Comparison and Identification of the Aroma-Active Compounds in the Root of Angelica dahurica
Abstract
:1. Introduction
2. Results and Discussion
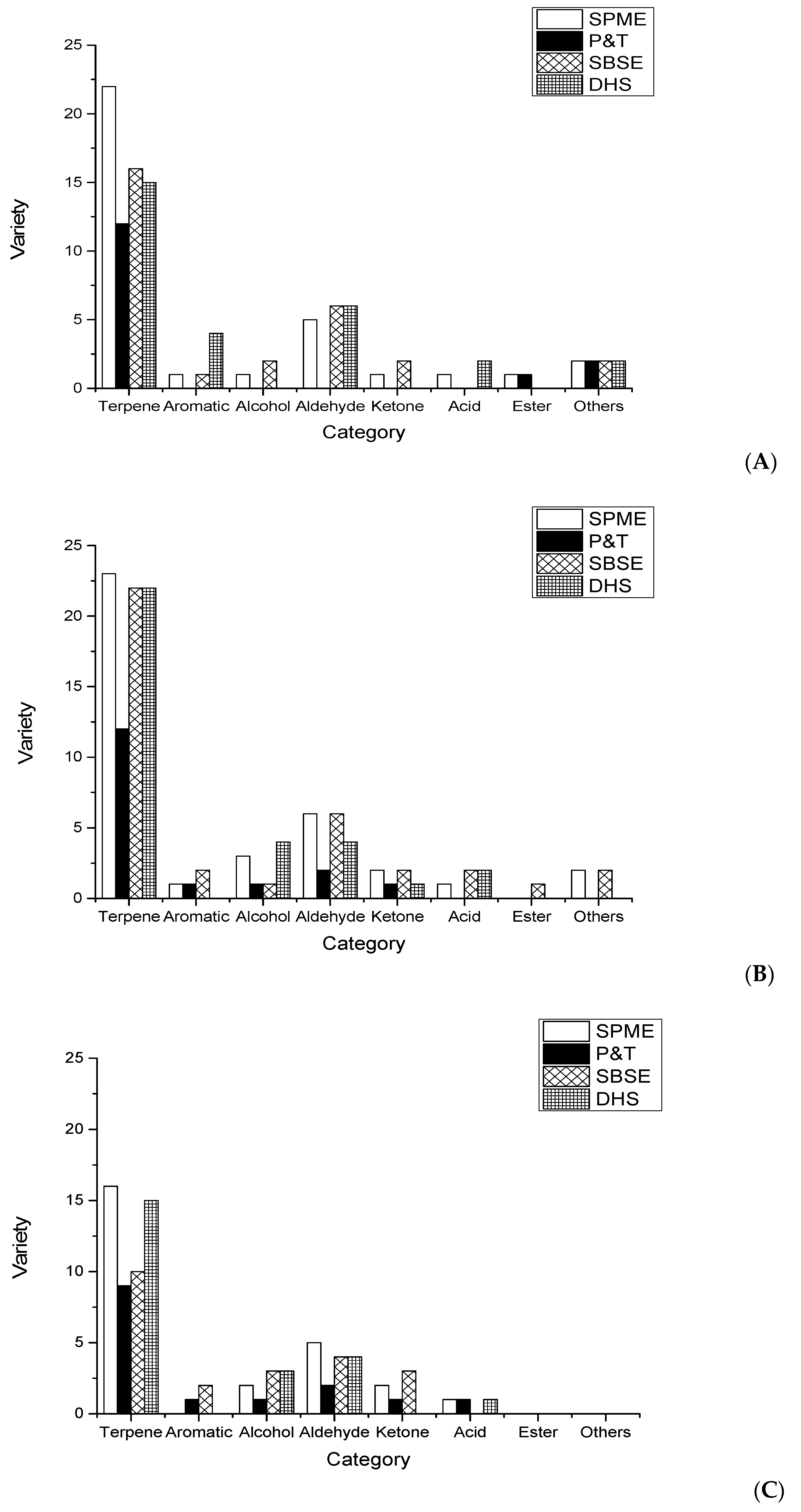
2.1. Comparison of Quantity of Aroma Compounds by the Different Extraction Methods
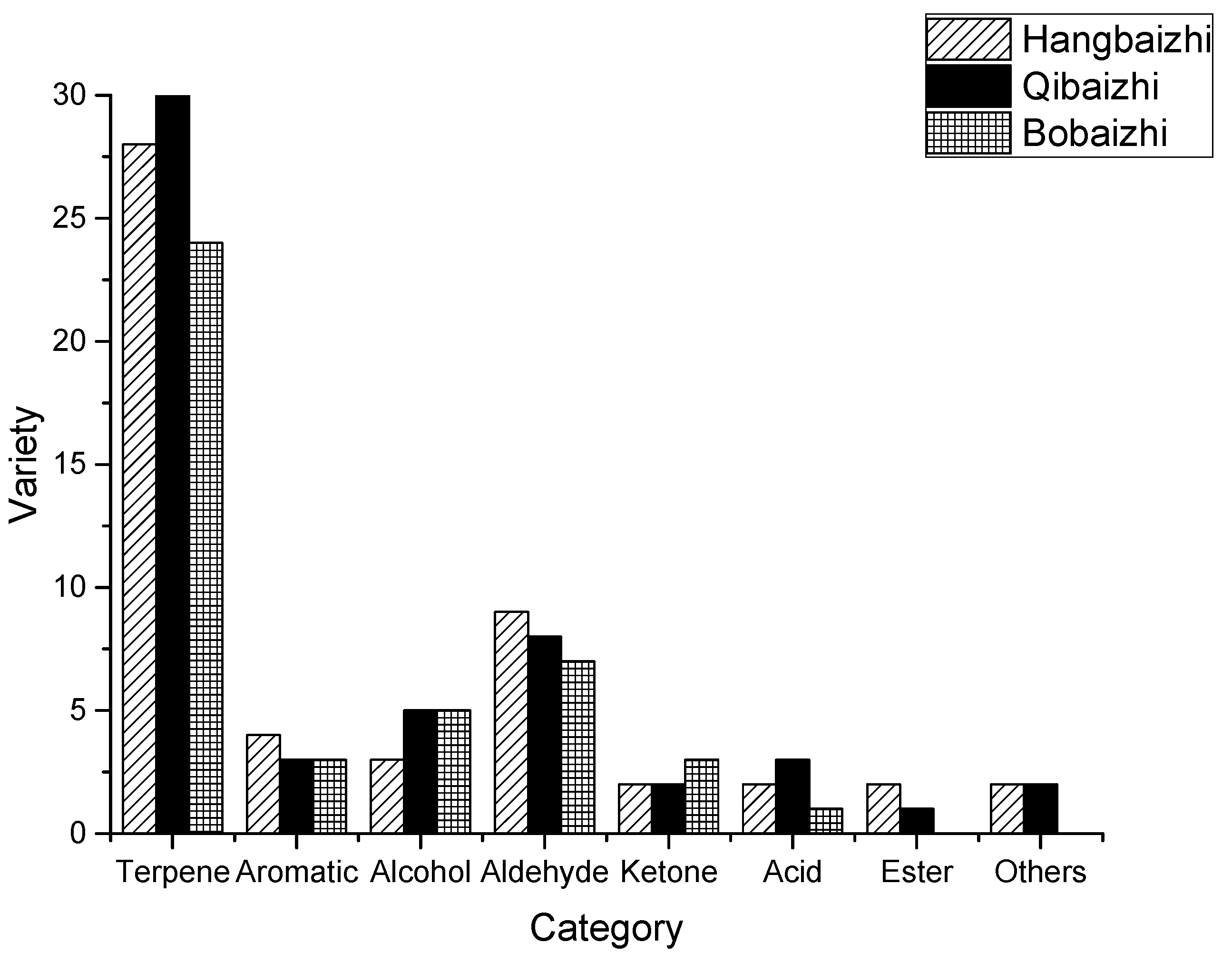
2.2. Comparison of Quality of Aroma Compounds by the Different Extraction Methods
2.3. Key Aroma-Active Compounds Identified by SPME-AEDA
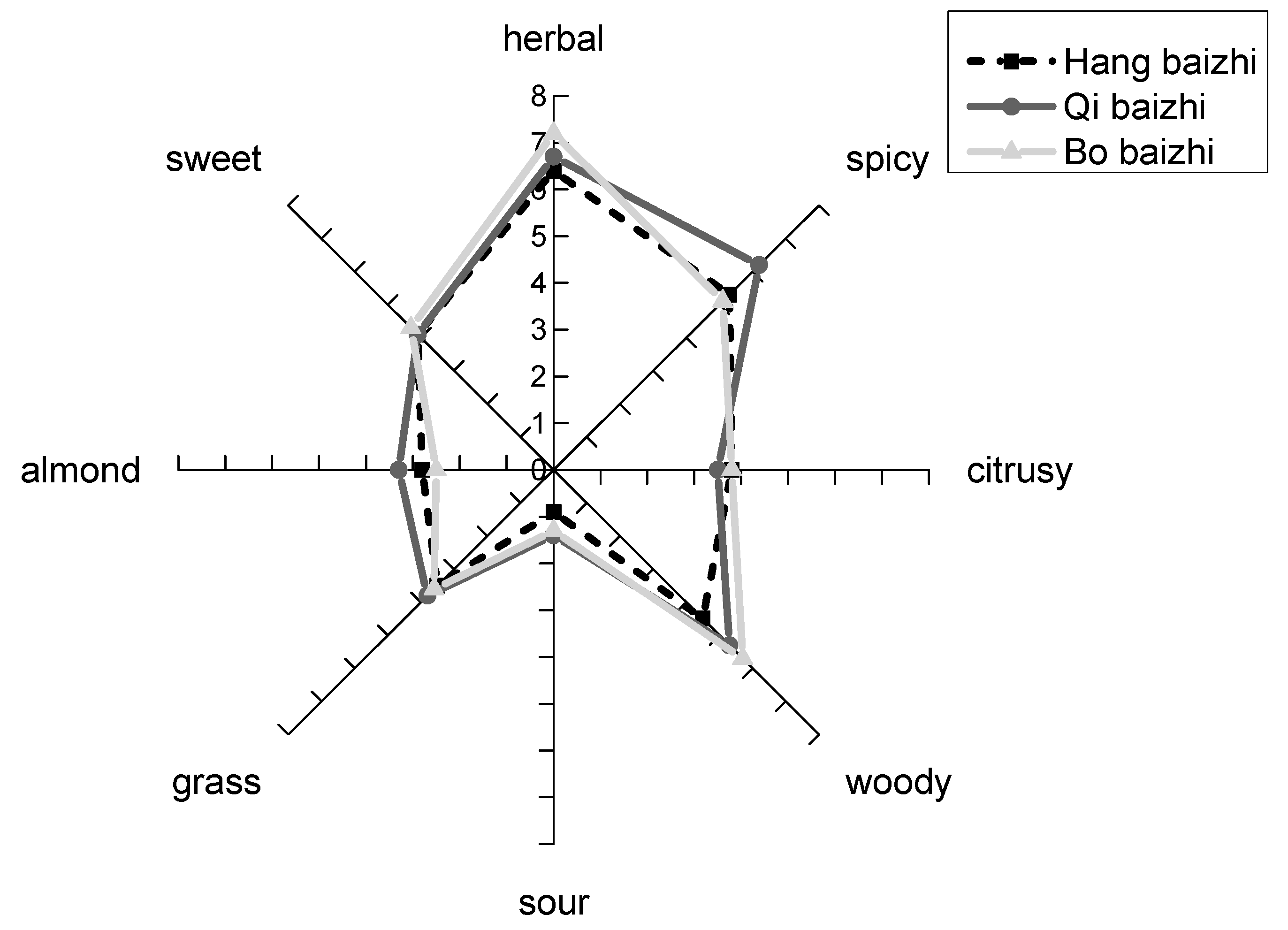
2.4. Sensory Evaluation
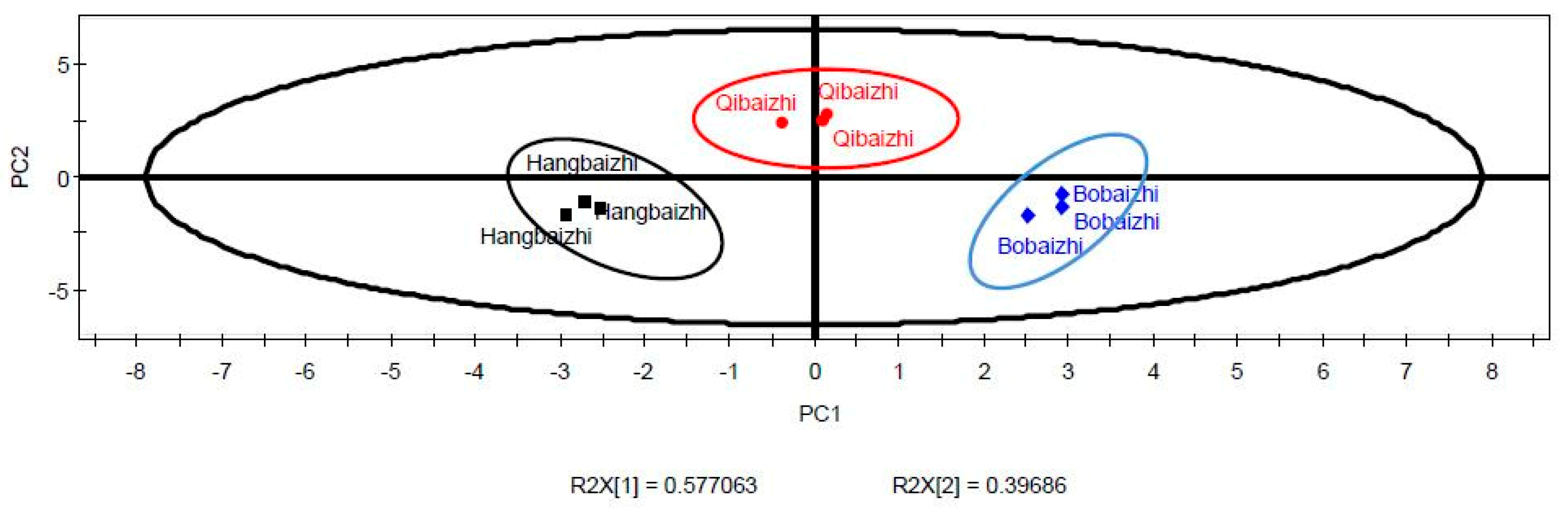
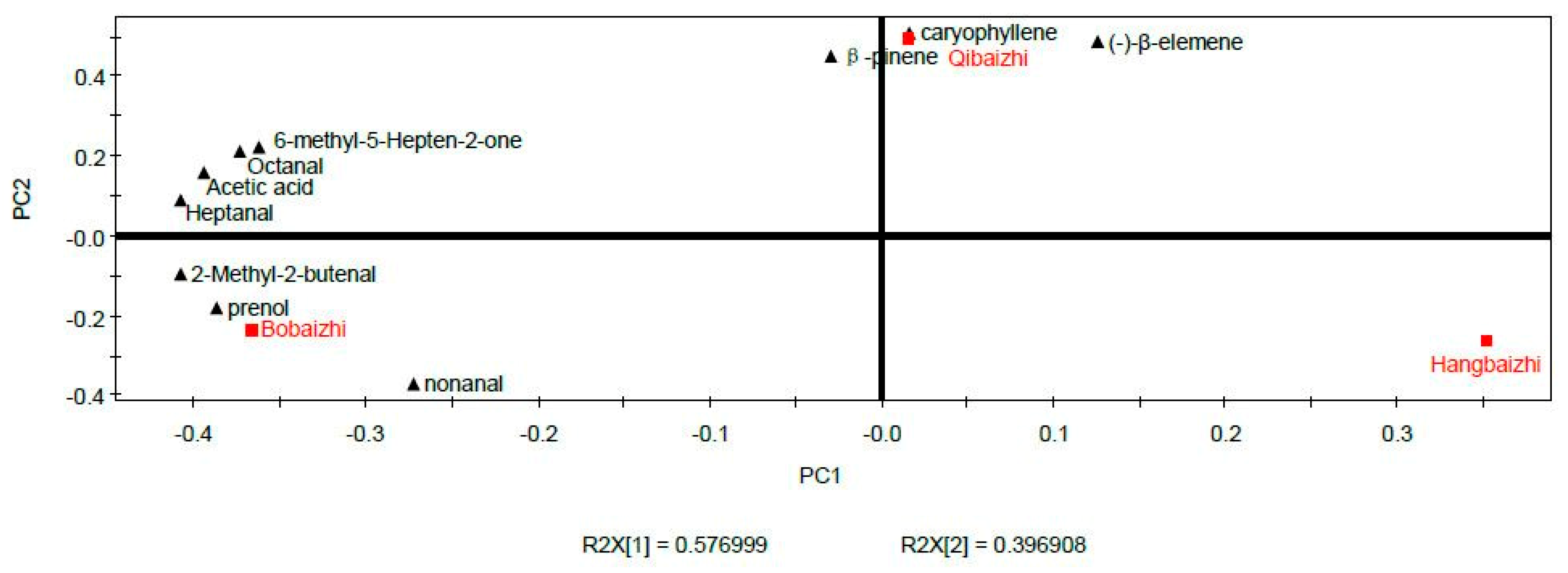
2.5. PCA and PLS-DA of the Aroma Compounds of A. dahurica
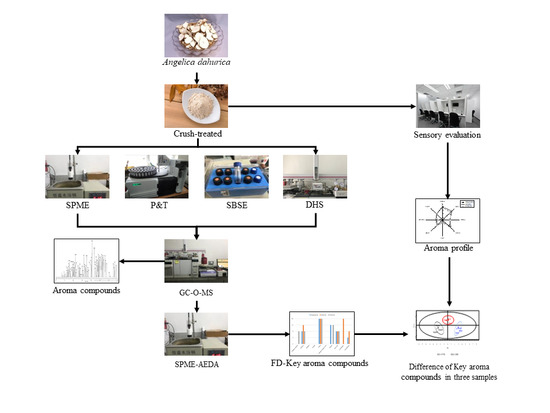
3. Materials and Methods
3.1. Preparation of A. dahurica
3.2. Chemicals
3.3. Extraction of the Volatile Compounds
3.3.1. Extraction of the Aroma Compounds from A. dahurica by SPME
3.3.2. Extraction of the Aroma Compounds from A. dahurica by P&T
3.3.3. Extraction of the Aroma Compounds from A. dahurica by SBSE
3.3.4. Extraction of the Aroma Compounds from A. dahurica by DHS
3.3.5. GC-O-MS Analysis
3.4. Qualitative Analysis of the Volatile Compounds in the Root of A. dahurica
3.5. SPME-AEDA
3.6. Quantitative Analysis of the Key Aroma Compounds in the Root of A. dahurica
3.7. Sensory Evaluation
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chau, C.F.; Wu, S.H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci. Technol. 2006, 17, 313–323. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Wang, G.-H.; Chen, C.-Y.; Tsai, T.-H.; Chen, C.-K.; Cheng, C.-Y.; Huang, Y.-H.; Hsieh, M.-C.; Chung, Y.-C. Evaluation of tyrosinase inhibitory and antioxidant activities of Angelica dahurica root extracts for four different probiotic bacteria fermentations. J. Biosci. Bioeng. 2017, 123, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, I.; Murauer, A.; Ganzera, M. Determination of coumarins in the roots of Angelica dahurica by supercritical fluid chromatography. J. Pharm. Biomed. Anal. 2016, 129, 246–251. [Google Scholar] [CrossRef]
- Wang, X.-H.; Xie, P.-S.; Lam, C.W.K.; Yan, Y.-Z.; Yu, Q.-X. Study of the destructive effect to inherent quality of Angelicae dahuricae radix (Baizhi) by sulfur-fumigated process using chromatographic fingerprinting analysis. J. Pharm. Biomed. Anal. 2009, 49, 1221–1225. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Medical-Pharmaceutical Science & Technology Publishing House: Beijing, China, 2010; p. 97. [Google Scholar]
- Wei, L.; Zhan-Guo, L.; Dan, F.; Peng-Jun, W. Study on aroma components of baizhi angelica by HS-SPME and GC-MS. China Condiment. 2012, 37, 109–112. [Google Scholar]
- Zhao, A.H.; Yang, X.B.; Yang, X.W.; Tao, H.Y.; Yu, J.L.; Wang, W.Q. GC-MS analysis of the chemical components of volatile oil from the root of Angelica dahurica. Chin. J. Pharm. Anal. 2012, 32, 763–768. [Google Scholar]
- Li, L.; Lv, L.; Zhang, W.; Zhao, J.X.; Lv, D.Y.; Zhao, J.Y. GC-MS combined with PCA analysis of the essential oil from two varieties of Radix Angelicae Dahuricae. Chin. J. Pharm. Anal. 2011, 31, 112–118. [Google Scholar]
- Matsushita, T.; Zhao, J.J.; Igura, N.; Shimoda, M. Authentication of commercial spices based on the similarities between gas chromatographic fingerprints. J. Sci. Food Agric. 2018, 98, 2989–3000. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Amanpour, A.; Kelebek, H.; Selli, S. The most aroma-active compounds in shade-dried aerial parts of basil obtained from Iran and Turkey. Ind. Crop. Prod. 2018, 124, 692–698. [Google Scholar] [CrossRef]
- Omar, J.; Olivares, M.; Alonso, I.; Vallejo, A.; Aizpurua-Olaizola, O.; Etxebarria, N. Quantitative Analysis of Bioactive Compounds from Aromatic Plants by Means of Dynamic Headspace Extraction and Multiple Headspace Extraction-Gas Chromatography-Mass Spectrometry. J. Food Sci. 2016, 81, C867–C873. [Google Scholar] [CrossRef] [PubMed]
- Raffo, A.; Masci, M.; Moneta, E.; Nicoli, S.; del Pulgar, J.S.; Paoletti, F. Characterization of volatiles and identification of odor-active compounds of rocket leaves. Food Chem. 2018, 240, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.; Bucking, M.; Steinhart, H. Novel analytical tools for food flavours. Food Res. Int. 2000, 33, 199–209. [Google Scholar] [CrossRef]
- Hoz, K.S.D.L.; Salinas, M.R.; Ferrandino, A. Different coatings for the HS-SBSE grape volatile analysis in model solution: Preliminary results. Food Chem. 2016, 212, 814–820. [Google Scholar]
- Zhao, Y.; Xie, Z.; Niu, Y.; Shi, H.; Chen, P.; Yu, L. Chemical compositions, HPLC/MS fingerprinting profiles and radical scavenging properties of commercial Gynostemma pentaphyllum (Thunb.) Makino samples. Food Chem. 2012, 134, 180–188. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis—A marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef]
- Seonmi, L.; Heera, C.; Kwang-Geun, L. Volatile compounds as markers of tofu (soybean curd) freshness during storage. J. Agric. Food Chem. 2014, 62, 772–779. [Google Scholar]
- Cai, M.G.; Huang, P.A. Purge and Trap Gas Chromatographic Method for Detection of Chlorofluorocarbons in Seawater. Chin. J. Anal. Chem. 2013, 41, 268–272. [Google Scholar] [CrossRef]
- Waehrens, S.S.; Zhang, S.; Hedelund, P.I.; Petersen, M.A.; Byrne, D.V. Application of the fast sensory method ‘Rate-All-That-Apply’ in chocolate Quality Control compared with DHS-GC-MS. Int. J. Food Sci. Technol. 2016, 51, 1877–1887. [Google Scholar] [CrossRef]
- Huanlu, S.; Jianbin, L. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar]
- Ye, L.; Hang, S.; Huan-Lu, S. Comparison of four extraction methods, SPME, DHS, SAFE, Versus SDE, for the analysis of flavor compounds in Natto. Food Anal. Methods 2018, 11, 343–354. [Google Scholar]
- Kim, T.H.; Lee, S.M.; Kim, Y.S.; Kim, K.H.; Oh, S.; Lee, H.J. Aroma dilution method using GC injector split ratio for volatile compounds extracted by headspace solid phase microextraction. Food Chem. 2003, 83, 151–158. [Google Scholar]
- Tominaga, T.; Dubourdieu, D. A novel method for quantification of 2-methyl-3-furanthiol and 2-furanmethanethiol in wines made from Vitis vinifera grape varieties. J. Agric. Food Chem. 2006, 54, 29–33. [Google Scholar] [CrossRef]
- Zhu, M.; Li, E.; He, H. Determination of volatile chemical constitutes in tea by simultaneous distillation extraction, vacuum hydrodistillation and thermal desorption. Chromatographia 2008, 68, 603–610. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| No | Compound | Odour 1 | RI 2 | Extract Method/Identification 3 | |||
|---|---|---|---|---|---|---|---|
| DB-Wax | DB-5 | Hangbaizhi | Qibaizhi | Bobaizhi | |||
| Terpenes | |||||||
| 1 | α-Pinene | pine, turpentine | 1032 | 939 | SPME, SBSE, DHS/MS, RI | SPME, SBSE, DHS/MS, RI | SBSE, DHS/MS, RI |
| 2 | Camphene | camphor | 1075 | P&T, DHS/MS, RI, O | P&T, SBSE, DHS/MS, RI, O | P&T/MS, RI, O | |
| 3 | β-Pinene | pine, resin | 1116 | 981 | SPME, P&T, SBSE, DHS/MS, RI, O, STD | SPME, P&T, SBSE, DHS/MS, RI, O, STD | SPME, P&T, SBSE, DHS/MS, RI, O, STD |
| 4 | Sabinene | turpentine, woody | 1123 | 972 | SBSE/MS, RI | SPME, P&T, SBSE, DHS/MS, RI | - |
| 5 | β-Myrcene | balsamic, spice | 1145 | 992 | SPME, SBSE, DHS/MS, RI | SPME, DHS/MS, RI | - |
| 6 | α-Phellandrene | turpentine, spice | 1166 | SPME, P&T/MS, RI | DHS/MS, RI | - | |
| 7 | α-Terpinene | lemon | 1178 | - | SPME, SBSE/MS, RI | - | |
| 8 | d-Limonene | citrus, mint | 1201 | 1030 | SPME, P&T, SBSE, DHS/MS, RI | SPME, P&T, SBSE, DHS/MS, RI | SPME, P&T, SBSE, DHS/MS, RI |
| 9 | β-Phellandrene | mint, turpentine | 1209 | SPME, P&T, SBSE/MS, RI | SPME, P&T, SBSE, DHS/MS, RI | P&T, DHS/MS, RI | |
| 10 | Eucalyptol | mint, sweet | 1213 | SBSE/MS, RI | P&T, SBSE/MS, RI | SBSE/MS, RI | |
| 11 | γ-Terpinene | gasoline, turpentine | 1238 | SBSE/MS, RI | SPME, SBSE, DHS/MS, RI | - | |
| 12 | trans-β-Ocimene | sweet, herb | 1242 | 1038 | SPME, P&T, SBSE, DHS/MS, RI | SPME, P&T, SBSE/MS, RI, O | SPME, P&T/MS, RI, O |
| 13 | p-Cymene | solvent, citrus | 1261 | SPME, SBSE/MS, RI | SPME, SBSE, DHS/MS, RI | P&T, SBSE, DHS/MS, RI | |
| 14 | α-Copaene | woody, spice | 1488 | SPME, P&T/MS, RI, O | SPME, P&T, SBSE, DHS/MS, RI, O | SPME, P&T, DHS/MS, RI, O | |
| 15 | Linalool | flower, lavender | 1537 | - | - | SBSE/MS, RI | |
| 16 | β-Cubebene | citrus, fruit | 1546 | - | SBSE/MS, RI | - | |
| 17 | Selina-5,11-diene | woody | 1553 | SPME/MS, O | - | - | |
| 18 | Longifolene-(V4) | woody | 1573 | SPME, DHS/MS, O | - | - | |
| 19 | (−)-β-Elemene | herb | 1583 | 1398 | SPME, P&T, SBSE, DHS/MS, RI, O, STD | SPME, SBSE, DHS/MS, O, STD | SPME, SBSE, DHS/MS, RI, O, STD |
| 20 | Caryophyllene | woody, spice | 1594 | 1467 | SPME, P&T, DHS/MS, RI, O, STD | SPME, P&T, SBSE, DHS/MS, RI, O, STD | SPME, P&T, SBSE, DHS/MS, RI, O, STD |
| 21 | Aromandendrene | woody | 1600 | 1475 | SPME, P&T, DHS/MS, RI | SPME, P&T, DHS/MS, RI | SPME/MS, RI, O |
| 22 | γ-Elemene | green, woody, oil | 1636 | 1425 | SPME, SBSE, DHS/MS, RI | SPME, SBSE, DHS/MS, RI, O | SPME, P&T, SBSE, DHS/MS, RI |
| 23 | cis-β-Farnesene | citrus, green | 1648 | SPME, SBSE/MS, RI | SPME/MS, RI | - | |
| 24 | Humulene | woody | 1663 | 1467 | SPME, P&T/MS, RI, O | SPME, P&T, SBSE, DHS/MS, RI, O | SPME, DHS/MS, RI |
| 25 | γ-Muurolene | herb, woody, spice | 1681 | 1475 | SPME/MS, RI | SPME, SBSE, DHS/MS, RI, O | - |
| 26 | δ-Elemene | woody | 1688 | 1340 | SPME, DHS/MS, RI | SPME, P&T, SBSE, DHS/MS, RI | SPME, DHS/MS, RI |
| 27 | β-Selinene | herb | 1711 | 1436 | SPME, P&T, DHS/MS, RI | SPME, SBSE, DHS/MS, RI | SPME, DHS/MS, RI |
| 28 | δ-Cadinene | thyme, woody | 1749 | - | DHS/MS, RI | DHS/MS, RI | |
| 29 | α-Gurjunene | woody, balsamic | 1760 | - | DHS/MS, RI | SPME, DHS/MS, RI, O | |
| 30 | α-Guaiene | woody, balsamic | 1801 | - | SPME/MS, RI | - | |
| 31 | γ-Selinene | woody | 1803 | 1455 | SBSE, DHS/MS, RI, O | - | SPME/MS, RI |
| 32 | β-Guaiene | woody, spice | 1831 | 1483 | - | - | SPME, DHS/MS, RI |
| 33 | Germacrene B | woody, earth, spice | 1864 | 1562 | SPME/MS, RI | SPME, DHS/MS, RI | SPME/MS, RI |
| 34 | Caryophyllene oxide | herb, sweet, spice | 1962 | SPME, SBSE/MS, RI, O | SPME, SBSE/MS, RI, O | SPME/MS, RI, O | |
| 35 | Spathulenol | herb, fruit | 2129 | SBSE, DHS/MS, RI | SBSE/MS, RI | SBSE/MS, RI | |
| Aromatics | |||||||
| 36 | Toluene | paint | 1042 | 773 | DHS/MS, RI | - | - |
| 37 | Benzene, 1,3-dimethyl- | plastic | 1150 | 802 | DHS/MS, RI | - | - |
| 38 | o-Xylene | geranium | 1183 | 888 | DHS/MS, RI | - | - |
| 39 | α-p-Dimethylstyrene | citrus, pine | 1414 | - | - | P&T/MS, RI | |
| 40 | α,3-Dimethylstyrene | cocoa | 1430 | - | P&T/MS, RI | - | |
| 41 | Estragole | licorice, anise | 1655 | 1200 | - | - | SBSE/MS, RI |
| 42 | Anethole | spice | 1809 | - | SBSE/MS, RI | - | |
| 43 | Isoelemicin | spice, flower | 1944 | 1596 | SPME, SBSE, DHS/MS, RI | SPME, SBSE/MS, RI | SBSE/MS, RI |
| Alcohols | |||||||
| 44 | 3-Buten-2-ol, 2-methyl- | herb | 968 | - | DHS/MS, RI | DHS/MS, RI | |
| 45 | Prenol | herb | 1127 | 779 | SPME/MS, RI, O, STD | SPME, DHS/MS, RI, O, STD | SPME, P&T, DHS/MS, RI, O, STD |
| 46 | 1-Hexanol | resin, flower | 1360 | - | P&T, DHS/MS, RI, O | - | |
| 47 | Terpinen-4-ol | turpentine, nutmeg | 1591 | 1179 | SBSE/MS, RI | - | SBSE/MS, RI |
| 48 | Benzyl alcohol | sweet, flower | 1865 | - | SPME/MS, RI | SPME, SBSE, DHS/MS, RI | |
| 49 | 1-Dodecanol | fat, wax | 1972 | 1577 | SBSE/MS, RI | SPME, SBSE, DHS/MS, RI | - |
| 50 | 1-Hexadecanol | wax, flower | 2378 | - | - | SBSE/MS, RI | |
| Aldehydes | |||||||
| 51 | Butanal, 3-methyl- | malt | 910 | DHS/MS, RI | - | DHS/MS, RI | |
| 52 | Hexanal | grass, tallow, fat | 1084 | 801 | SPME, SBSE, DHS/MS, RI | SPME, P&T, SBSE, DHS/MS, RI, O | SPME, SBSE, DHS/MS, RI |
| 53 | 2-Methyl-2-butenal | green, fruit | 1101 | 753 | SPME, DHS/MS, RI, O, STD | SPME, DHS/MS, RI, O, STD | SPME, DHS/MS, RI, O, STD |
| 54 | Heptanal | fat, citrus, rancid | 1174 | 903 | SPME, SBSE, DHS/MS, RI, O, STD | SPME, P&T, SBSE/MS, RI, O, STD | SPME, SBSE/MS, RI, O, STD |
| 55 | Octanal | fat, soap, lemon | 1280 | 1006 | SPME, SBSE/MS, RI, O, STD | SPME, SBSE, DHS/MS, RI, O, STD | SPME, P&T, SBSE, DHS/MS, RI, O, STD |
| 56 | (E)-2-Octenal | green, nut, fat | 1345 | 1060 | - | SBSE/MS, RI | - |
| 57 | Nonanal | fat, citrus, green | 1385 | 1104 | SPME, SBSE, DHS/MS, RI, O, STD | SPME, SBSE, DHS/MS, RI, O, STD | SPME, P&T/MS, RI, O, STD |
| 58 | Decanal | soap, orange peel | 1484 | 1209 | - | SPME/MS, RI | - |
| 59 | Benzaldehyde | almond, sugar | 1495 | 960 | DHS/MS, RI | - | - |
| 60 | (E)-2-Nonenal | cucumber, fat | 1527 | 1162 | SBSE/MS, RI | SBSE/MS, RI | SBSE/MS, RI, O |
| 61 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | lemon | 1667 | SBSE/MS, RI | - | - | |
| Ketones | |||||||
| 62 | 6-Methyl-5-hepten-2-one | pepper, mushroom, rubber | 1336 | 974 | SPME, SBSE/MS, RI, O, STD | SPME, SBSE/MS, RI, O, STD | SPME, SBSE/MS, RI, O, STD |
| 63 | 2-Nonanone | hot milk, green | 1388 | 1093 | SBSE/MS, RI | SPME, P&T, SBSE, DHS/MS, RI | SPME, SBSE/MS, RI |
| 64 | Camphor | camphor | 1491 | - | - | P&T, SBSE/MS, RI | |
| Acids | |||||||
| 65 | Acetic acid | sour | 1450 | SPME, DHS/MS, RI, O, STD | SPME, SBSE, DHS/MS, RI, O, STD | SPME, P&T, DHS/MS, RI, O, STD | |
| 66 | Hexanoic acid | sweat | 1829 | 1019 | - | DHS/MS, RI | - |
| 67 | Oleic Acid | fat | 2430 | 2082 | - | SBSE/MS, RI | - |
| 68 | Dodecanoic acid | metal | 2517 | DHS/MS, RI | - | - | |
| Esters | |||||||
| 69 | Vinyl acetate | sour | 990 | P&T/MS, O | - | - | |
| 70 | ethyl-(E)-cinnamate | flower, honey | 2097 | SPME/MS, RI | - | - | |
| 71 | γ-Decalactone | peach, fat | 2103 | - | SBSE/MS, RI | - | |
| Other Compounds | |||||||
| 72 | UN1 | amaretto | 1286 | SPME, P&T, SBSE, DHS/O | SPME, SBSE/O | - | |
| 73 | UN2 | grass | 1478 | SPME, P&T, SBSE, DHS/O | SPME, SBSE/O | - | |
| No | Compound | CAS | RI | Odor | FD | |||
|---|---|---|---|---|---|---|---|---|
| DB-Wax | DB-5 | Hangbaizhi | Qibaizhi | Bobaizhi | ||||
| 1 | 2-Methyl-2-butenal | 1115-11-3 | 1101 | 753 | green, fruity | 1 | 1 | 9 |
| 2 | β-Pinene | 127-91-3 | 1116 | 981 | pine, resin, turpentine | 9 | 27 | 9 |
| 3 | Prenol | 556-82-1 | 1127 | 779 | herb | 81 | 81 | 81 |
| 4 | Heptanal | 111-71-7 | 1174 | 903 | fatty, citrusy, rancid | 1 | 1 | 1 |
| 5 | trans-β-Ocimene | 3779-61-1 | 1242 | 1038 | sweetie, herb | - | 1 | 1 |
| 6 | Octanal | 124-13-0 | 1280 | 1006 | fatty, soap, lemon | 1 | 1 | 1 |
| 7 | 6-Methyl-5-hepten-2-one | 110-93-0 | 1336 | 974 | mushroom | 1 | 1 | 1 |
| 8 | Nonanal | 124-19-6 | 1385 | 1104 | citrusy, green | 27 | 1 | 27 |
| 9 | Acetic acid | 64-19-7 | 1450 | sour | 9 | 9 | 9 | |
| 10 | α-Copaene | 3856-25-5 | 1488 | woody, spice | 27 | 3 | 27 | |
| 11 | Caryophyllene | 87-44-5 | 1594 | 1467 | woody, spice | 3 | 9 | 1 |
| 12 | (−)-β-Elemene | 515-13-9 | 1595 | 1398 | spice | 1 | 81 | 1 |
| 13 | Aromadendrene | 489-39-4 | 1600 | 1475 | woody | - | - | 9 |
| 14 | γ-Elemene | 29873-99-2 | 1636 | 1425 | green, woody, oil | - | 1 | - |
| 15 | Humulene | 6753-98-6 | 1663 | 1467 | woody | - | 9 | - |
| 16 | γ-Muurolene | 30021-74-0 | 1681 | 1475 | herb, woody, spice | - | 27 | - |
| 17 | α-Gurjunene | 489-40-7 | 1760 | woody | - | - | 81 | |
| 18 | Caryophyllene oxide | 1139-30-6 | 1962 | herb, sweetie, spice | 3 | 3 | 1 | |
| No | Compound | Y | R2 | Hangbaizhi | Qibaizhi | Bobaizhi | |||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (ng/g) | FD | Concentration (ng/g) | FD | Concentration (ng/g) | FD | ||||
| 1 | 2-Methyl-2-butenal | y = 11,238x + 468,104 | 0.9981 | 124.13 ± 9.19 a | 1 | 179.65 ± 10.26 b | 1 | 304.83 ± 15.53 c | 9 |
| 2 | β-Pinene | y = 8207.2x + 15,568 | 0.9904 | 20.63 ± 1.29 a | 9 | 26.59 ± 0.48 b | 27 | 21.37 ± 2.60 a | 9 |
| 3 | Heptanal | y = 2918.3x − 29,754 | 0.9969 | 45.99 ± 2.35 a | 1 | 105.64 ± 6.88 b | 1 | 140.09 ± 5.67 c | 1 |
| 4 | Octanal | y = 5022x + 33,019 | 0.9989 | 50.62 ± 2.61 a | 1 | 141.46 ± 13.48 b | 1 | 155.08 ± 7.19 b | 1 |
| 5 | Prenol | y = 1560.4x + 10,598 | 0.9917 | 109.15 ± 6.42 a | 81 | 142.78 ± 20.28 a | 81 | 361.94 ± 22.46 b | 81 |
| 6 | 6-Methyl-5-hepten-2-one | y = 32,472x + 5788.4 | 0.9983 | 3.24 ± 0.09 a | 1 | 4.36 ± 0.22 b | 1 | 4.48 ± 0.13 b | 1 |
| 7 | Nonanal | y = 7734.4x + 41,903 | 0.9985 | 80.44 ± 4.61 b | 27 | 67.37 ± 5.06 a | 1 | 104.88 ± 3.26 c | 27 |
| 8 | Acetic acid | y = 29,396x + 8 × 106 | 0.9952 | 148.03 ± 1.32 a | 9 | 286.38 ± 11.27 b | 9 | 331.44 ± 14.04 c | 9 |
| 9 | (−)-β-Elemene | y = 15,828x − 354,790 | 0.9954 | 310.28 ± 13.09 b | 1 | 789.51 ± 21.95 c | 81 | 126.29 ± 0.84 a | 1 |
| 10 | Caryophyllene | y = 26,536x + 71,101 | 0.9951 | 21.6 ± 2.46 a | 3 | 73.04 ± 1.71 b | 9 | 21.47 ± 0.71 a | 1 |
| Principal Component | Eigenvalue | Contribution (%) | Cumulative Contribution (%) |
|---|---|---|---|
| 1 | 5.19 | 57.7 | 57.7 |
| 2 | 3.57 | 39.7 | 97.4 |
| 3 | 0.155 | 1.72 | 99.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Guo, J.; Li, T.; Zhao, M.; Zou, T.; Song, H.; Alim, A. Comparison and Identification of the Aroma-Active Compounds in the Root of Angelica dahurica. Molecules 2019, 24, 4352. https://doi.org/10.3390/molecules24234352
Hu D, Guo J, Li T, Zhao M, Zou T, Song H, Alim A. Comparison and Identification of the Aroma-Active Compounds in the Root of Angelica dahurica. Molecules. 2019; 24(23):4352. https://doi.org/10.3390/molecules24234352
Chicago/Turabian StyleHu, Die, Junrui Guo, Ting Li, Mu Zhao, Tingting Zou, Huanlu Song, and Aygul Alim. 2019. "Comparison and Identification of the Aroma-Active Compounds in the Root of Angelica dahurica" Molecules 24, no. 23: 4352. https://doi.org/10.3390/molecules24234352
APA StyleHu, D., Guo, J., Li, T., Zhao, M., Zou, T., Song, H., & Alim, A. (2019). Comparison and Identification of the Aroma-Active Compounds in the Root of Angelica dahurica. Molecules, 24(23), 4352. https://doi.org/10.3390/molecules24234352