Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production
Abstract
1. Introduction
2. Results
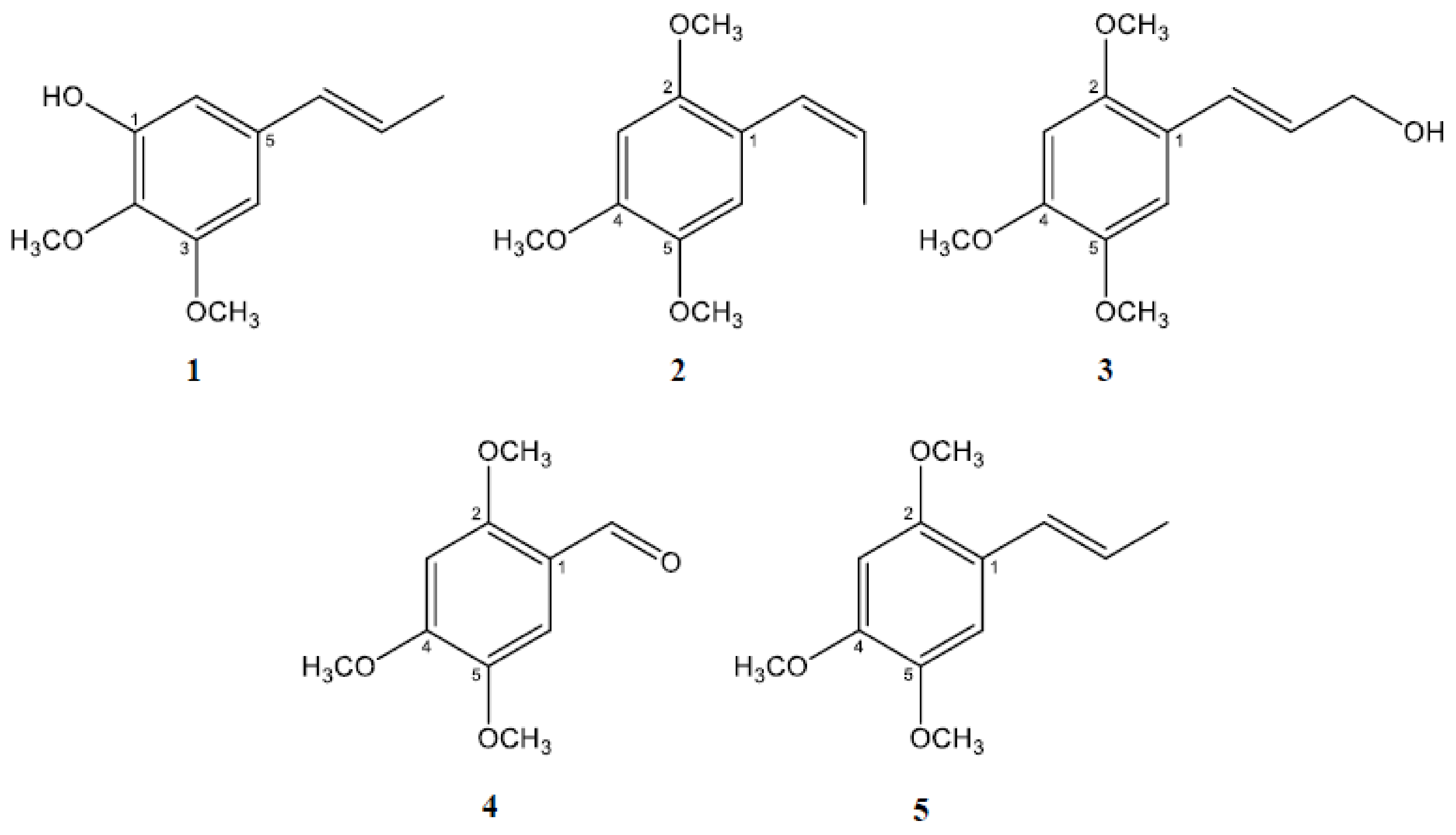
2.1. Isolation and Characterization of the Active Constituents Inhibiting Aβ Aggregation
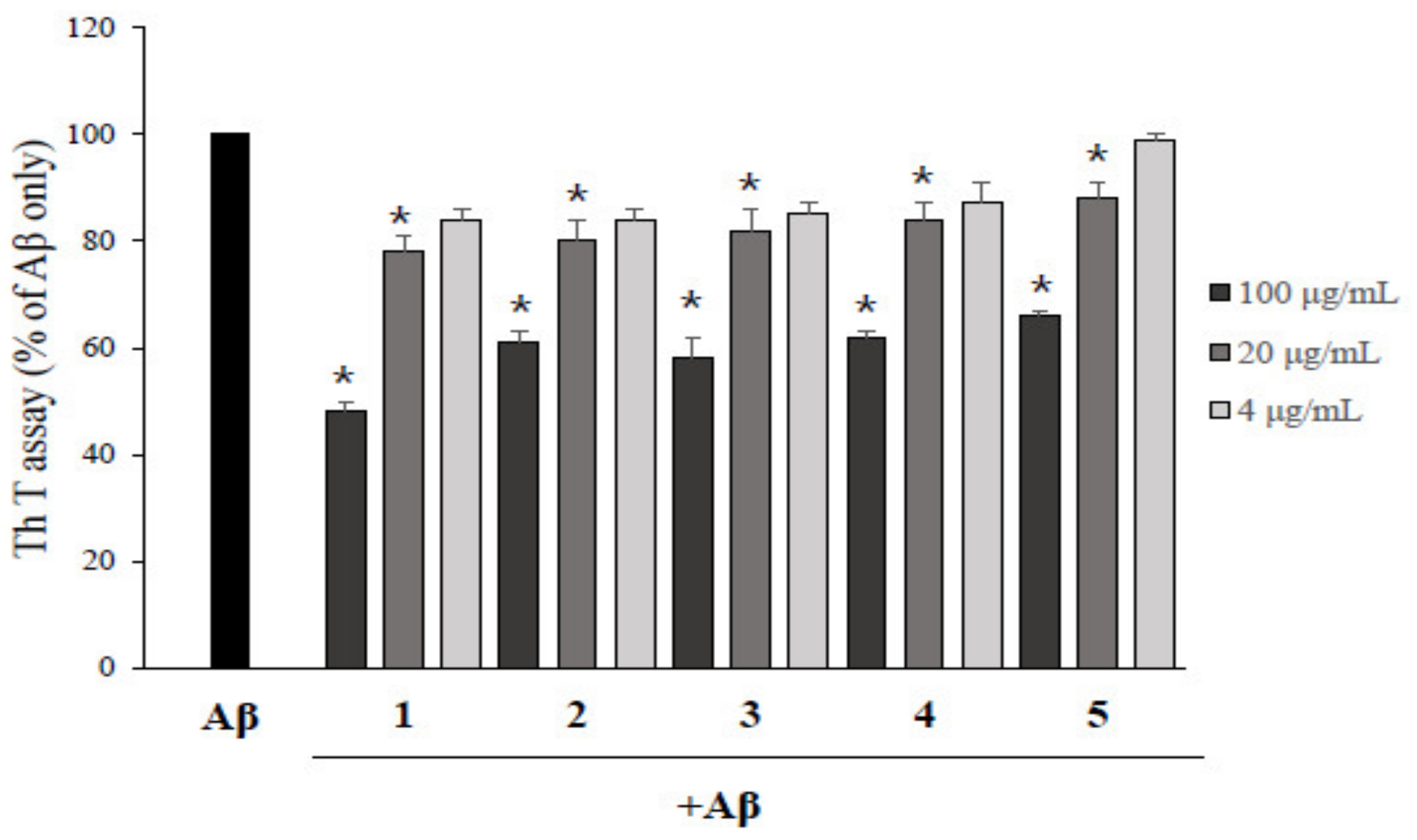
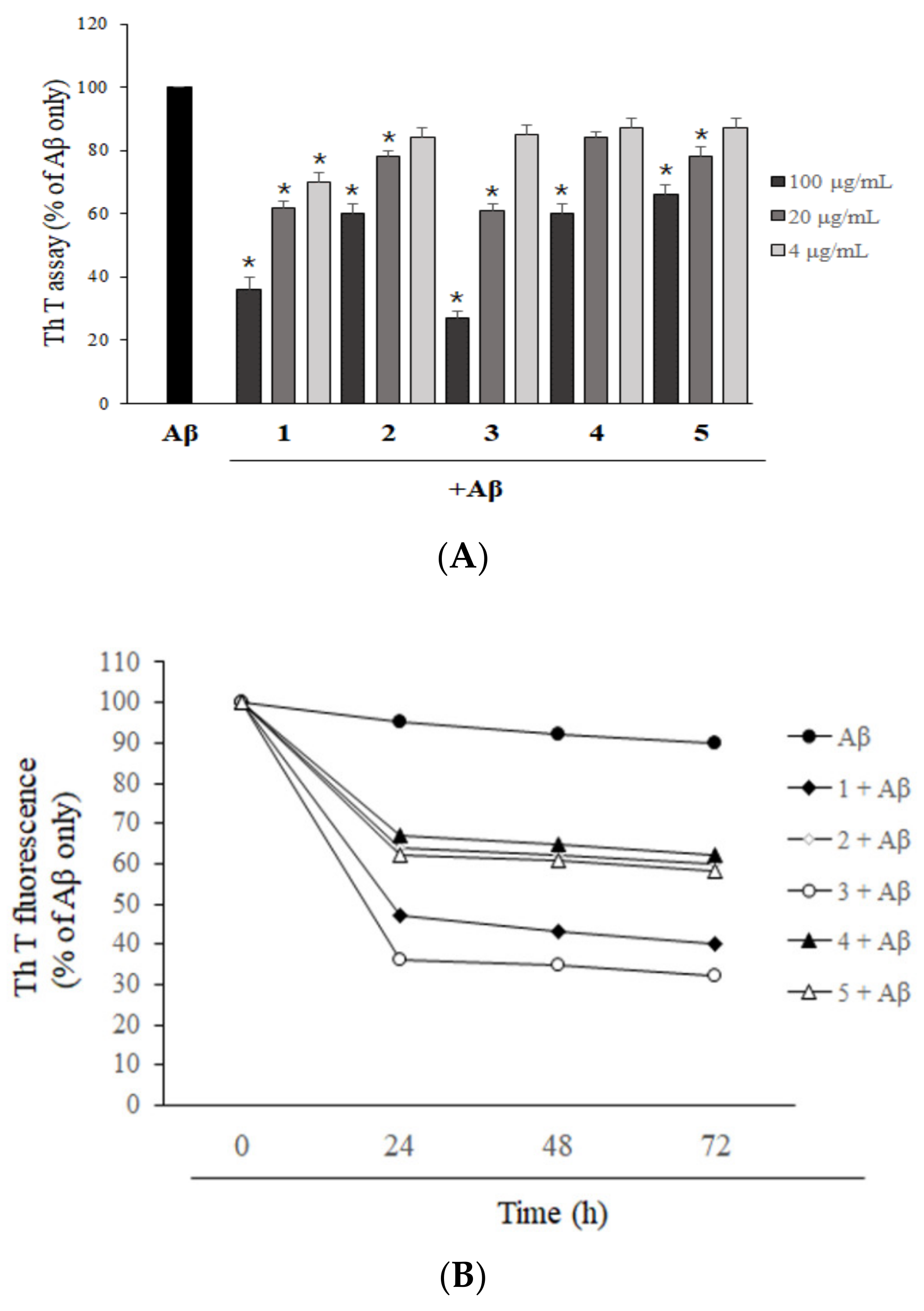
2.2. Asarone Derivatives Inhibit Aβ Aggregation
2.3. Asarone Derivatives Increase the Disaggregation of Pre-Aggregated Aβ
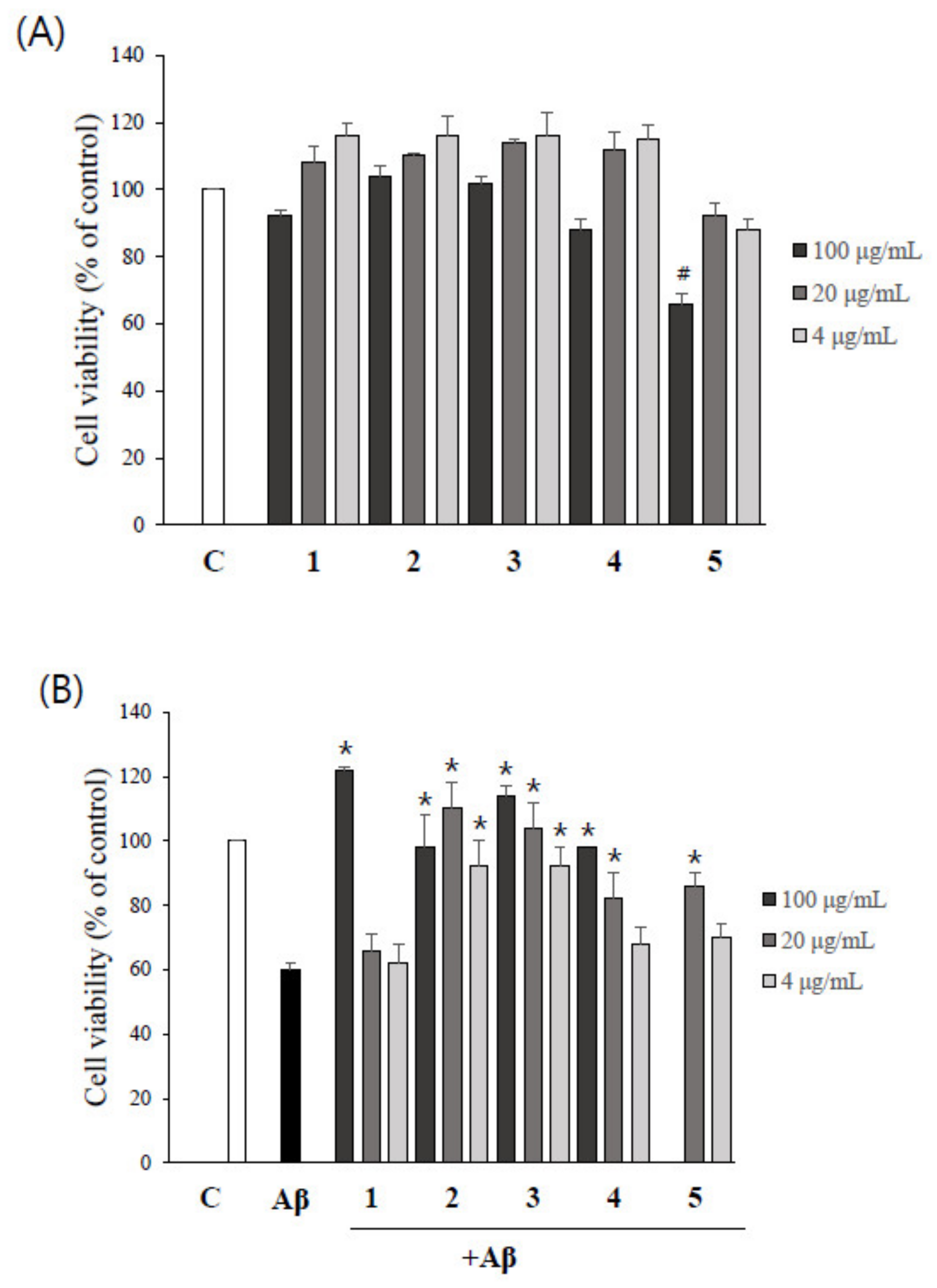
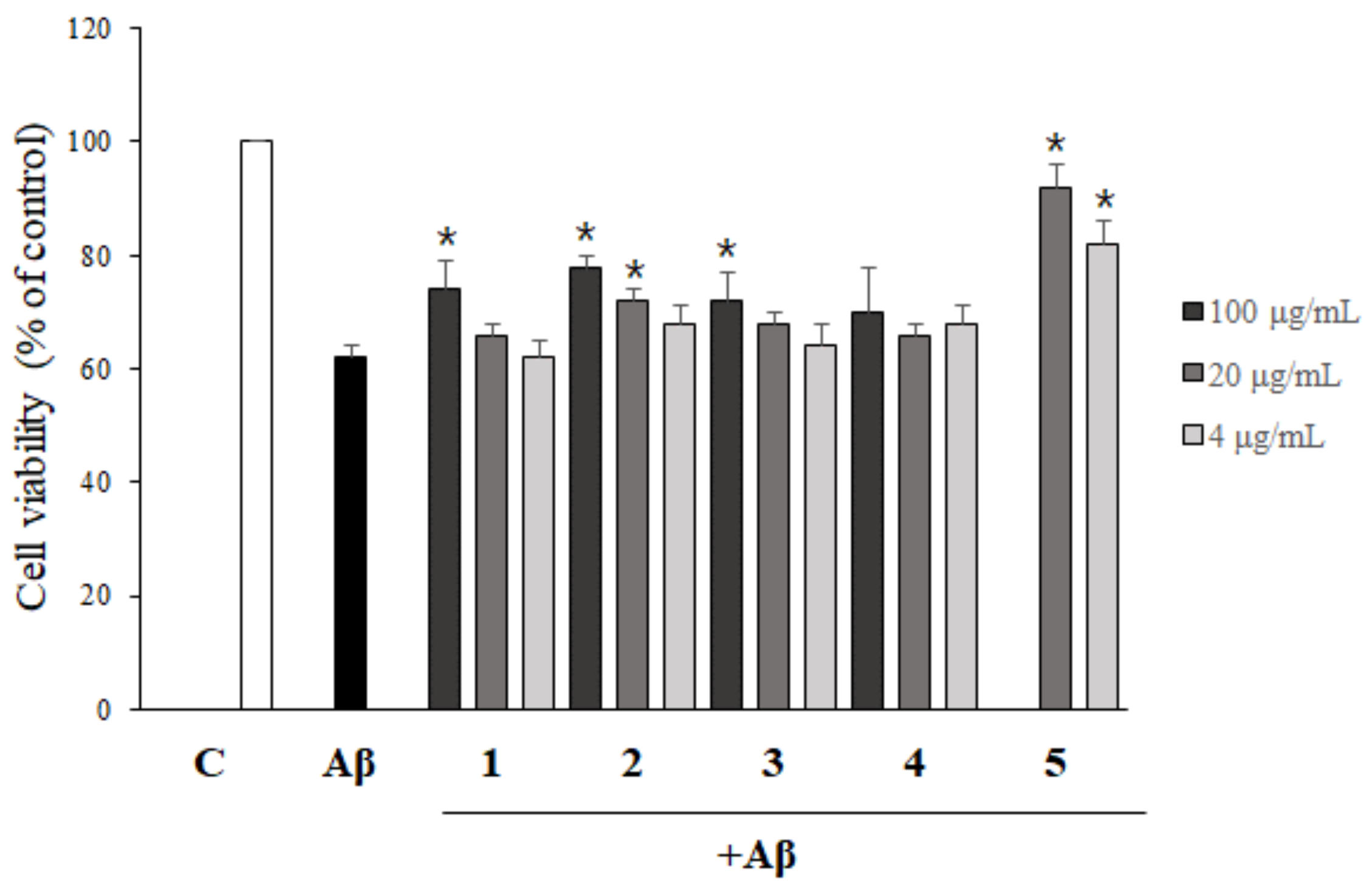
2.4. Asarone Derivatives Protect PC12 Cells from Aβ-Induced Toxicity
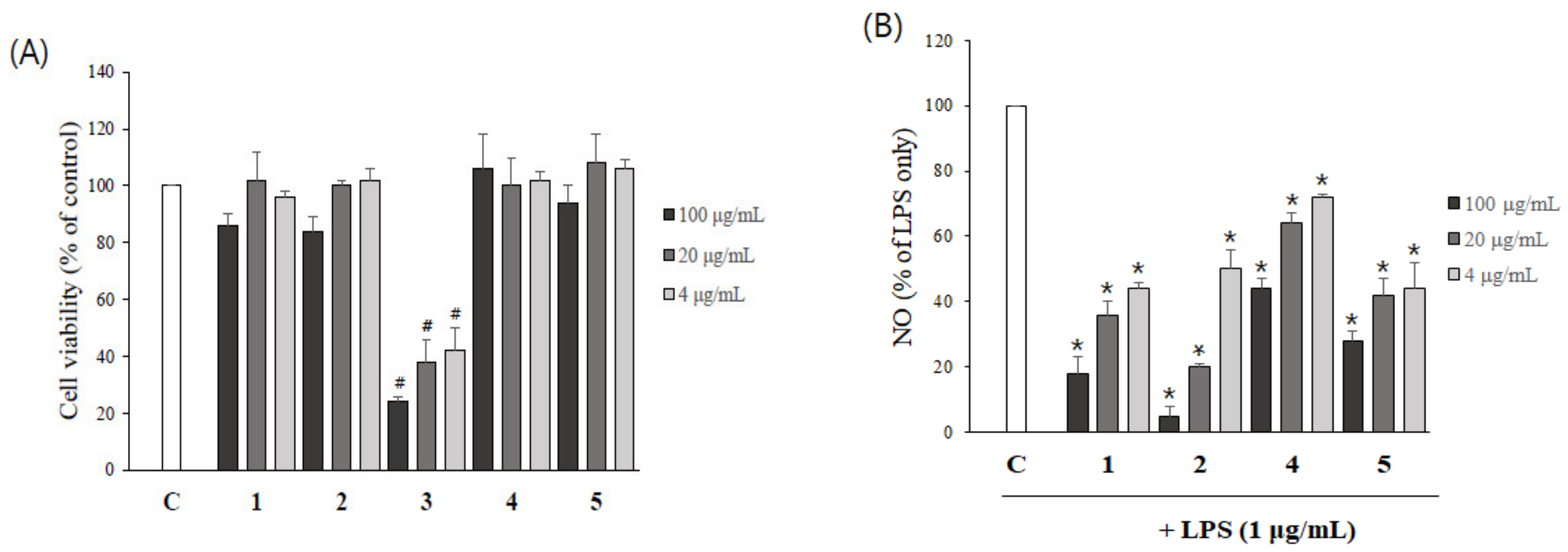
2.5. The Inhibition of Aβ Aggregation by Asarone Derivatives Rescues PC12 Cells from Aβ Toxicity
2.6. Asarone Derivatives Reduce NO Production in LPS-Stimulated Microglial Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Extracts and Isolation
4.4. Thioflavin T (ThT) Assay
4.5. Cell Viabiltiy Assay
4.6. Determination of NO production
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992, 256, 184. [Google Scholar] [CrossRef]
- Mudher, A.; Lovestone, S. Alzheimer’s disease–do tauists and baptists finally shake hands? Trends Neurosci. 2002, 25, 22–26. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Olsson, F.; Schmidt, S.; Althoff, V.; Munter, L.M.; Jin, S.; Rosqvist, S.; Lundkvist, J. Characterization of Intermediate Steps in Amyloid Beta (Aβ) Production under Near-native Conditions. J. Bio. Chem. 2014, 289, 1540–1550. [Google Scholar] [CrossRef]
- Yang, S.G.; Wang, W.Y.; Ling, T.J.; Feng, Y.; Du, X.T.; Zhang, X.; Liu, R.T. Alpha-tocopherol quinone inhibits beta-amyloid aggregation and cytotoxicity, disaggregates preformed fibrils and decreases the production of reactive oxygen species, NO and inflammatory cytokines. Neurochem. Int. 2010, 57, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla Frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Woo, K.W.; Lee, K.R.; Lee, S.K.; Kim, H.P. Inhibition of proinflammatory cytokine generation in lung inflammation by the leaves of Perilla frutescens and its constituents. Biomol. Ther. 2014, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Furuta, Y.; Wakushima, H.; Fujii, H.; Saito, K.I.; Kano, Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytoth. Res. 2003, 17, 240–243. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol. Pharm. Bull. 2002, 25, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol. Pharm. Bull. 2003, 26, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Hur, J.M.; Yang, E.J.; Jun, M.; Park, H.J.; Lee, K.B.; Song, K.S. β-Secretase (BACE1) inhibitors from Perilla frutescens var. acuta. Arch. Pharm. Rer. 2008, 31, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, M.S.; Kim, S.; Hwang, K.W.; Park, S.Y. Anti-amyloidogenic effects of Perilla frutescens var. acutaon beta-amyloid aggregation and disaggregation. J. Food Biochem. 2017, 41, e12393. [Google Scholar] [CrossRef]
- Sairafianpour, M.; Kayser, O.; Christensen, J.; Asfa, M.; Witt, M.; Stærk, D.; Jaroszewski, J.W. Leishmanicidal and Antiplasmodial Activity of Constituents of Smirnowia i ranica. J. Nat. Prod. 2002, 65, 1754–1758. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.Y.; Yun, B.S.; Hwang, B.K. Antifungal activity of β-asarone from rhizomes of Acorus gramineus. J. Agr. Food Chem. 2004, 52, 776–780. [Google Scholar] [CrossRef]
- Cartus, A.T.; Stegmuller, S.; Simson, N.; Wahl, A.; Neef, S.; Kelm, H.; Schrenk, D. Hepatic Metabolism of Carcinogenic β-asarone. Chem. Res. Toxicol. 2015, 28, 1760–1773. [Google Scholar] [CrossRef]
- Czepa, A.; Hofmann, T. Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree. J. Agr. Food Chem. 2003, 51, 3865–3873. [Google Scholar] [CrossRef]
- Siergiejczyk, L.; Poplawski, J.; Lozowicka, B.; Dubis, A.; Lachowska, B. 1H and 13C NMR spectral analysis of (E)-asarone and its isomers. Mag. Reson. Chem. 2000, 38, 1037–1038. [Google Scholar] [CrossRef]
- Kirkitadze, M.D.; Bitan, G.; Teplow, D.B. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: The emerging role of oligomeric assemblies. J. Neurosci. Res. 2002, 69, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N–terminus of β–amyloid. Nat. Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Sisodia, S.S.; Koo, E.H.; Beyreuther, K.; Unterbeck, A.; Price, D.L. Evidence that beta-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science 1990, 248, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Nisha, N. Phytomedicines as potential inhibitors of β amyloid aggregation: significance to Alzheimer’s disease. Chin. J. Nat. Med. 2014, 12, 801–818. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcuminlipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N. Synthetic Curcumin Analogs as Inhibitors of β -Amyloid Peptide Aggregation: Potential Therapeutic and Diagnostic Agents for Alzheimer’s Disease. Mini. Rev. Med. Chem. 2015, 15, 1110–1121. [Google Scholar] [CrossRef]
- Rong, H.; Liang, Y.; Niu, Y. Rosmarinic acid attenuates β-amyloid-induced oxidative stress via Akt/GSK-3β/Fyn-mediated Nrf2 activation in PC12 cells. Free Radic. Biol. Med. 2018, 120, 114–123. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Carrillo-Hormaza, L.; Osorio, E.; Cardona-Gómez, G.P. Effects of biflavonoids from Garcinia madruno on a triple transgenic mouse model of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 128–138. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory activity on amyloid-β aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J. Agr. Food Chem. 2006, 53, 8762–8768. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Lin, B.; Cheng, Z.Y.; Bai, M.; Shi, S.; Huang, X.X.; Song, S.J. Phenylpropanoids and lignans from Prunus tomentosa seeds as efficient β-amyloid (Aβ) aggregationinhibitors. Bioorg. Chem. 2019, 84, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.J.; Wang, G.; Liu, J.C.; Dong, M.X.; Li, X.M.; Zhang, C.; Zhou, L.; Wang, R.; Niu, Y.C. Beta-asarone attenuates beta-amyloid-induced apoptosis through the inhibition of the activation of apoptosis signal-regulating kinase 1 in SH-SY5Y cells. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 44–51. [Google Scholar]
- Park, C.H.; Kim, K.H.; Lee, I.K.; Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, K.R. Phenolic constituents of Acorus gramineus. Arch. Pharm. Res. 2011, 34, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, G.; Salazar, M.; Tamariz, J.; Labarrios, F. Dominant lethal study of alpha-asarone in male and female mice after sub-chronic treatment. Phytother 1999. [Google Scholar] [CrossRef]
- Bhat, S.D.; Ashok, B.K.; Acharya, R.N.; Ravishankar, B. Anticonvulsant activity of raw and classically processed Vacha (Acorus calamus Linn.) rhizomes. Ayu 2012, 33, 119–122. [Google Scholar] [CrossRef]
- Chellian, R.; Pandy, V.; Mohamed, Z. Biphasic Effects of α-Asarone on Immobility in the Tail Suspension Test: Evidence for the Involvement of the Noradrenergic and Serotonergic Systems in Its Antidepressant-Like Activity. Front. Pharmacol. 2016, 7, 72. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Alpha-Asarone, a Major Component of Acorus gramineus, Attenuates Corticosterone-Induced Anxiety-Like Behaviours via Modulating TrkB Signaling Process. Korean J. Physiol. Pharmacol. 2014, 18, 191–200. [Google Scholar] [CrossRef]
- An, H.M.; Li, G.W.; Lin, C.; Gu, C.; Jin, M.; Sun, W.X.; Qiu, M.F.; Hu, B. Acorus tatarinowii Schott extract protects PC12 cells from amyloid-beta induced neurotoxicity. Die Pharm. Int. J. Pharm. Sci. 2014, 69, 391–395. [Google Scholar]
- Mdina-Franco, J.L.; Lopez-Vallejo, F.; Rodriguez-Morales, S.; Castillo, R.; Chamorro, G.; Tamariz, J. Molecular docking of the highly hypolipidemic agent alpha-asarone with catalytic portion of HMG-CoA reductase. Bioorg. Med. Chem. Lett. 2005, 15, 989–994. [Google Scholar] [CrossRef]
- Feng, X.-L.; Yu, Y.; Qin, D.-P.; Gao, H.; Yao, X.-S. Acorus Linnaeus: A review of traditional uses, phytochemistry and neuropharmacology. RSC Adv. 2015, 5, 5173–5182. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, W.C.; Li, H.; Mao, S.J. Asarone injection in treating respiratory disease: A meta-analysis. Chin. J. Evid. -Based Med. 2010, 10, 1174–1181. [Google Scholar]
- Liu, L.; Wang, J.; Shi, L.; Zhang, W.; Du, X.; Wang, Z.; Zhang, Y. beta-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine 2013, 20, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, R.W.; Miller, E.C.; Miller, J.A.; Liem, A. Structure-activity studies of the hepatocarcinogenicities of alkenylbenzene derivatives related to estragole and safrole on administration to preweanling male C58BL/6 x C3H/He J F1 mice. Cancer Res. 1987, 47, 2275–2283. [Google Scholar] [PubMed]
- Chen, Q.X.; Miao, J.K.; Li, C.; Li, X.W.; Wu, X.M.; Zhang, X.P. Anticonvulsant activitiy of acute and chronic treatment with a-asarone from Acorus gramineus in seizure models. Biol. Pharm. Bull. 2013, 36, 23–30. [Google Scholar] [CrossRef]
- Chellian, R.; Pandy, V.; Mohamed, Z. Pharmacology and toxicology of α- and β-Asarone: A review of preclinical evidence. Phytomedicin 2017, 32, 41–58. [Google Scholar] [CrossRef]
- Li, C.; Xing, G.; Dong, M.; Zhou, L.; Li, J.; Wang, G.; Niu, Y. Beta-asarone protection against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur. J. Pharmacol. 2010, 635, 96–102. [Google Scholar] [CrossRef]
- Deng, M.; Huang, L.; Ning, B.; Wang, N.; Zhang, Q.; Zhu, C.; Fang, Y. β-asarone improves learning and memory and reduces Acetyl Cholinesterase and Beta-amyloid42 levels in APP/PS1 transgenic mice by regulating Beclin-1-dependent autophagy. Brain Res. 2016, 1652, 188–194. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Kumar, H.; Park, J.Y.; Kim, I.W.; More, S.V.; Choi, D.K. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. [Google Scholar] [CrossRef]
- Lee, S.R.; Kim, M.S.; Kim, S.; Hwang, K.W.; Park, S.Y. Constituents from Scutellaria barbata Inhibiting Nitric OxideProduction in LPS-Stimulated Microglial Cells. Chem. Biodivers. 2017, 14, e1700231. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2 and 5 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Kim, N.; Yeo, J.Y.; Seo, D.-G.; Kim, S.; Lee, J.-S.; Hwang, K.W.; Park, S.-Y. Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules 2019, 24, 4297. https://doi.org/10.3390/molecules24234297
Lee JE, Kim N, Yeo JY, Seo D-G, Kim S, Lee J-S, Hwang KW, Park S-Y. Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules. 2019; 24(23):4297. https://doi.org/10.3390/molecules24234297
Chicago/Turabian StyleLee, Jae Eun, Nayeon Kim, Ji Yun Yeo, Dae-Gun Seo, Sunggun Kim, Jae-Sun Lee, Kwang Woo Hwang, and So-Young Park. 2019. "Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production" Molecules 24, no. 23: 4297. https://doi.org/10.3390/molecules24234297
APA StyleLee, J. E., Kim, N., Yeo, J. Y., Seo, D.-G., Kim, S., Lee, J.-S., Hwang, K. W., & Park, S.-Y. (2019). Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules, 24(23), 4297. https://doi.org/10.3390/molecules24234297




