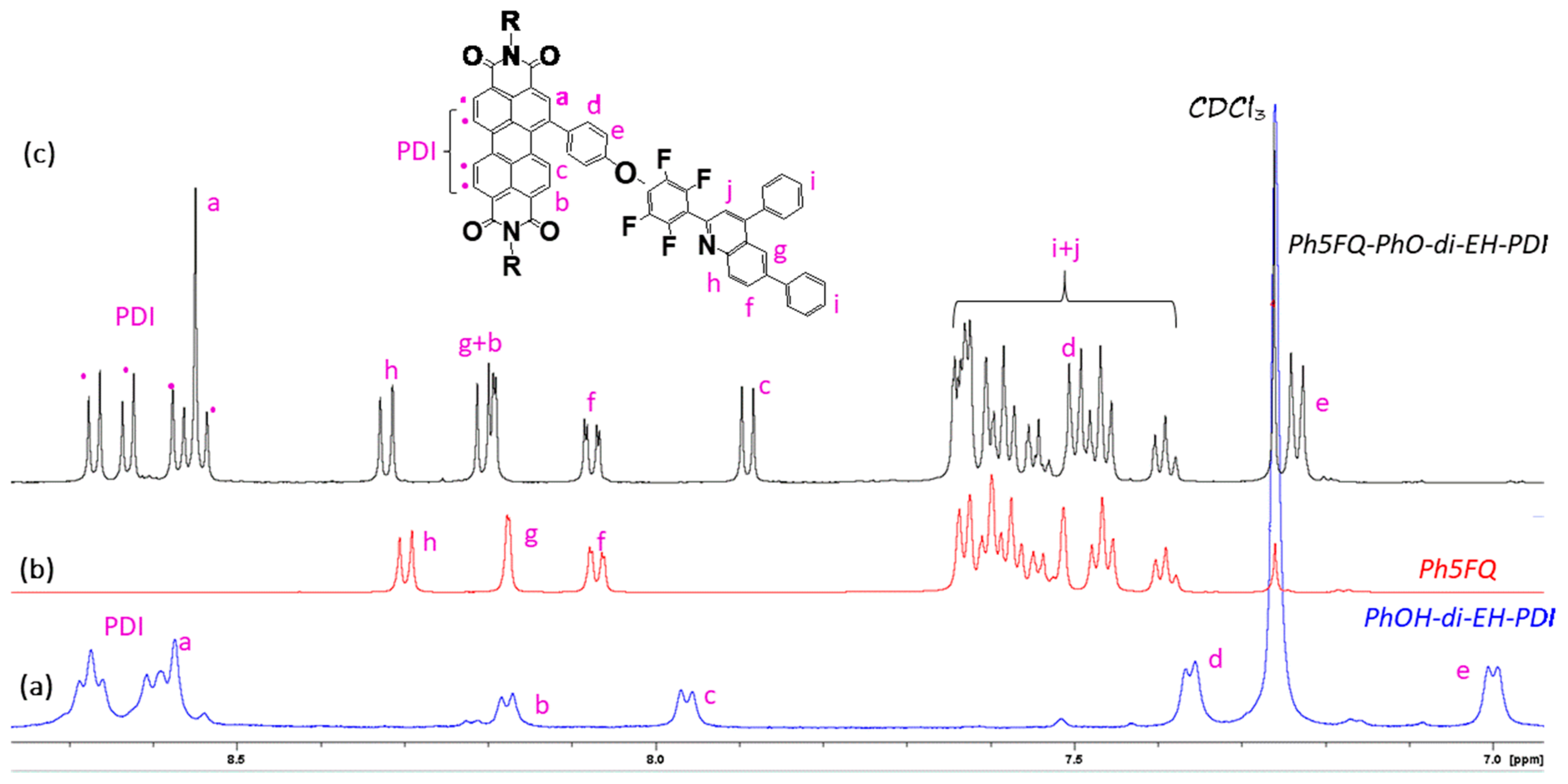
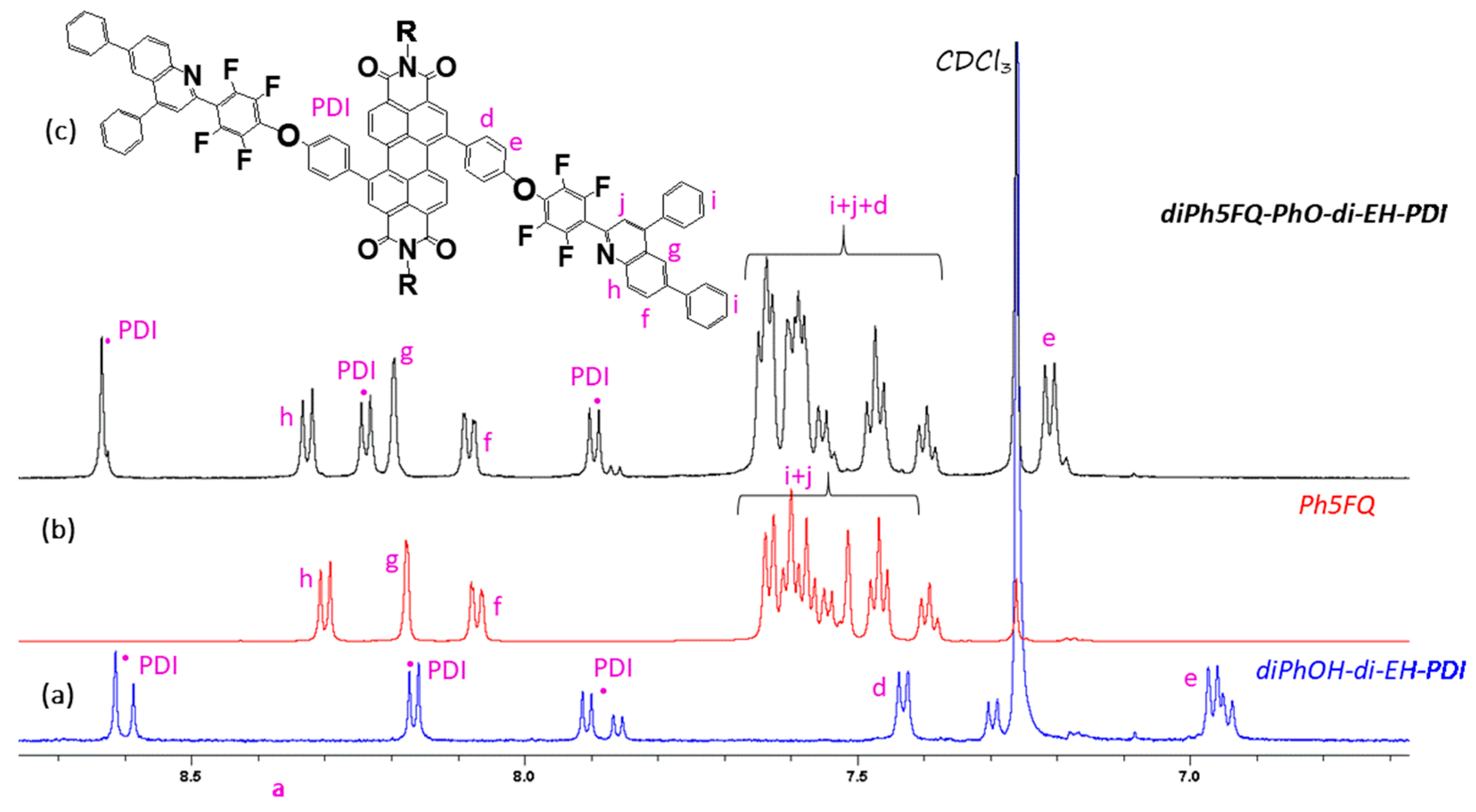
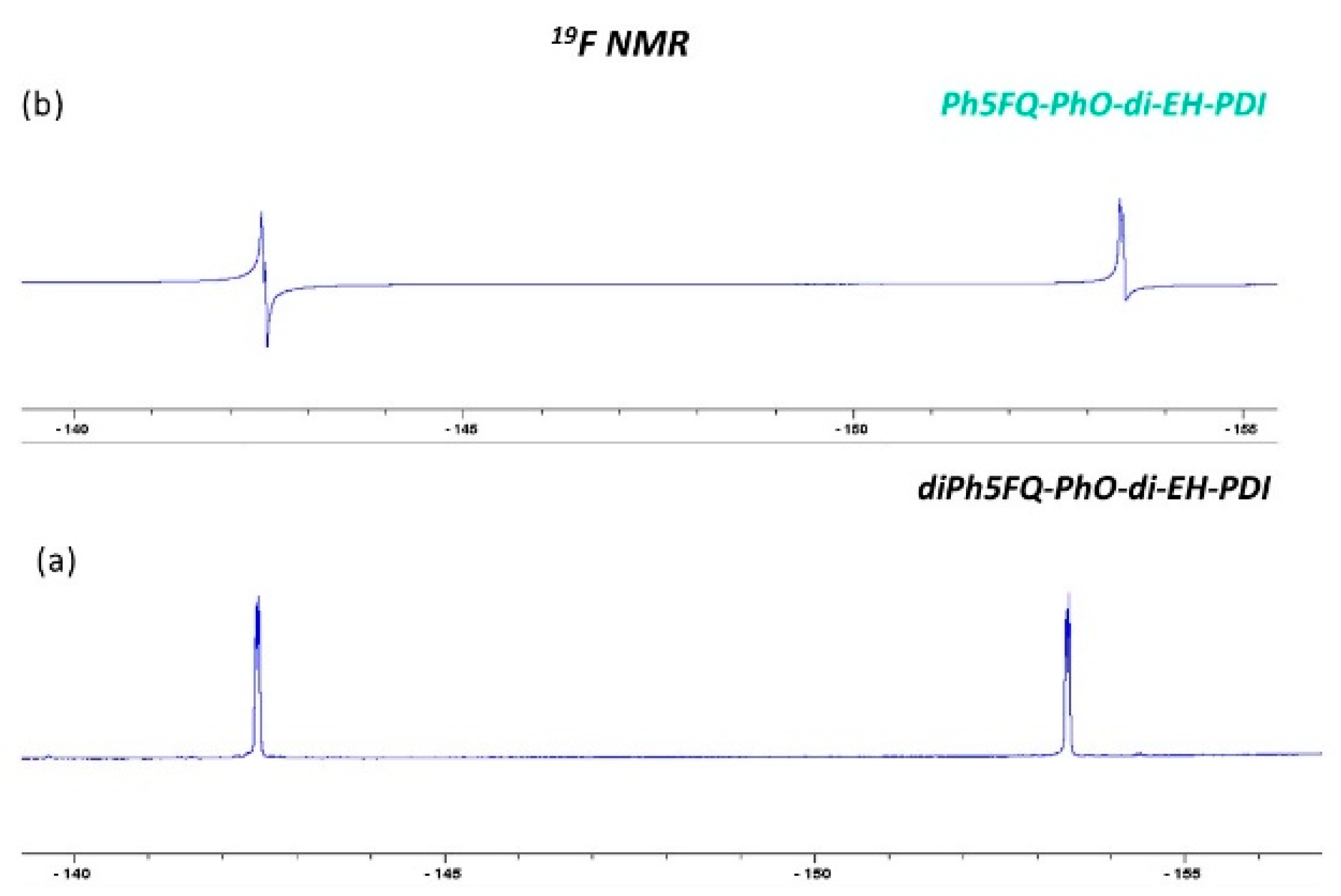
2.2. Synthesis of Quinoline Bay-Substituted Perylene Diimide Derivatives
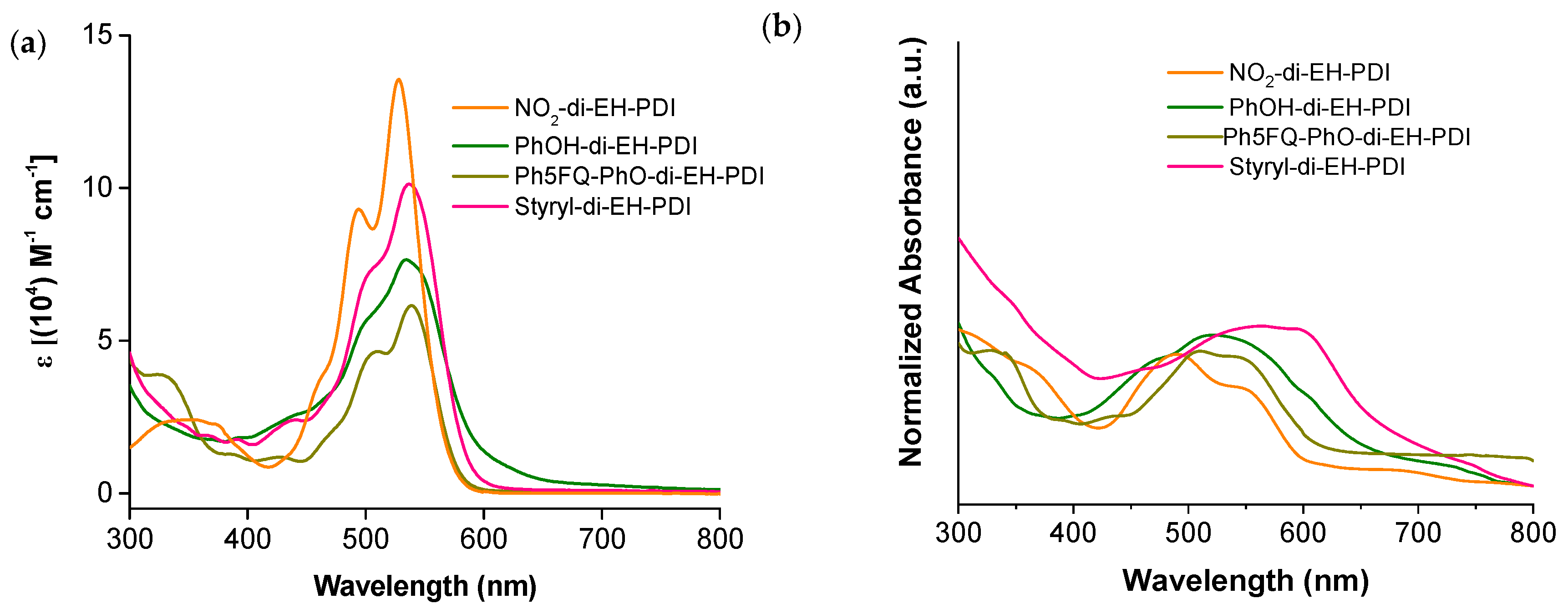
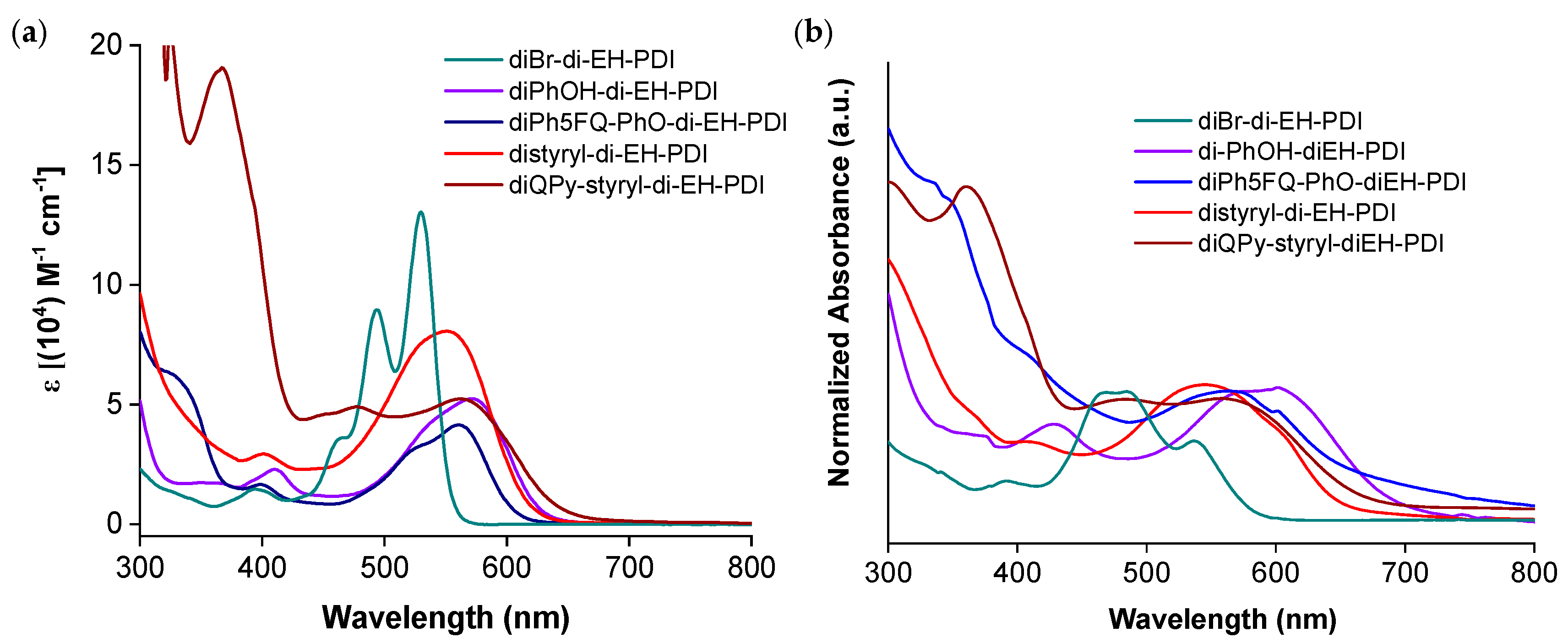
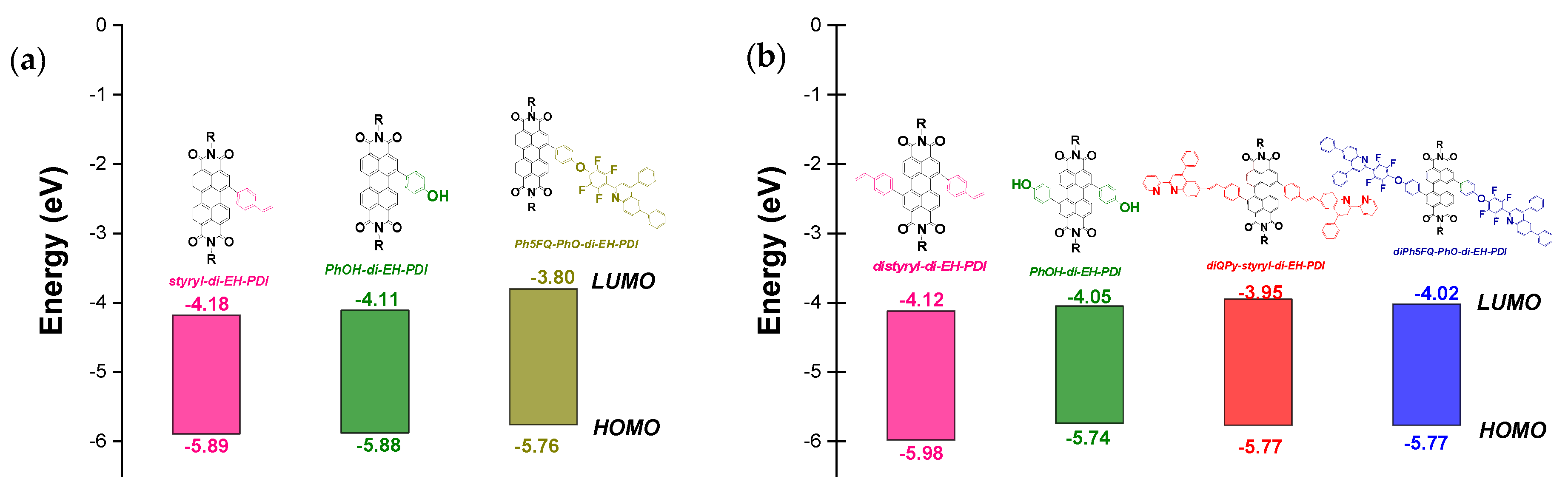
2.3. Optical and Electrochemical Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis of Functional Perylene Diimide Molecules and Perylene-Quinoline Based Molecules.
3.3.1. Synthesis of Styryl-di-EH-PDI
3.3.2. Synthesis of PhOH-di-EH-PDI
3.3.3. Synthesis of diPhOH-di-EH-PDI
3.3.4. Synthesis of distyryl-di-EH-PDI
3.3.5. Synthesis of diQPy-styryl-di-EH-PDI
3.3.6. Synthesis of Ph5FQ-PhO-di-EH-PDI
3.3.7. Synthesis of di5FQ-PhO-di-EH-PDI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, C.; Wonneberger, H. Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-Assembly of Perylene Imide Molecules into 1D Nanostructures: Methods, Morphologies, and Applications. Chem. Rev. 2015, 115, 1196–11998. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; Yin, Y.; Zhang, Y.; Zhou, E.; Guoa, F.; Zhao, L. Novel perylene diimide-based polymers with electron-deficient segments as the comonomer for efficient all-polymer solar cells. J. Mater. Chem. A 2018, 6, 414–422. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, T.; Cheng, W.; Wu, K.; Xie, D.; Huo, L.; Sun, Y.; Yang, C. A three-dimensional thiophene-annulated perylene bisimide as a fullerene-free acceptor for a high performance polymer solar cell with the highest PCE of 8.28% and a VOC over 1.0 V. J. Mater. Chem. C 2018, 6, 1136–1142. [Google Scholar] [CrossRef]
- Ego, C.; Marsitzky, D.; Becker, S.; Zhang, J.; Grimsdale, A.C.; Müllen, K.; MacKenzie, J.D.; Silva, C.; Friend, R.H. Attaching Perylene Dyes to Polyfluorene: Three Simple, Efficient Methods for Facile Color Tuning of Light-Emitting Polymers. J. Am. Chem. Soc. 2011, 125, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Stolte, M. Naphthalene and perylene diimides for organic transistors. Chem. Commun. 2011, 47, 5109–5115. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Oh, J.H.; Sun, Y.S.; Deppisch, M.; Krause, A.M.; Radacki, K.; Braunschweig, H.; Könemann, M.; Erk, P.; Bao, Z.; et al. High-Performance Air-Stable n-Channel Organic Thin Film Transistors Based on Halogenated Perylene Bisimide Semiconductors. J. Am. Chem. Soc. 2009, 131, 6215–6228. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Silicon MOS Optoelectronic Micro-Nano Structure Based on Reverse-Biased PN Junction. Phys. Status Solidi A 2019, 216, 1800868. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, E. Development of Polymer Acceptors for Organic Photovoltaic Cells. Polymers 2014, 6, 382–407. [Google Scholar] [CrossRef]
- Welsh, T.A.; Laventure, A.; Welch, G.C. Direct (Hetero) Arylation for the Synthesis of Molecular Materials: Coupling Thieno [3,4-c]pyrrole-4,6-dione with Perylene Diimide to Yield Novel Non-Fullerene Acceptors for Organic Solar Cells. Molecules 2018, 23, 931. [Google Scholar] [CrossRef]
- Hou, J.; Inganäs, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Króla, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic Acid Diimides: Synthesis, Physical Properties, and Use in Organic Electronics. J.Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Lu, Q.; Qiu, M.; Zhao, M.; Li, Z.; Li, Y. Modification of NFA-conjugated Bridges with symmetric structures for High-efficiency Non-fullerene PSCs. Polymers 2019, 11, 958. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; et al. Fast Charge Separation in a Non-Fullerene Organic Solar Cell with a Small Driving Force. Nat. Energy 2016, 1, 16089. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Huang, J.; Hu, H.; Zhang, G.; Ma, T.; Chow, P.C.Y.; Ade, H.; Pan, D.; Yan, H. Ring-Fusion of Perylene Diimide Acceptor Enabling Efficient Nonfullerene Organic Solar Cells with a Small Voltage Loss. J. Am. Chem. Soc. 2017, 139, 16092–16095. [Google Scholar] [CrossRef]
- Rajaram, S.; Shivanna, R.; Kandappa, S.K.; Narayan, K.S. Nonplanar Perylene Diimides as Potential Alternatives to Fullerenes in Organic Solar Cells. J. Phys. Chem. Lett. 2012, 3, 2405–2408. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Chi, Y.; Yu, H.; Li, Y.; Jiang, T.; Wei, X.; Shi, J. Self-assembly, optical and electrical properties of perylene diimide dyes bearing unsymmetrical substituents at bay position. Sci. Rep. 2018, 8, 8208. [Google Scholar] [CrossRef]
- Hartnett, P.E.; Timalsina, A.; Matte, H.S.; Zhou, N.; Guo, X.; Zhao, W.; Facchetti, A.; Chang, R.P.; Hersam, M.C.; Wasielewski, M.R.; et al. Slip-stacked perylene diimides as an alternative strategy for high efficiency non fullerene acceptors in organic photovoltaics. J. Am. Chem. Soc. 2014, 136, 16345–16356. [Google Scholar] [CrossRef] [PubMed]
- Cann, J.; Dayneko, S.; Sun, J.-P.; Hendsbee, A.D.; Hill, I.G.; Welch, G.C. N-Annulated perylene diimide dimers: Acetylene linkers as a strategy for controlling structural conformation and the impact on physical, electronic, optical and photovoltaic properties. J. Mater. Chem. C 2017, 5, 2074–2083. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Holliday, S.; Chen, H.Y.; Cryer, S.J.; McCulloch, I. Non-Fullerene Electron Acceptors for Use in Organic Solar Cells. Acc. Chem. Res. 2015, 48, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Aluicio-Sarduy, E.; Kan, Z.; Ye, T.; MacKenzie, R.C.I.; Keivanidis, P.E. Fullerene-Free Organic Solar Cells with an Efficiency of 3.7% Based on a Low-Cost Geometrically Planar Perylene Diimide Monomer. J. Mater. Chem. A 2014, 2, 14348–14353. [Google Scholar] [CrossRef]
- Venkateswararao, A.; Liua, S.-W.; Wong, K.-T. Organic polymeric and small molecular electron acceptors for organic solar cells. Mater. Sci. Eng. R 2018, 124, 1–57. [Google Scholar]
- McAfee, S.M.; Dayneko, S.V.; Hendsbee, A.D.; Josse, P.; Blanchard, P.; Cabanetos, C.; Welch, G.C. Applying direct heteroarylation synthesis to evaluate organic dyes as the core component in PDI-based molecular materials for fullerene-free organic solar cells. J. Mater. Chem. A 2017, 5, 11623–11633. [Google Scholar] [CrossRef]
- Cai, Y.; Huo, L.; Sun, X.; Wei, D.; Tang, M.; Sun, Y. High Performance Organic Solar Cells Based on a Twisted Bay-Substituted Tetraphenyl Functionalized Perylenediimide Electron Acceptor. Adv. Energy Mater. 2015, 5, 1500032. [Google Scholar] [CrossRef]
- Han, H.; Ma, L.K.; Zhang, L.; Guo, Y.; Li, Y.; Yu, H.; Ma, W.; Yan, H.; Zhao, D. Tweaking the Molecular Geometry of a Tetraperylenediimide Acceptor. ACS Appl. Mater. Interfaces 2019, 11, 6970–6977. [Google Scholar] [CrossRef]
- Lin, H.; Chen, S.; Hu, H.; Zhang, L.; Ma, T.; Lai, J.Y.L.; Li, Z.; Qin, A.; Huang, X.; Tang, B.; et al. Reduced Intramolecular Twisting Improves the Performance of 3D Molecular Acceptors in Non-Fullerene Organic Solar Cells. Adv. Mater. 2016, 28, 8546–8551. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Xia, S.; Zhou, F.; Luo, Z.; Luo, J.; He, F.; Yang, C. 9,9′-Bifluorenylidene-Core Perylene Diimide Acceptors for As-Cast Non-Fullerene Organic Solar Cells: The Isomeric Effect on Optoelectronic Properties. Chem. Eur. J. 2018, 24, 4149–4156. [Google Scholar] [CrossRef]
- Yadav, M.R.; Nagaoka, M.; Kashihara, M.; Zhong, R.L.; Miyazaki, T.; Sakaki, S.; Nakao, Y. The Suzuki–Miyaura Coupling of Nitroarenes. J. Am. Chem. Soc. 2017, 139, 9423–9426. [Google Scholar] [CrossRef] [PubMed]
- Stefopoulos, A.A.; Kourkouli, S.N.; Economopoulos, S.; Ravani, F.; Andreopoulou, A.K.; Papagelis, K.; Siokou, A.; Kallitsis, J.K. Polymer and hybrid electron accepting materials based on a semiconducting perfluorophenylquinoline. Macromolecules 2010, 43, 4827–4828. [Google Scholar] [CrossRef]
- Kakogianni, S.; Kourkouli, S.N.; Andreopoulou, A.K.; Kallitsis, J.K. A versatile approach for creating hybrid semiconducting polymer–fullerene architectures for organic electronics. J. Mater. Chem. A 2014, 2, 8110–8117. [Google Scholar] [CrossRef]
- Aivali, S.; Kakogianni, S.; Anastasopoulos, C.; Andreopoulou, A.K.; Kallitsis, J.K. Copolymers and Hybrids Based on Carbazole Derivatives and Their Nanomorphology Investigation. Nanomaterials 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Kourkouli, S.N.; Siokou, A.; Stefopoulos, A.A.; Ravani, F.; Plocke, T.; Müller, M.; Maultzsch, J.; Thomsen, C.; Papagelis, K.; Kallitsis, J.K. Electronic Properties of Semiconducting Polymer-Functionalized Single Wall Carbon Nanotubes. Macromolecules 2013, 46, 2590–2598. [Google Scholar] [CrossRef]
- Economopoulos, S.P.; Andreopoulou, A.K.; Gregoriou, V.G.; Kallitsis, J.K. Synthesis and optical properties of new end-functionalized polyquinolines. Chem. Mater. 2005, 17, 1063–1071. [Google Scholar] [CrossRef]
- Andreopoulou, A.K.; Kallitsis, J.K. An “Attachment through Coordination” approach to side chain dendritic polymers. Eur. J. Org. Chem. 2005, 20, 4448–4458. [Google Scholar] [CrossRef]
- Ganesamoorthy, R.; Vijayaraghavana, R.; Ramki, K.; Pachagounder, S. Synthesis, characterization of bay-substituted perylene diimide based D-A-D type small molecules and their applications as a non-fullerene electron acceptor in polymer solar cells. J. Sci. Adv. Mater. Devices 2018, 3, 99–106. [Google Scholar] [CrossRef]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, J.W.L.; Rybtchinski, B.J. Selective Bromination of Perylene Diimides under Mild Conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chang, C.-W.; Cheng, K.-Y. 1,6- and 1,7-Regioisomers of Asymmetric and Symmetric Perylene Bisimides: Synthesis, Characterization and Optical Properties. Molecules 2014, 19, 327–341. [Google Scholar] [CrossRef]
- Andreopoulou, A.K.; Kallitsis, J.K. From Terphenyl-Dendronized Macromonomers to Aromatic−Aliphatic Polyethers Bearing Two Pendant Dendrons per Repeating Unit. Macromolecules 2002, 35, 5808–5815. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; Sun, J.-P.; Law, W.K.; Yan, H.; Hill, I.G.; Spasyuk, D.M.; Welch, G.C. Synthesis, Self-Assembly, and Solar Cell Performance of N-Annulated Perylene Diimide Non-Fullerene Acceptors. Chem. Mater. 2016, 28, 7098–7109. [Google Scholar] [CrossRef]
- El-Berjawi, R.; Hudhomme, P. Synthesis of a perylenediimide-fullerene C60 dyad: A simple use of a nitro leaving group for a Suzuki-Miyaura coupling reaction. Dyes Pigm. 2018, 159, 551–556. [Google Scholar] [CrossRef]
- Albeniz, A.C.; Espinet, P.; Martın-Ruiz, B.; Milstein, D. Catalytic System for the Heck Reaction of Fluorinated Haloaryls. Organometallics 2005, 24, 3679–3684. [Google Scholar] [CrossRef]
- Kallitsis, K.J.; Nannou, R.; Andreopoulou, A.K.; Daletou, M.K.; Papaioannou, D.; Neophytides, S.G.; Kallitsis, J.K. Crosslinked wholly aromatic polyether membranes based on quinoline derivatives and their application in high temperature polymer electrolyte membrane fuel cells. J. Power Sour. 2018, 379, 144–154. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Y.; Okhai, A.T.; Snyman, W.L. Micro optical sensors based on avalanching silicon light-emitting devices monolithically integrated on chips. Opt. Mater. Express 2019, 9, 3985–3997. [Google Scholar] [CrossRef]
- Coulson, D.R.; Satek, L.C.; Grim, S. Tetrakis (triphenylphosphine) palladium (0). In Inorganic Syntheses; Cotton, F.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1972; Volume 13, ISBN 9780470132449. [Google Scholar]
- Al-Ibrahim, M.; Roth, H.-K.; Schroedner, M.; Konkin, A.; Zhokhavets, U.; Gobsch, G.; Scharff, P.; Sensfuss, S. The influence of the optoelectronic properties of poly (3-alkylthiophenes) on the device parameters in flexible polymer solar cells. Org. Electron. 2005, 6, 65–77. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds diQPy-styryl-di-EH-PDI, Ph5FQ-PhO-di-EH-PDI and di5FQ-PhO-di-EH-PDI are available from the authors. |

| Solvent | Styryl-di-EH-PDI | PhOH-di-EH-PDI | Ph5FQ-PhO-di-EH-PDI |
|---|---|---|---|
| o-DCB | 3% | 3% | 3% |
| CHCl3 | 3% | 3% | 3% |
| DCM | 2% | 3% | - |
| THF | 2% | 3% | - |
| Toluene | 2% | 1% | - |
| Solvent | distyryl-di-EH-PDI | diQPy-styryl-di-EH-PDI | diPhOH-di-EH-PDI | diPh5FQ-PhO-di-EH-PDI |
|---|---|---|---|---|
| o-DCB | 3% | 3% | 0.5% | 2% |
| CHCl3 | 3% | 3% | 0.5% | 2% |
| DCM | 3% | 2% | 0.5% | 2% |
| THF | 3% | 3% | 3% | 2% |
| Toluene | 3% | 2% | 0.5% | 2% |
| λ (nm) | Eopt d (eV) | ERED e (eV) | LUMO (eV) | Homo (eV) | |||
|---|---|---|---|---|---|---|---|
| Solution a | Film b | Onset c | |||||
| distyryl-di-EH-PDI | 556 | 545 | 665 | 1.86 | −0.48 | −4.12 | −5.98 |
| diPhOH-di-EH-PDI | 570 | 595 | 730 | 1.69 | −0.55 | −4.05 | −5.74 |
| diQPy-styryl-di-EH-PDI | 365/565 | 365/565 | 680 | 1.82 | −0.65 | −3.95 | −5.77 |
| diPh5FQ-PhO-di-EH-PDI | 326/558 | 566 | 706 | 1.75 | −0.58 | −4.02 | −5.77 |
| Styryl-di-EH-PDI | 550 | 570 | 726 | 1.71 | −0.43 | −4.18 | −5.89 |
| PhOH-di-EH-PDI | 540 | 535 | 702 | 1.77 | −0.49 | −4.11 | −5.88 |
| Ph5FQ-PhO-di-EH-PDI | 330/535 | 348/500 | 690 | 1.96 | −0.80 | −3.80 | −5.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aivali, S.; Tsimpouki, L.; Anastasopoulos, C.; Kallitsis, J.K. Synthesis and Optoelectronic Characterization of Perylene Diimide-Quinoline Based Small Molecules. Molecules 2019, 24, 4406. https://doi.org/10.3390/molecules24234406
Aivali S, Tsimpouki L, Anastasopoulos C, Kallitsis JK. Synthesis and Optoelectronic Characterization of Perylene Diimide-Quinoline Based Small Molecules. Molecules. 2019; 24(23):4406. https://doi.org/10.3390/molecules24234406
Chicago/Turabian StyleAivali, Stefania, Loukia Tsimpouki, Charalampos Anastasopoulos, and Joannis K. Kallitsis. 2019. "Synthesis and Optoelectronic Characterization of Perylene Diimide-Quinoline Based Small Molecules" Molecules 24, no. 23: 4406. https://doi.org/10.3390/molecules24234406
APA StyleAivali, S., Tsimpouki, L., Anastasopoulos, C., & Kallitsis, J. K. (2019). Synthesis and Optoelectronic Characterization of Perylene Diimide-Quinoline Based Small Molecules. Molecules, 24(23), 4406. https://doi.org/10.3390/molecules24234406