Inhibition of Porcine Aminopeptidase M (pAMP) by the Pentapeptide Microginins
Abstract
1. Introduction
2. Results
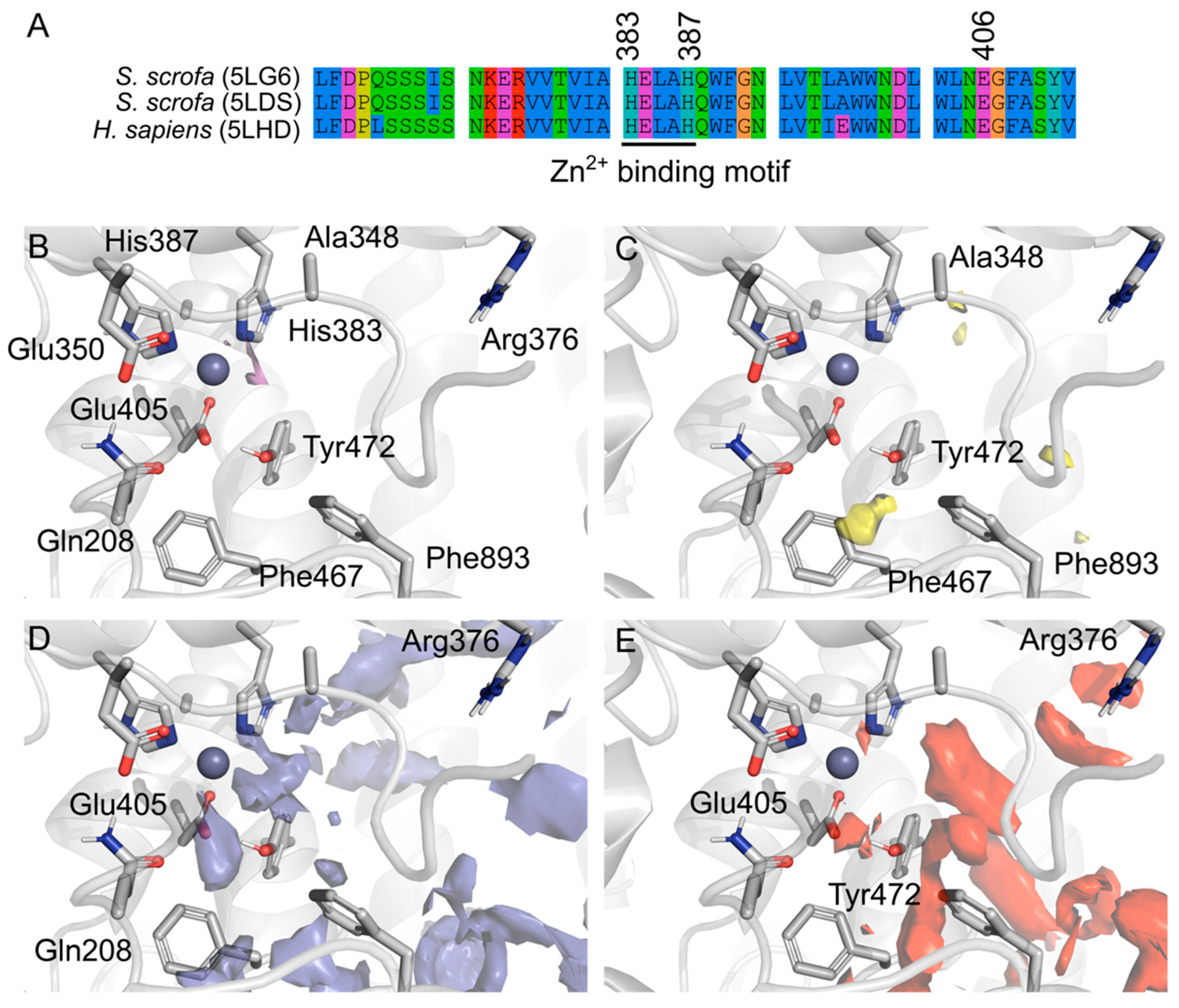
2.1. Porcine and Human Aminopeptidase Have Similar Active Sites
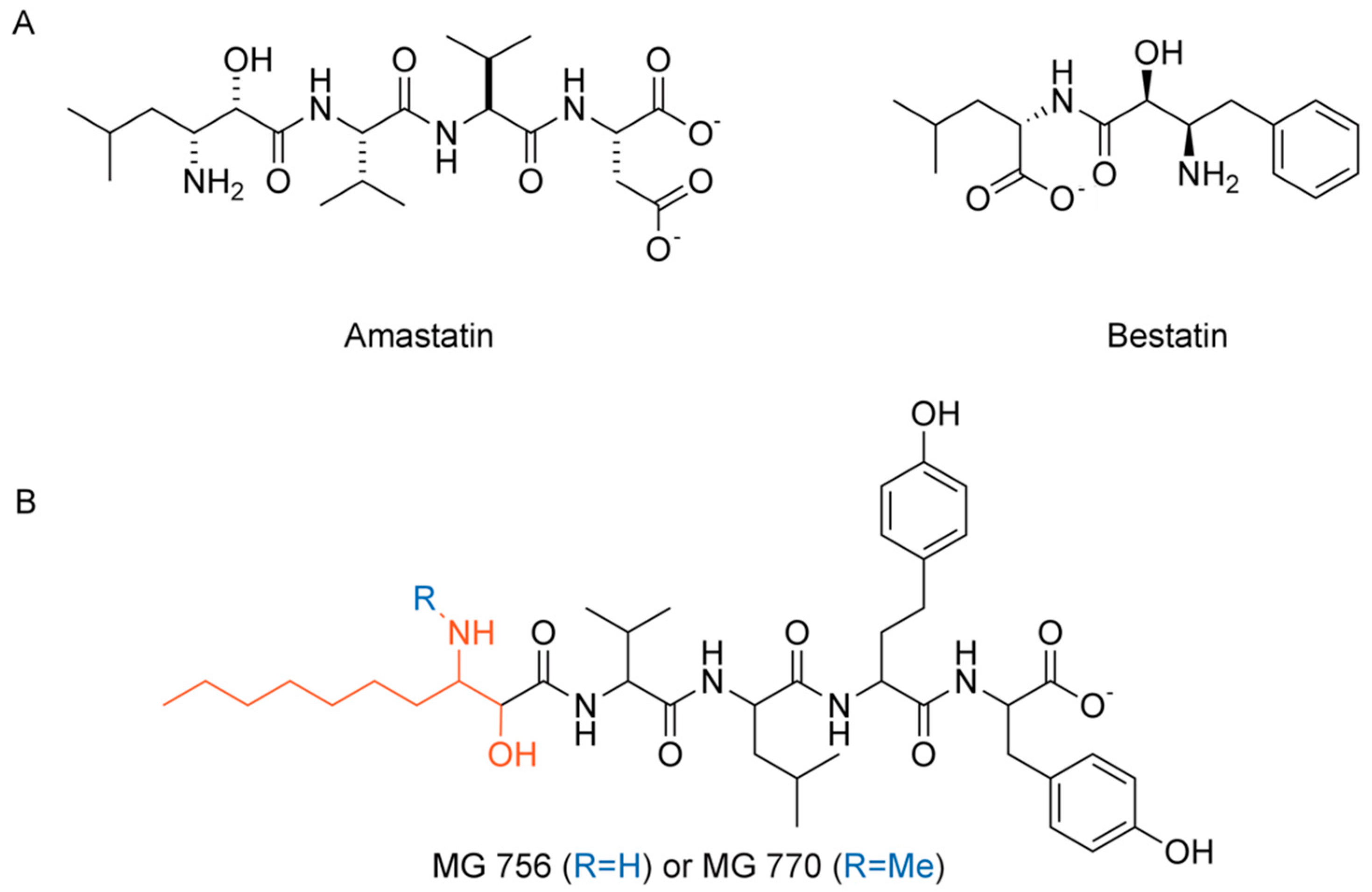
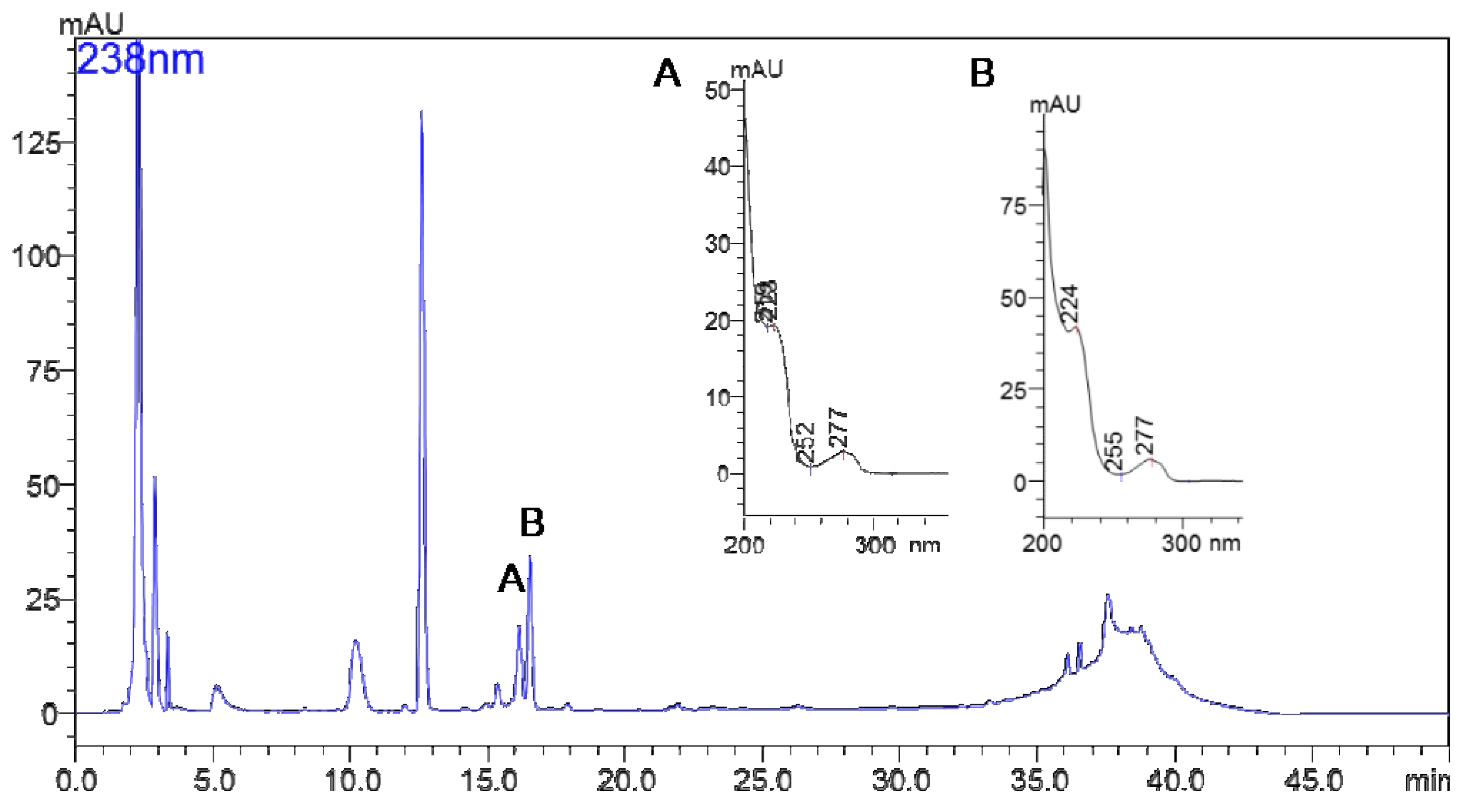
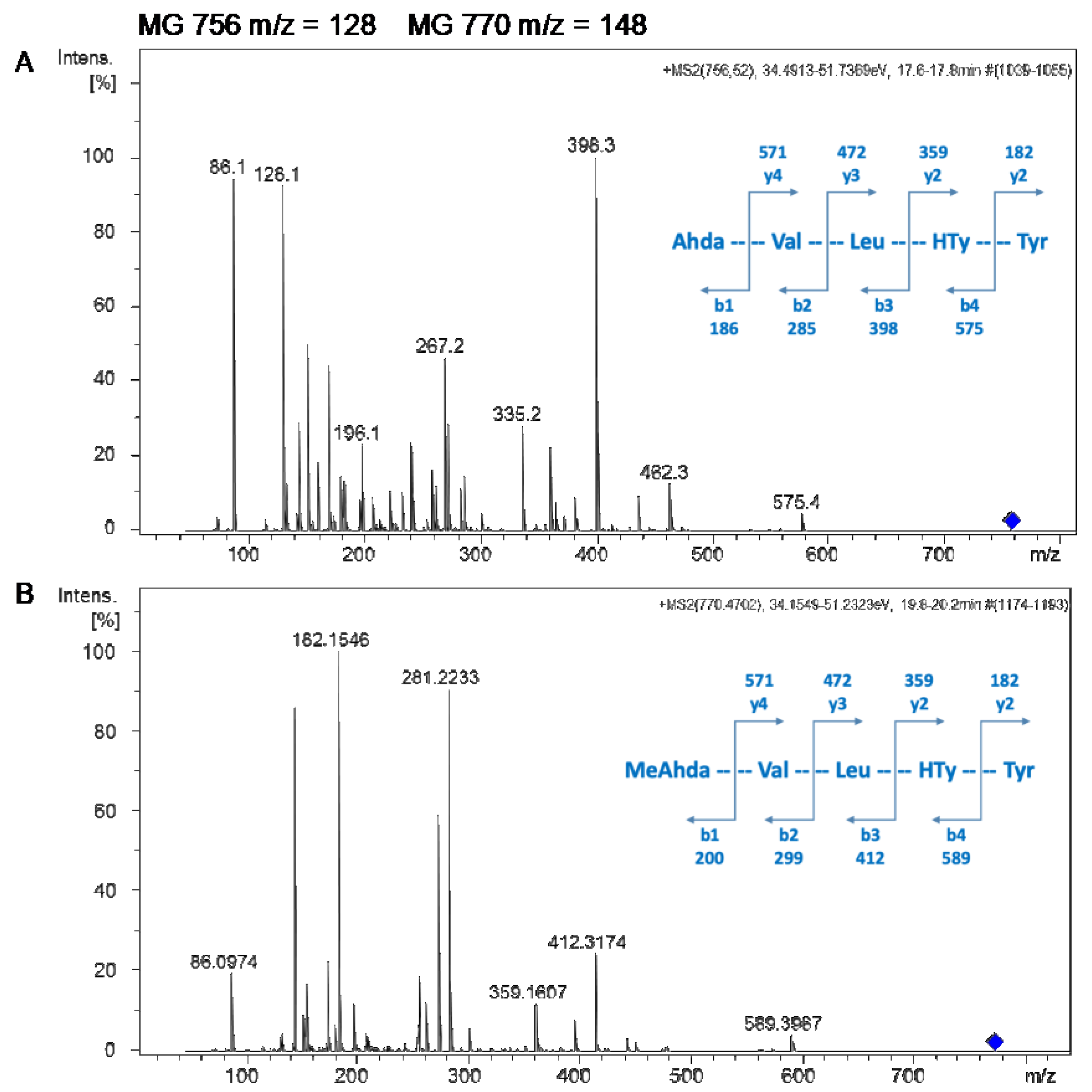
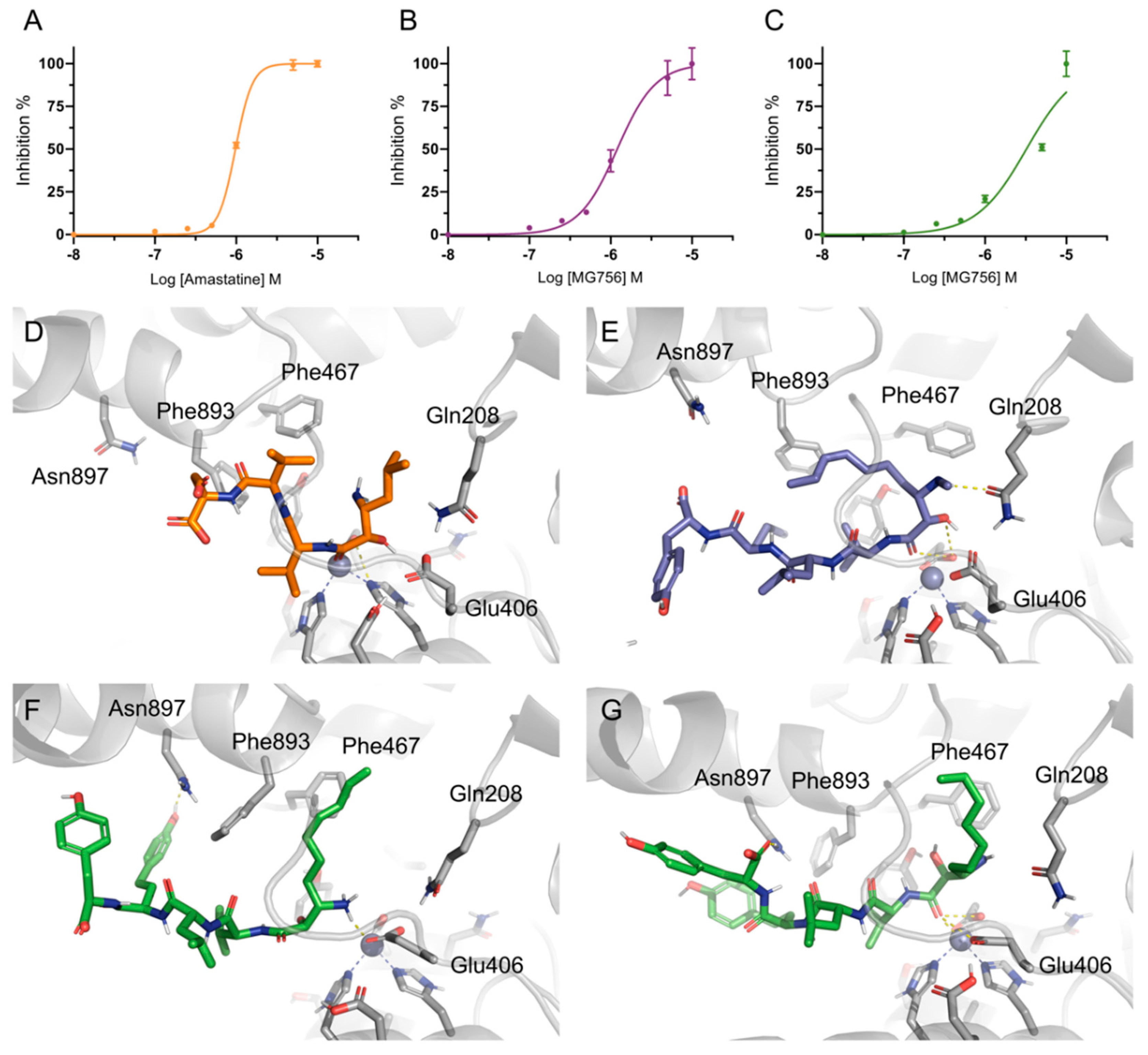
2.2. Isolation and Identification of MG756 and MG770 from Microcystis aeroginosa LTPNA 08 and pAMP Inhibition
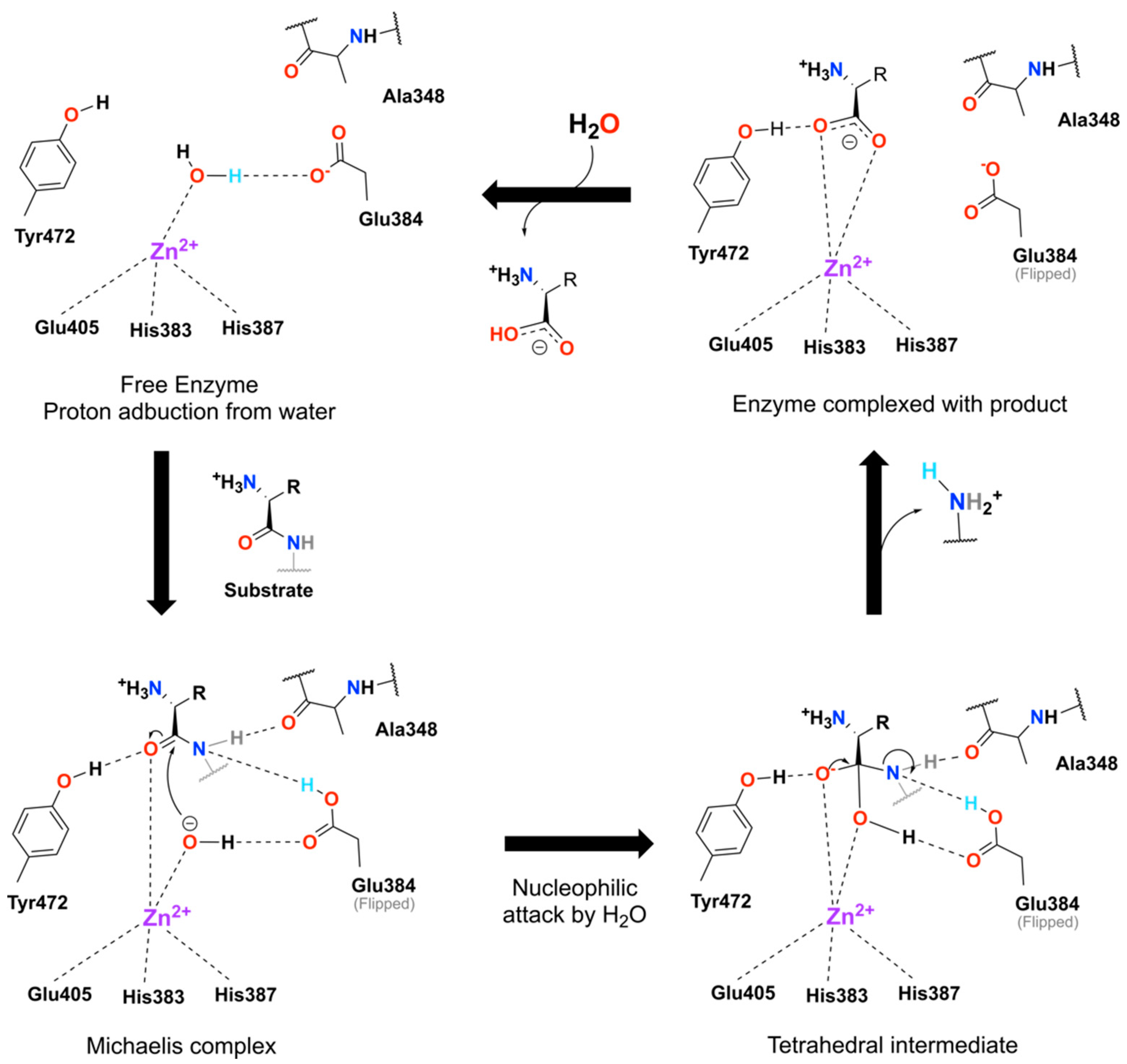
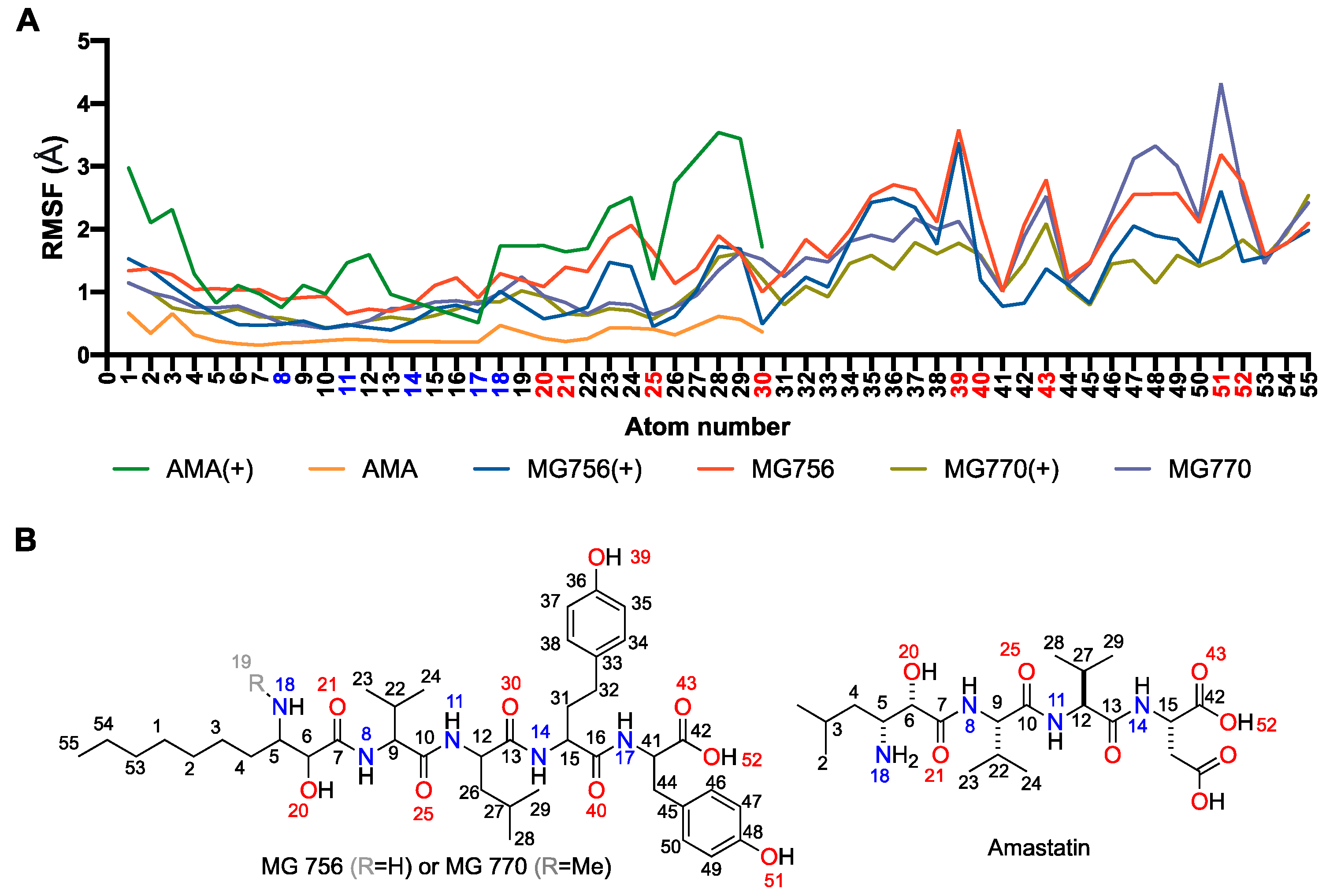
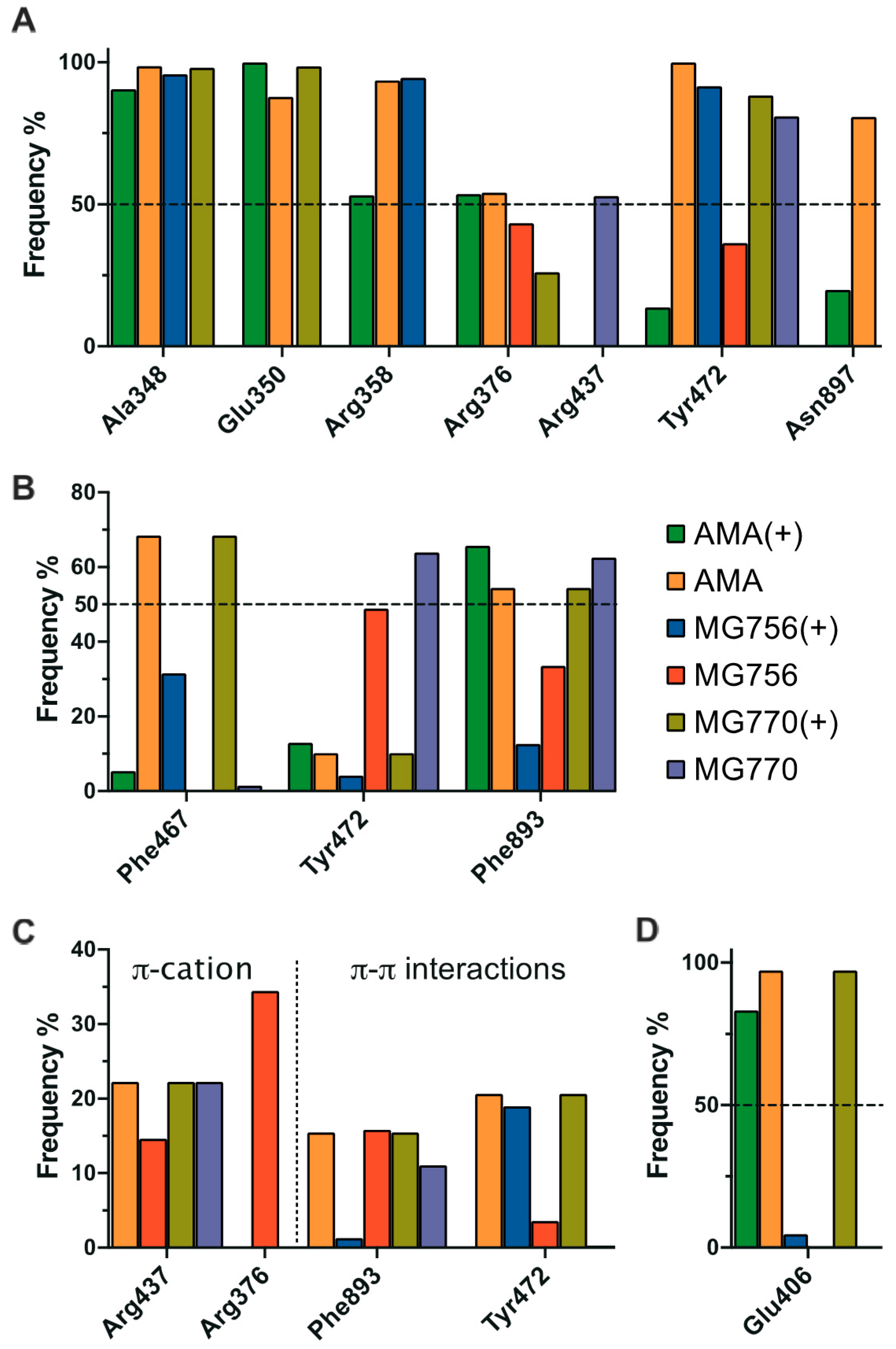
2.3. Single Methylation Can Change the MG’s Binding Mode in the Porcine Aminopeptidase
3. Discussion
4. Materials and Methods
4.1. Growth and Cell Harvesting
4.2. Extraction, Isolation and Characterization of Microginins
4.3. Aminopeptidase M Inhibition
4.4. Molecular Modelling—System Preparation
4.5. Molecular Docking
4.6. Molecular Dynamics Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santiago, C.; Mudgal, G.; Reguera, J.; Recacha, R.; Albrecht, S.; Enjuanes, L.; Casasnovas, J.M. Allosteric inhibition of aminopeptidase N functions related to tumor growth and virus infection. Sci. Rep. 2017, 7, 46045. [Google Scholar] [CrossRef]
- Reguera, J.; Santiago, C.; Mudgal, G.; Ordoño, D.; Enjuanes, L.; Casasnovas, J.M. Structural Bases of Coronavirus Attachment to Host Aminopeptidase N and Its Inhibition by Neutralizing Antibodies. PLoS Pathog. 2012, 8, e1002859. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Browning, T.; Facey, D. CD13 (GP150; aminopeptidase-N): Predominant functional activity in blood is localized to plasma and is not cell-surface associated. Exp. Hematol. 1993, 21, 1695–1701. [Google Scholar]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.H.; Zhou, D.; Rini, J.M. The X-ray Crystal Structure of Human Aminopeptidase N Reveals a Novel Dimer and the Basis for Peptide Processing. J. Biol. Chem. 2012, 287, 36804–36813. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nakajima, Y.; Onohara, Y.; Takeo, M.; Nakashima, K.; Matsubara, F.; Ito, T.; Yoshimoto, T. Crystal Structure of Aminopeptidase N (Proteobacteria Alanyl Aminopeptidase) from Escherichia coli and Conformational Change of Methionine 260 Involved in Substrate Recognition. J. Biol. Chem. 2006, 281, 33664–33676. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, Y.-L.; Peng, G.; Li, F. Structural basis for multifunctional roles of mammalian aminopeptidase N. Proc. Natl. Acad. Sci. USA 2012, 109, 17966–17971. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Rojas, L.; Rangel, R.; Salameh, A.; Edwards, J.K.; Dondossola, E.; Kim, Y.-G.; Saghatelian, A.; Giordano, R.J.; Kolonin, M.G.; Staquicini, F.I.; et al. Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2012, 109, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.V.; Petrovic, N.; Okamoto, Y.; Shapiro, L.H. The angiogenic regulator CD13/APN is a transcriptional target of Ras signaling pathways in endothelial morphogenesis. Blood 2003, 101, 1818–1826. [Google Scholar] [CrossRef]
- Ashmun, R.A.; Look, A.T. Metalloprotease activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood 1990, 75, 462–469. [Google Scholar] [CrossRef]
- Kehlen, A.; Lendeckel, U.; Dralle, H.; Langner, J.; Hoang-Vu, C. Biological significance of aminopeptidase N/CD13 in thyroid carcinomas. Cancer Res. 2003, 63, 8500–8506. [Google Scholar] [PubMed]
- Bhagwat, S.V.; Lahdenranta, J.; Giordano, R.; Arap, W.; Pasqualini, R.; Shapiro, L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 2001, 97, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Hitzerd, S.M.; Verbrugge, S.E.; Ossenkoppele, G.; Jansen, G.; Peters, G.J. Positioning of aminopeptidase inhibitors in next generation cancer therapy. Amino Acids 2014, 46, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Wickström, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Incyte, Inc. A Phase 1 Study of INCMGA00012 in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03059823 (accessed on 2 July 2019).
- Amin, S.A.; Adhikari, N.; Jha, T. Design of Aminopeptidase N Inhibitors as Anti-cancer Agents. J. Med. Chem. 2018, 61, 6468–6490. [Google Scholar] [CrossRef]
- Dörr, F.A.; Pinto, E.; Soares, R.M.; Feliciano de Oliveira e Azevedo, S.M. Microcystins in South American aquatic ecosystems: Occurrence, toxicity and toxicological assays. Toxicon 2010, 56, 1247–1256. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.d.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and Effects on Aquatic Animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Fernandes, K.; Gomes, A.; Calado, L.; Yasui, G.; Assis, D.; Henry, T.; Fonseca, A.; Pinto, E. Toxicity of Cyanopeptides from Two Microcystis Strains on Larval Development of Astyanax altiparanae. Toxins 2019, 11, 220. [Google Scholar] [CrossRef]
- Neilan, B.A.; Dittmann, E.; Rouhiainen, L.; Bass, R.A.; Schaub, V.; Sivonen, K.; Börner, T. Nonribosomal peptide synthesis and toxigenicity of cyanobacteria. J. Bacteriol. 1999, 181, 4089–4097. [Google Scholar]
- Ji, C.-M.; Wang, B.; Zhou, J.; Huang, Y.-W. Aminopeptidase-N-independent entry of porcine epidemic diarrhea virus into Vero or porcine small intestine epithelial cells. Virology 2018, 517, 16–23. [Google Scholar] [CrossRef]
- Halgren, T.A. Identifying and Characterizing Binding Sites and Assessing Druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.L.; Dörr, F.A.; Dörr, F.; Bortoli, S.; Delherbe, N.; Vásquez, M.; Pinto, E. Co-occurrence of microcystin and microginin congeners in Brazilian strains of Microcystis sp. FEMS Microbiol. Ecol. 2012, 82, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Paiva, F.; Ferreira, G.; Trossini, G.; Pinto, E. Identification, In Vitro Testing and Molecular Docking Studies of Microginins’ Mechanism of Angiotensin-Converting Enzyme Inhibition. Molecules 2017, 22, 1884. [Google Scholar] [CrossRef] [PubMed]
- Coutsias, E.A.; Seok, C.; Dill, K.A. Using quaternions to calculate RMSD. J. Comput. Chem. 2004, 25, 1849–1857. [Google Scholar] [CrossRef]
- Harding, J.W.; Felix, D. The effects of the aminopeptidase inhibitors amastatin and bestatin on angiotensin-evoked neuronal activity in rat brain. Brain Res. 1987, 424, 299–304. [Google Scholar] [CrossRef]
- Ishida, K.; Kato, T.; Murakami, M.; Watanabe, M.; Watanabe, M.F. Microginins, Zinc Metalloproteases Inhibitors from the Cyanobacterium Microcystis aeruginosa. Tetrahedron 2000, 56, 8643–8656. [Google Scholar] [CrossRef]
- Luciani, N.; Marie-Claire, C.; Ruffet, E.; Beaumont, A.; Roques, B.P.; Fournié-Zaluski, M.-C. Characterization of Glu350 as a Critical Residue Involved in the N-Terminal Amine Binding Site of Aminopeptidase N (EC 3.4.11.2): Insights into Its Mechanism of Action. Biochemistry 1998, 37, 686–692. [Google Scholar] [CrossRef]
- Sakamoto, K.; Sugimoto, K.; Sudoh, T.; Fujimura, A. Different Effects of Imidapril and Enalapril on Aminopeptidase P Activity in the Mouse Trachea. Hypertens. Res. 2005, 28, 243–247. [Google Scholar] [CrossRef]
- Patchett, A.A.; Harris, E.; Tristram, E.W.; Wyvratt, M.J.; Wu, M.T.; Taub, D.; Peterson, E.R.; Ikeler, T.J.; ten Broeke, J.; Payne, L.G.; et al. A new class of angiotensin-converting enzyme inhibitors. Nature 1980, 288, 280–283. [Google Scholar] [CrossRef]
- Tavares, M.T.; Primi, M.C.; Polli, M.C.; Ferreira, E.I.; Parise-Filho, R. Drug-receptor interactions: In silico approaches applied to experimental classes regarding the evolution of angiotensin converting enzyme inhibitors. Química Nova 2015, 38, 1117–1124. [Google Scholar]
- Lodin-Friedman, A.; Carmeli, S. Microginins from a Microcystis sp. Bloom Material Collected from the Kishon Reservoir, Israel. Mar. Drugs 2018, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U. Microginin FR1, a linear peptide from a water bloom of Microcystis species. FEMS Microbiol. Lett. 1997, 153, 475–478. [Google Scholar] [CrossRef]
- Kraft, M.; Schleberger, C.; Weckesser, J.; Schulz, G.E. Binding structure of the leucine aminopeptidase inhibitor microginin FR1. FEBS Lett. 2006, 580, 6943–6947. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Marsálek, B.; Sejnohová, L.; von Döhren, H. Detection and identification of oligopeptides in Microcystis (cyanobacteria) colonies: Toward an understanding of metabolic diversity. Peptides 2006, 27, 2090–2103. [Google Scholar] [CrossRef]
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Microginins 299-A and -B, leucine aminopeptidase inhibitors from the cyanobacterium Microcystis aeruginosa (NIES-299). Tetrahedron 1997, 53, 10281–10288. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters, Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; ACM: New York, NY, USA, 2006. [Google Scholar]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Tobe, H.; Morishima, H.; Naganawa, H.; Takita, T.; Aoyagi, T.; Umezawa, H. Structure and Chemical Synthesis of Amastatin. Agric. Biol. Chem. 1979, 43, 591–596. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors, due to the low production yield. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, G.M.; Kronenberger, T.; de Almeida, É.C.; Sampaio, J.; Terra, C.F.; Pinto, E.; Trossini, G.H.G. Inhibition of Porcine Aminopeptidase M (pAMP) by the Pentapeptide Microginins. Molecules 2019, 24, 4369. https://doi.org/10.3390/molecules24234369
Ferreira GM, Kronenberger T, de Almeida ÉC, Sampaio J, Terra CF, Pinto E, Trossini GHG. Inhibition of Porcine Aminopeptidase M (pAMP) by the Pentapeptide Microginins. Molecules. 2019; 24(23):4369. https://doi.org/10.3390/molecules24234369
Chicago/Turabian StyleFerreira, Glaucio Monteiro, Thales Kronenberger, Éryka Costa de Almeida, Joseane Sampaio, Clélia Ferreira Terra, Ernani Pinto, and Gustavo Henrique Goulart Trossini. 2019. "Inhibition of Porcine Aminopeptidase M (pAMP) by the Pentapeptide Microginins" Molecules 24, no. 23: 4369. https://doi.org/10.3390/molecules24234369
APA StyleFerreira, G. M., Kronenberger, T., de Almeida, É. C., Sampaio, J., Terra, C. F., Pinto, E., & Trossini, G. H. G. (2019). Inhibition of Porcine Aminopeptidase M (pAMP) by the Pentapeptide Microginins. Molecules, 24(23), 4369. https://doi.org/10.3390/molecules24234369





