Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation of Compound 1
2.2. Bioactivity Evaluation
2.2.1. ABTS.+ Radical Scavenging Assay
2.2.2. ORAC Antioxidant Activity Assay
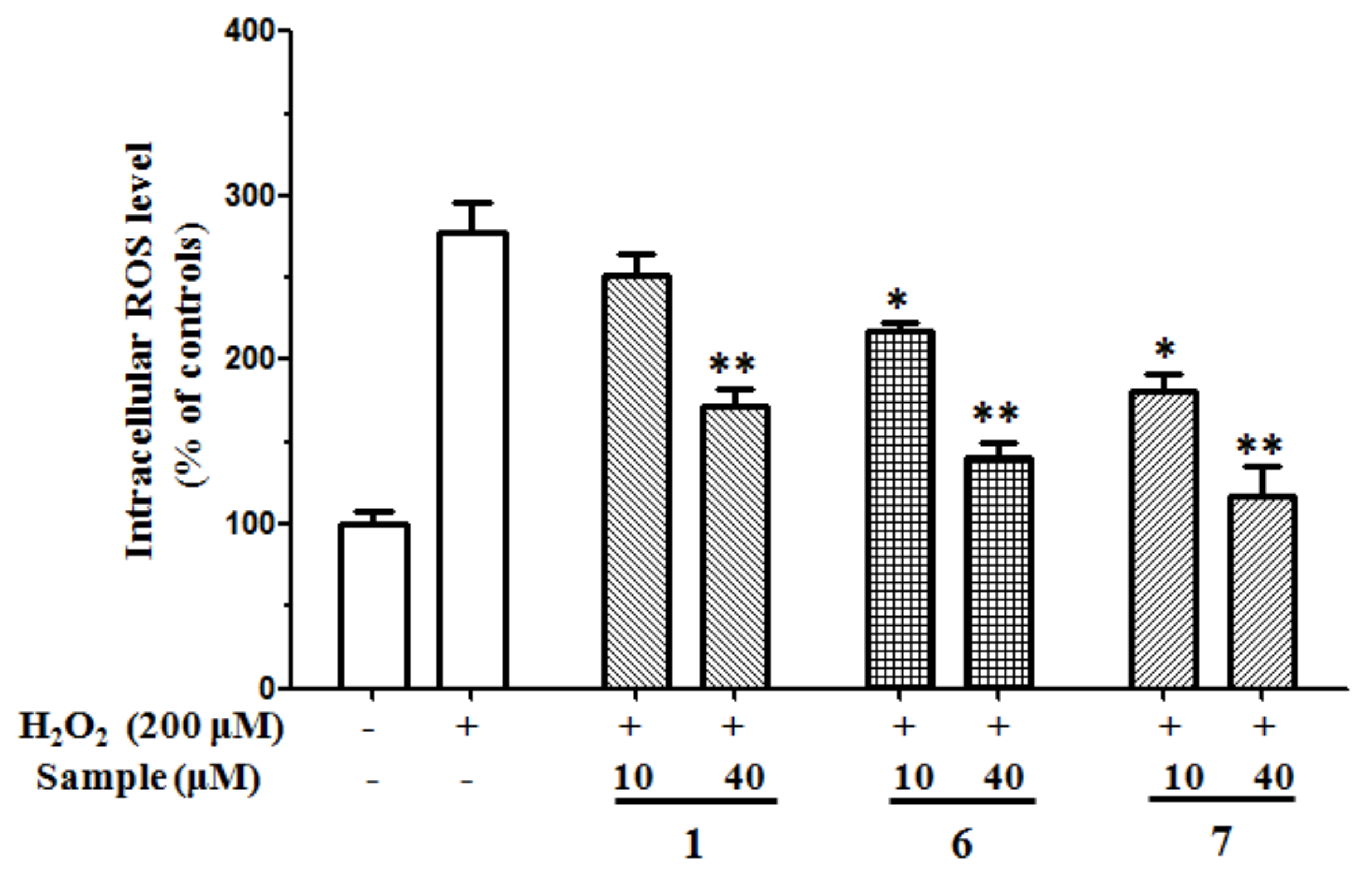
2.2.3. Antioxidant Effects on H2O2-Induced ROS Production in SH-SY5Y Cells
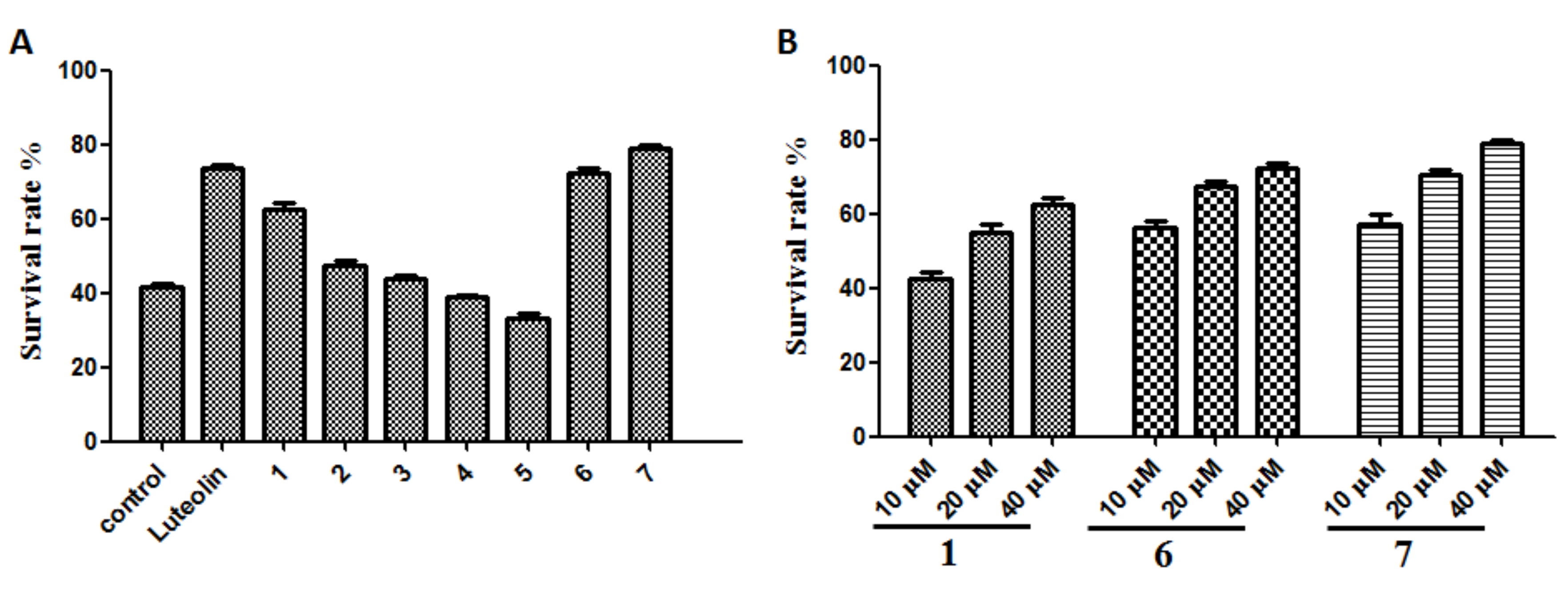
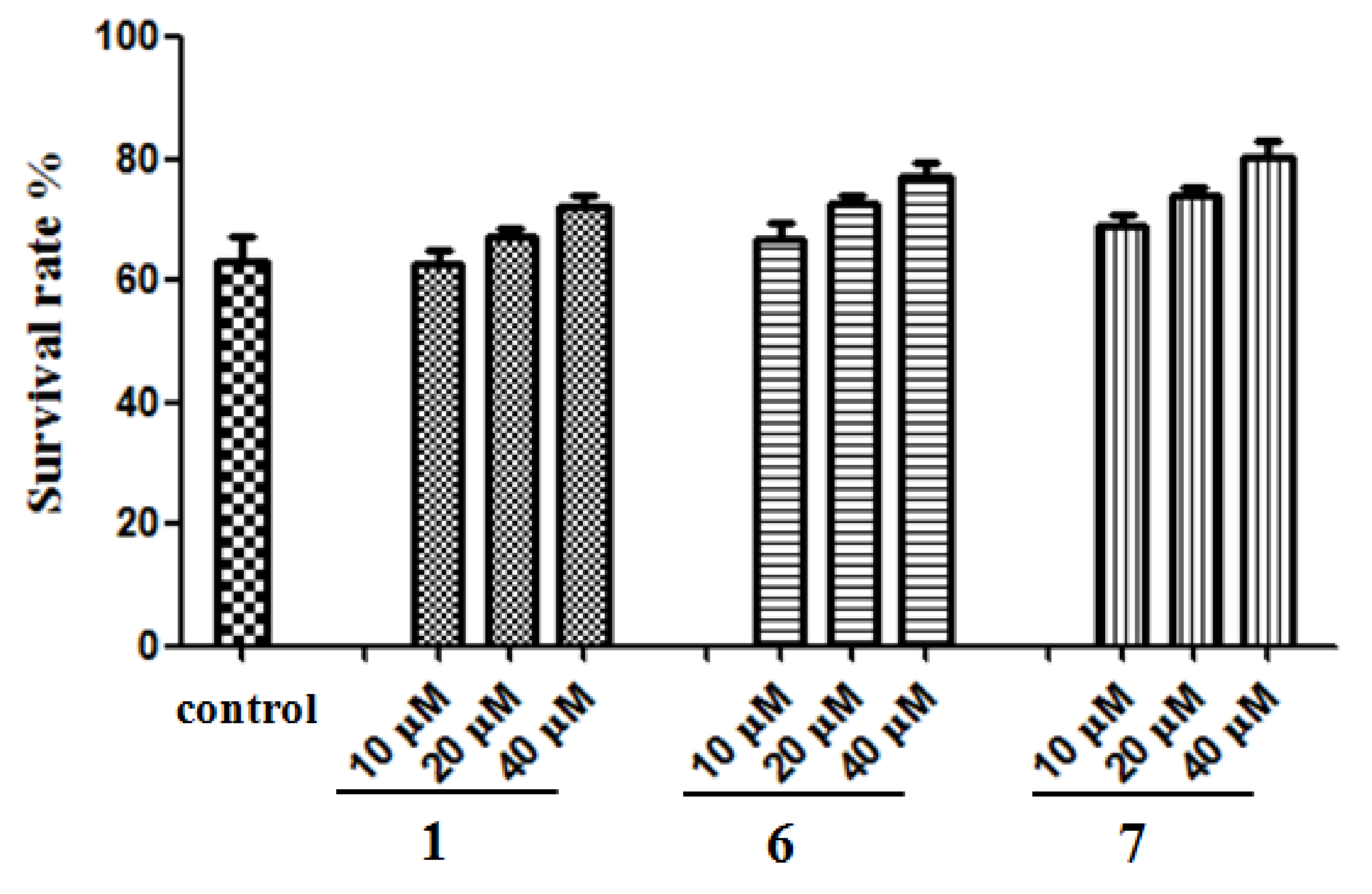
2.2.4. Protection of SH-SY5Y Cells Against Aβ-Induced Damage
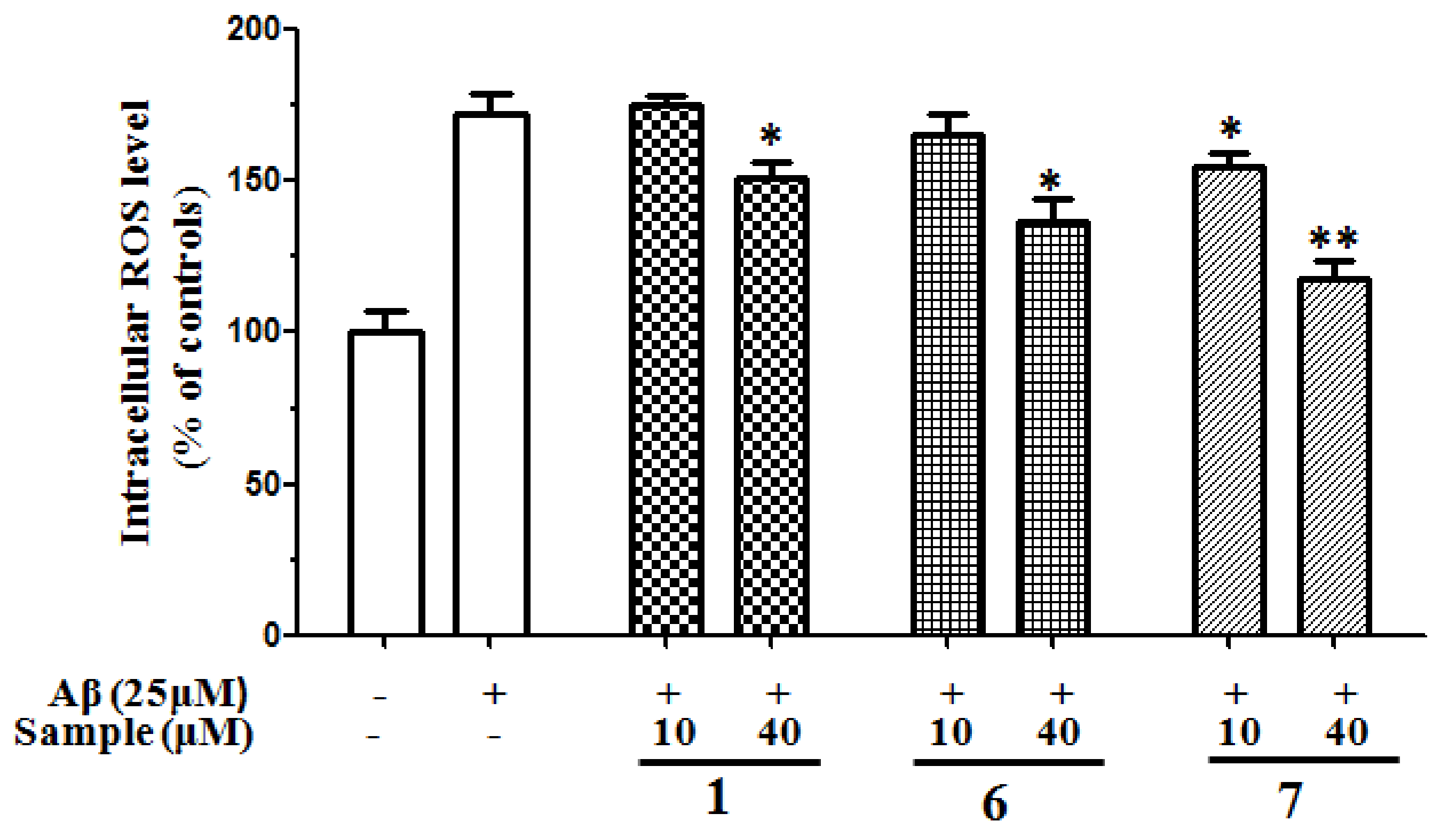
2.2.5. Inhibition of ROS Generation Induced by Aβ25–35 in SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagent
4.2. Apparatus and Chemicals
4.3. Extraction and Isolation
4.4. Bioactivity Evaluation
4.4.1. ABTS Radical Cation Scavenging Activity
4.4.2. Oxygen Radical Absorbance Capacity Assay (ORAC Assay)
4.4.3. Cell Culture
4.4.4. Cytoprotective Effects of Compounds 1–7 in SH-SY5Y
4.4.5. Neuroprotective Effects of Compounds 1, 6, and 7 in SH-SY5Y
4.4.6. Measurement of Intracellular ROS Level
4.4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krishnaiah, D.; Sarbatly, R.; Bono, A. Phytochemical antioxidants for health and medicine a move towards nature. Biotechnol. Mol. Biol. Rev. 2007, 2, 97–104. [Google Scholar]
- Selkoe, D.J. Developing preventive therapies for chronic diseases: Lessons learned from Alzheimer’s disease. Nutr. Rev. 2007, 65, S239–S243. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Dimayuga, E.; Keller, J.N. Oxidative damage, protein synthesis, and protein degradation in Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid β-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Mathew, J.; Sudheesh, N.P.; Rony, K.A.; Smina, T.P.; Janardhanan, K.K. Antioxidant and antitumor activities of cultured mycelium of culinary-medicinal paddy straw mushroom Volvariella volvacea (Bull.: Fr.) singer (agaricomycetideae). Int. J. Med. Mushrooms 2008, 10, 139–148. [Google Scholar] [CrossRef]
- Nitha, B.; De, S.; Adhikari, S.; Devasagayam, T.; Janardhanan, K. Evaluation of free radical scavenging activity of morel mushroom, Morchella esculenta mycelia: A potential source of therapeutically useful antioxidants. Pharm. Bio. 2010, 48, 453–460. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Yang, J.H.; Mau, J.L. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008, 107, 732–738. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Bio. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Wang, C.; Han, Z.; Shu, Y.; Li, X.; Zhou, L.; Qiu, M. Unusual prenylated phenols with antioxidant activities from Ganoderma cochlear. Food Chem. 2015, 171, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, L.; Wang, X.; Zhu, G.; Li, Z.; Qiu, M. Antioxidant farnesylated hydroquinones from Ganoderma capense. Fitoterapia 2016, 111, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Liu, J.Q.; Wan, L.S.; Li, X.N.; Yan, Y.X.; Qiu, M.H. Four new polycyclic meroterpenoids from Ganoderma cochlear. Org. Lett. 2014, 16, 4838–4841. [Google Scholar] [CrossRef]
- Luo, Q.; Luo, Q.; Di, L.; Lu, Q.; Yan, Y.M.; Yang, Z.L.; Li, R.T.; Cheng, Y.X. Applanatumin A, a new dimeric Meroterpenoid from Ganoderma applanatum that displays potent antifibrotic activity. Org. Lett. 2015, 17, 1110–1113. [Google Scholar] [CrossRef]
- Chen, D.; Liu, H.M.; Li, M.M.; Yan, Y.M.; Xu, W.D.; Li, X.N.; Cheng, Y.X.; Qin, H.B. Concise synthesis of (±)-Lingzhiol via epoxy-arene cyclization. Chem. Commun. 2015, 51, 14594–14596. [Google Scholar] [CrossRef]
- Dine, E.R.S.; El Halawany, A.M.; Ma, C.M.; Hattori, M. Inhibition of the dimerization and active site of HIV-1 protease by secondary metabolites from the Vietnamese Mushroom Ganoderma colossum. J. Nat. Prod. 2009, 72, 2019–2023. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhang, J.J.; Ren, J.W.; Wang, W.Z.; Xiong, W.P.; Zhang, Y.D.; Bao, L.; Liu, H.W. Triterpenes and Meroterpenes with neuroprotective effects from Ganoderma leucocontextum. Chem. Biodivers. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Qiu, J.M.; Wang, X.; Song, C.G. Neuroprotective and Antioxidant Lanostanoid Triterpenes from the Fruiting Bodies of Ganoderma atrum. Fitoterapia 2016, 109, 75–79. [Google Scholar] [CrossRef]
- Wang, C.F.; Liu, J.Q.; Yan, Y.X.; Chen, J.C.; Lu, Y.; Guo, Y.H.; Qiu, M.H. Three new triterpenoids containing four-membered ring from the fruiting body of Ganoderma sinense. Org. Lett. 2010, 12, 1656–1659. [Google Scholar] [CrossRef]
- Lian, C.; Wang, C.; Xiao, Q.; Xiao, L.; Xu, Y.; Liu, J. The triterpenes and steroids from the fruiting body Ganoderma duripora. Biochem. Syst. Ecol. 2017, 73, 50–53. [Google Scholar] [CrossRef]
- Liu, J.Q.; Lian, C.L.; Hu, T.Y.; Wang, C.F.; Xu, Y.; Xiao, L.; Liu, Z.Q.; Qiu, S.Q.; Cheng, B.H. Two new farnesyl phenolic compounds with anti-inflammatory activities from Ganoderma duripora. Food Chem. 2018, 263, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Matsuda, S.; Murai., Y.; Ogita, Z. Ganoderic acid G and I and ganolucidic acid A and B, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 2628–2631. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kanomi, S.; Murai, Y.; Kadota, S.; Tsubono, K.; Ogita, Z. Constituents of the fungus Ganoderma lucidum (FR.) Karst. III. Structures of Ganolucidic acids A and B, new lanostane-type triterpenoids. Chem. Pharm. Bull. 1986, 34, 4030–4036. [Google Scholar] [CrossRef]
- Li, P.; Deng, Y.P.; Wei, X.X.; Xu, J.H. Triterpenoids from Ganoderma lucidum and their cytotoxic activities. Nat. Prod. Res. 2013, 27, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shimizu, K.; Konishi, F.; Kumamoto, S.; Kondo, R. The anti-Androgen Effect of Ganoderol B (I)3 Isolated from the Fruiting Body of Ganoderma lucidum. Bioorgan. Med. Chem. 2007, 15, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.M.; Wang, X.L.; Luo, Q.; Jiang, L.P.; Yang, C.P.; Hou, B.; Zuo, Z.L.; Chen, Y.B.; Cheng, Y.X. Metabolites from the mushroom Ganoderma lingzhi as stimulators of neural stem cell proliferation. Phytochemistry 2015, 114, 155–162. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Kong, L. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2012, 131, 1056–1062. [Google Scholar] [CrossRef]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. B 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Risacher, S.L.; Saykin, A.J. Neuroimaging and other biomarkers for Alzheimer’s disease: The changing landscape of early detection. Annu. Rev. Clin. Psycho. 2013, 9, 621–648. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ramassamy, C.; Doré, S.; Christen, Y.; Poirier, J.; Quirion, R. The ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur. J. Neurosci. 2000, 12, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Folin, M.; Baiguera, S.; Fioravanzo, L.; Conconi, M.T.; Grandi, C.; Nussdorfer, G.G.; Parnigotto, P.P. Caspase-8 activation and oxidative stress are involved in the cytotoxic effect of β-amyloid on rat brain microvascular endothelial cells. Int. J. Mol. Med. 2006, 17, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Ning, R.; Lei, Y.; Liu, S.; Wang, H.; Zhang, R.; Wang, W.; Zhu, Y.; Zhang, H.; Zhao, W. Natural β-dihydroagarofuran-type sesquiterpenoids as cognition-enhancing and neuroprotective agents from medicinal plants of the genus Celastrus. J. Nat. Prod. 2015, 78, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Behl, C.; Davis, J.; Lesley, R.; Schubert, D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef]
- Paydar, M.; Wong, Y.L.; Moharam, B.A.; Wong, W.F.; Looi, C.Y. In vitro anti-oxidant and anti-cancer activity of methanolic extract from Sanchezia speciosa leaves. Pak. J. Biol. Sci. 2013, 16, 1212–1215. [Google Scholar]
- Lu, H.; Shi, J.X.; Zhang, D.M.; Shen, J.; Lin, Y.X.; Hang, C.H.; Yin, H.X. Hemolysate-Induced expression of intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 expression in cultured brain microvascular endothelial cells via through ROS-dependent NF-κB pathways. Cell. Mol. Neurobiol. 2009, 29, 87–95. [Google Scholar] [CrossRef]
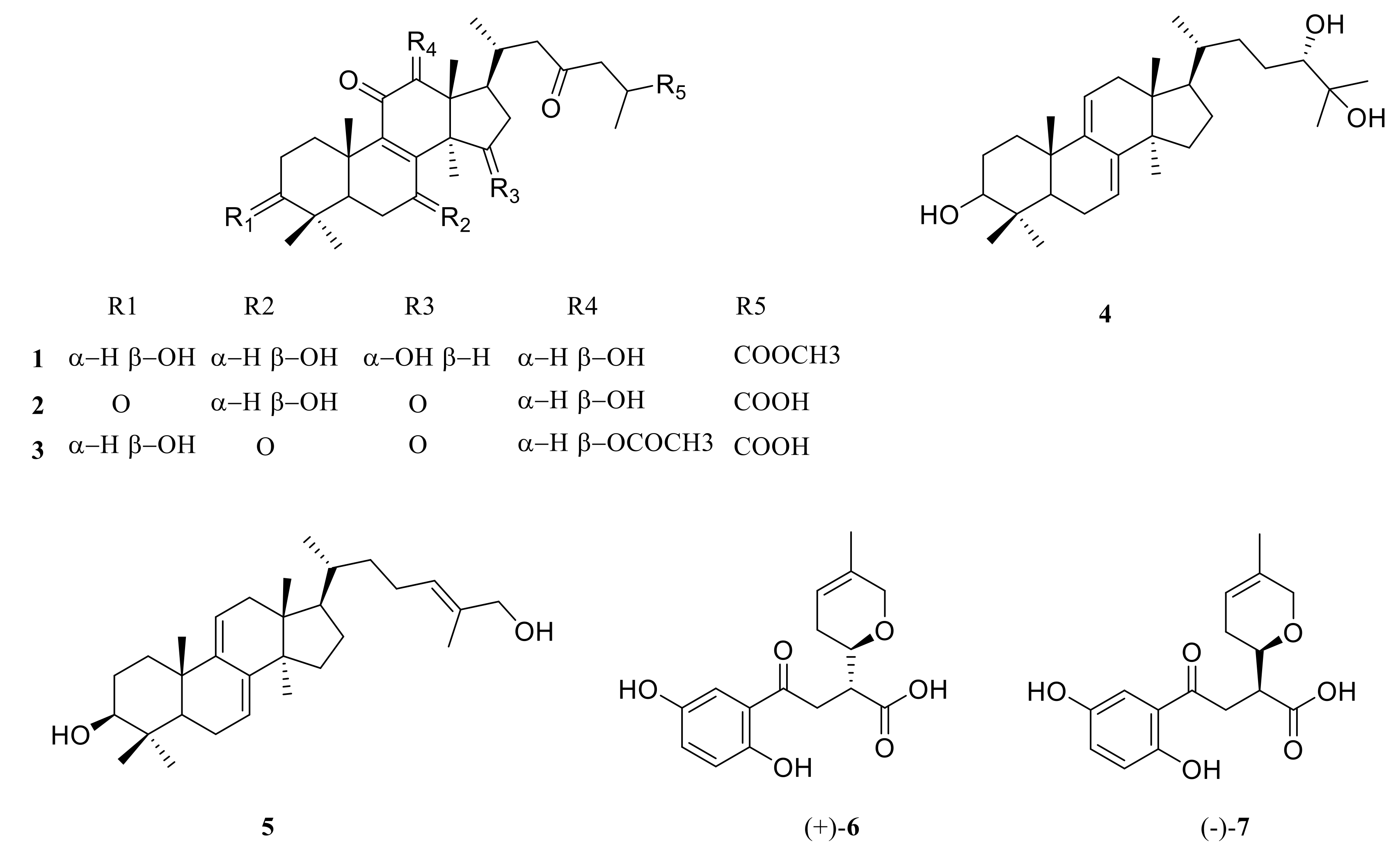
| Position | 1 | Position | 1 | ||
|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | ||
| 1 | 1.68 m | 34.61 | 16 | 1.79 m | 36.25 |
| 2.75 m | 2.50 m | ||||
| 2 | 2.15 m | 28.28 | 17 | 1.81 m | 48.10 |
| 18 | 0.95 s | 17.12 | |||
| 3 | 3.23 dd (10.8, 5.5) | 78.19 | 19 | 1.26 s | 19.38 |
| 4 | - | 38.61 | 20 | 2.15 m | 32.63 |
| 5 | 2.95 m | 49.07 | 21 | 0.89 d (6.4) | 19.62 |
| 6 | 1.64 m | 27.80 | 22 | 1.8 m | 49.72 |
| 2.13 m | |||||
| 7 | 4.57 dd (10.3, 7.2) | 69.52 | 23 | - | 208.53 |
| 8 | - | 157.78 | 24 | 2.47 m | 46.72 |
| 9 | - | 141.96 | 2.83 m | ||
| 10 | - | 38.52 | 25 | 2.08 m | 34.61 |
| 11 | - | 199.73 | 26 | - | 176.26 |
| 12 | 4.14 d (2.3) | 77.22 | 27 | 1.19 d (7.1) | 17.09 |
| 13 | - | 47.12 | 28 | 1.04 s | 28.15 |
| 14 | - | 53.90 | 29 | 0.86 s | 15.67 |
| 15 | 4.76 t (7.8) | 72.48 | 30 | 1.27 s | 19.48 |
| COOMe | 3.69 s | 51.91 | |||
| Samples | Antioxidant Capacity | |
|---|---|---|
| ABTS Radical Scavenging Assay(EC50 ± SD, mM) | ORAC Value (μmol TE/ μmol)(mean ± SD) | |
| 1 | 1.12 ± 0.17 | 1.35 ± 0.25 |
| 2 | 2.32 ± 0.16 | 0.36 ± 0.14 |
| 3 | 2.18 ± 0.15 | 0.25 ± 0.18 |
| 4 | 1.64 ± 0.18 | 0.51 ± 0.12 |
| 5 | 1.85 ± 0.14 | 0.59 ± 0.18 |
| 6 | 0.59 ± 0.15 | 5.42 ± 0.20 |
| 7 | 0.27 ± 0.05 | 7.24 ± 0.15 |
| Trolox | 0.42 ± 0.03 | - |
| Quercetin | - | 7.78 ± 0.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum. Molecules 2019, 24, 4353. https://doi.org/10.3390/molecules24234353
Wang C, Liu X, Lian C, Ke J, Liu J. Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum. Molecules. 2019; 24(23):4353. https://doi.org/10.3390/molecules24234353
Chicago/Turabian StyleWang, Cuifang, Xuemin Liu, Chenlei Lian, Jiaying Ke, and Jieqing Liu. 2019. "Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum" Molecules 24, no. 23: 4353. https://doi.org/10.3390/molecules24234353
APA StyleWang, C., Liu, X., Lian, C., Ke, J., & Liu, J. (2019). Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum. Molecules, 24(23), 4353. https://doi.org/10.3390/molecules24234353




