Comprehensive Analysis of the Copper Exchange Implemented in Ammonia and Protonated Forms of Mordenite Using Microwave and Conventional Methods
Abstract
1. Introduction
2. Results
2.1. Elemental Analysis
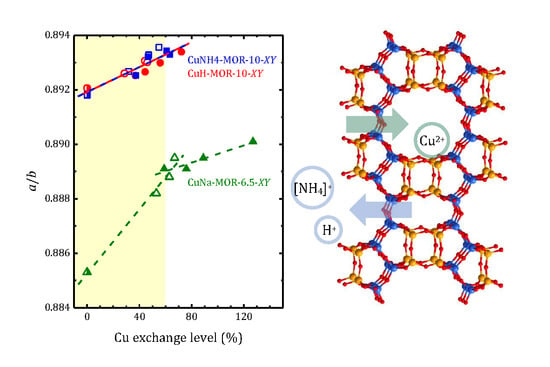
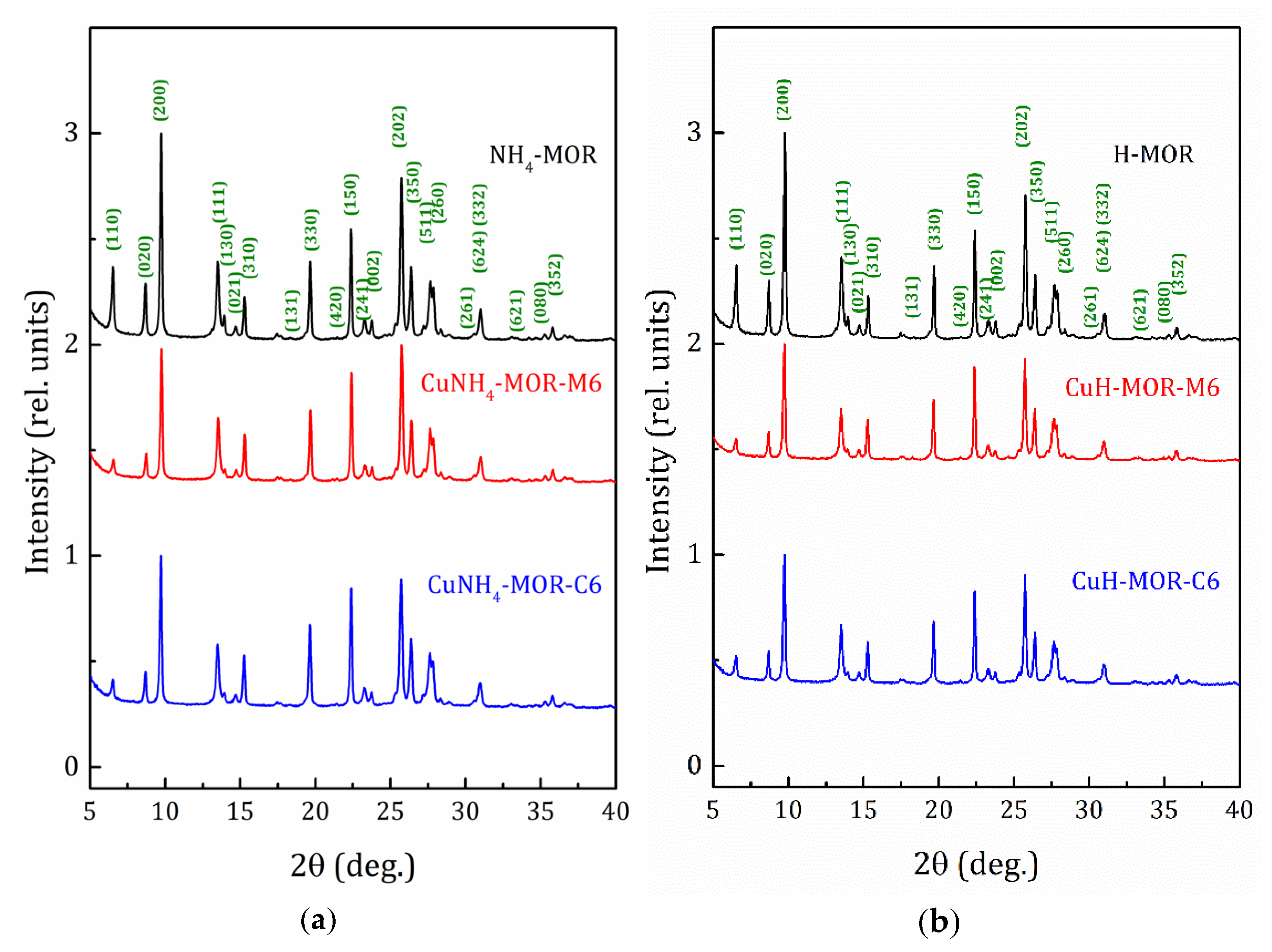
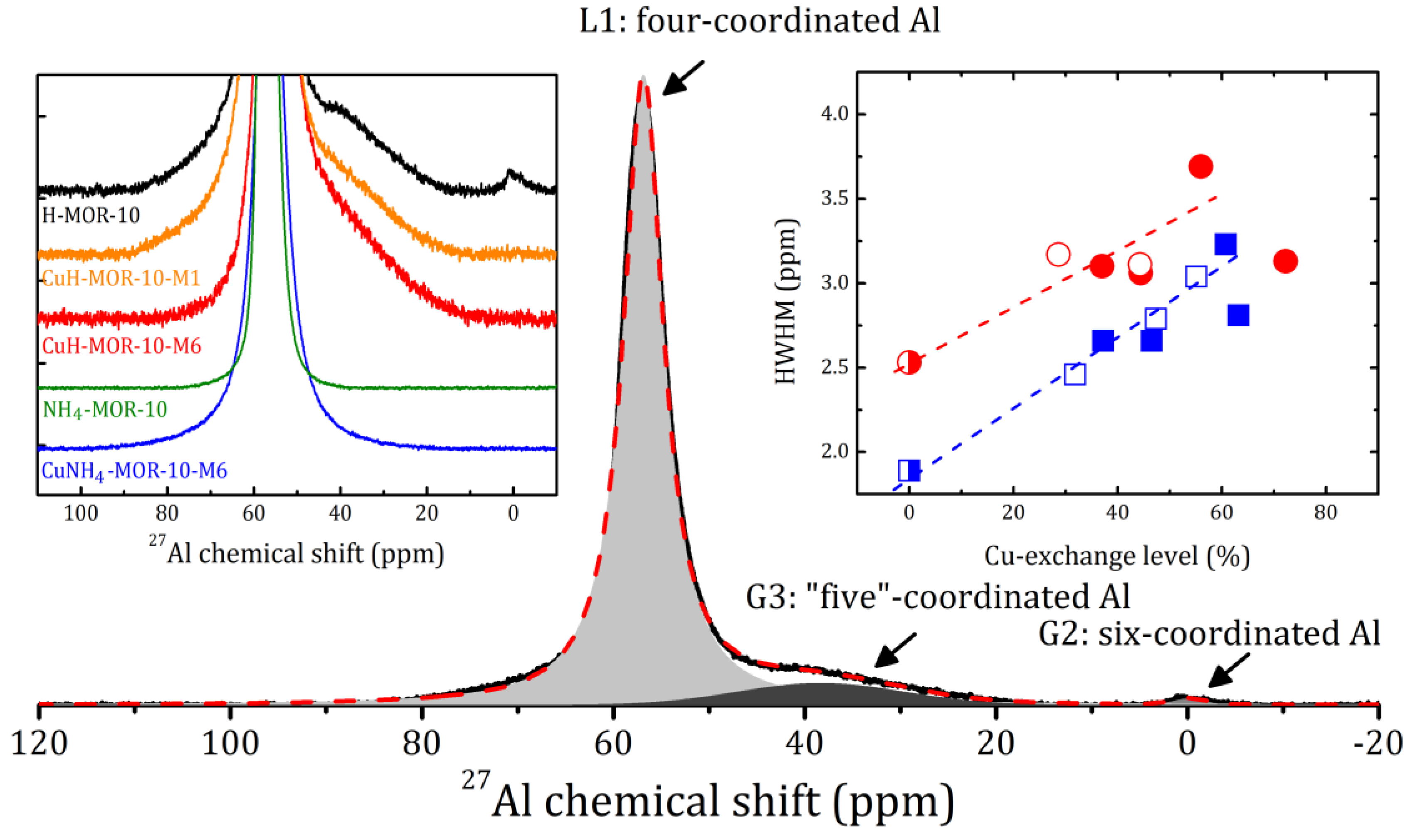
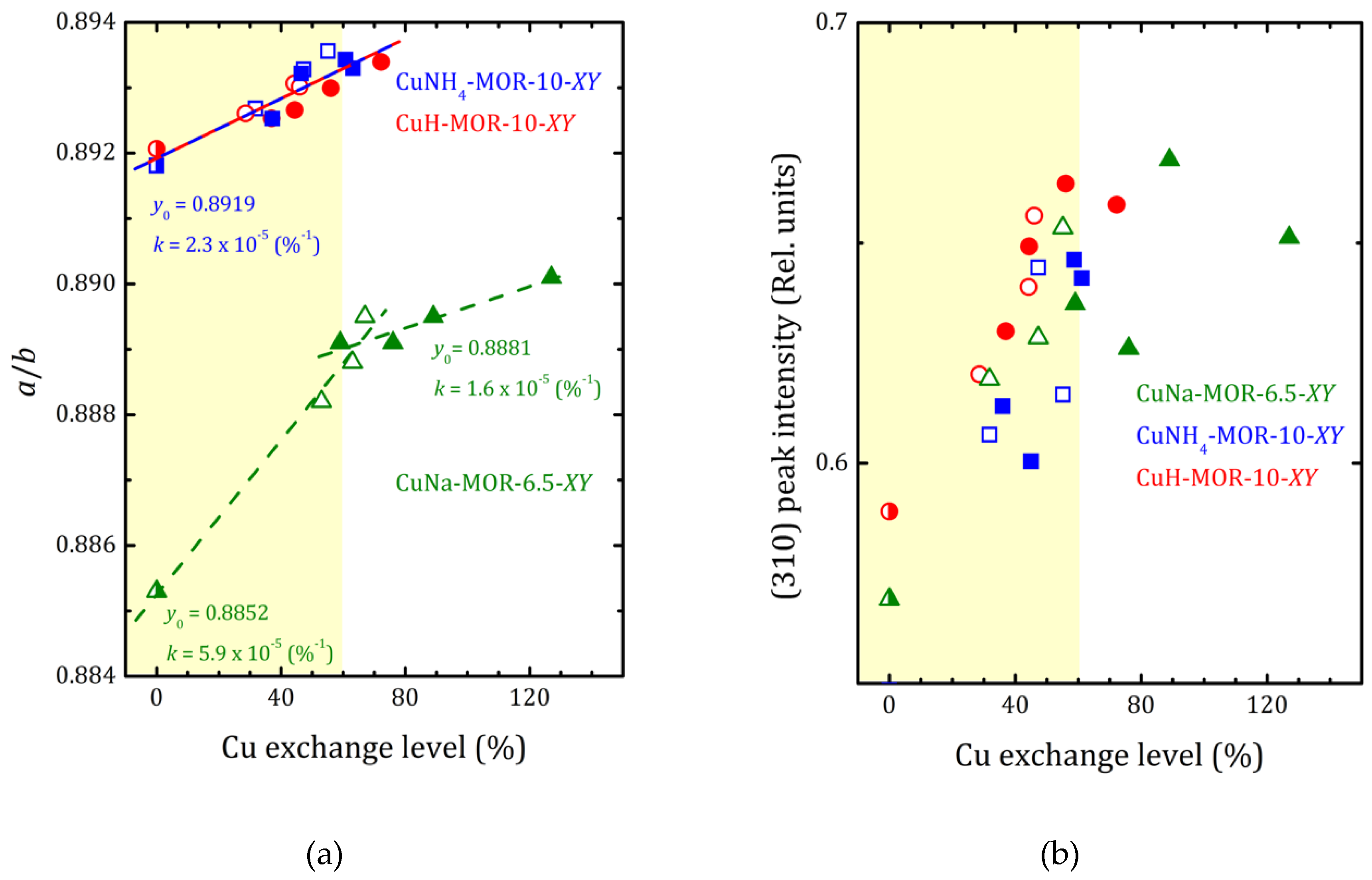
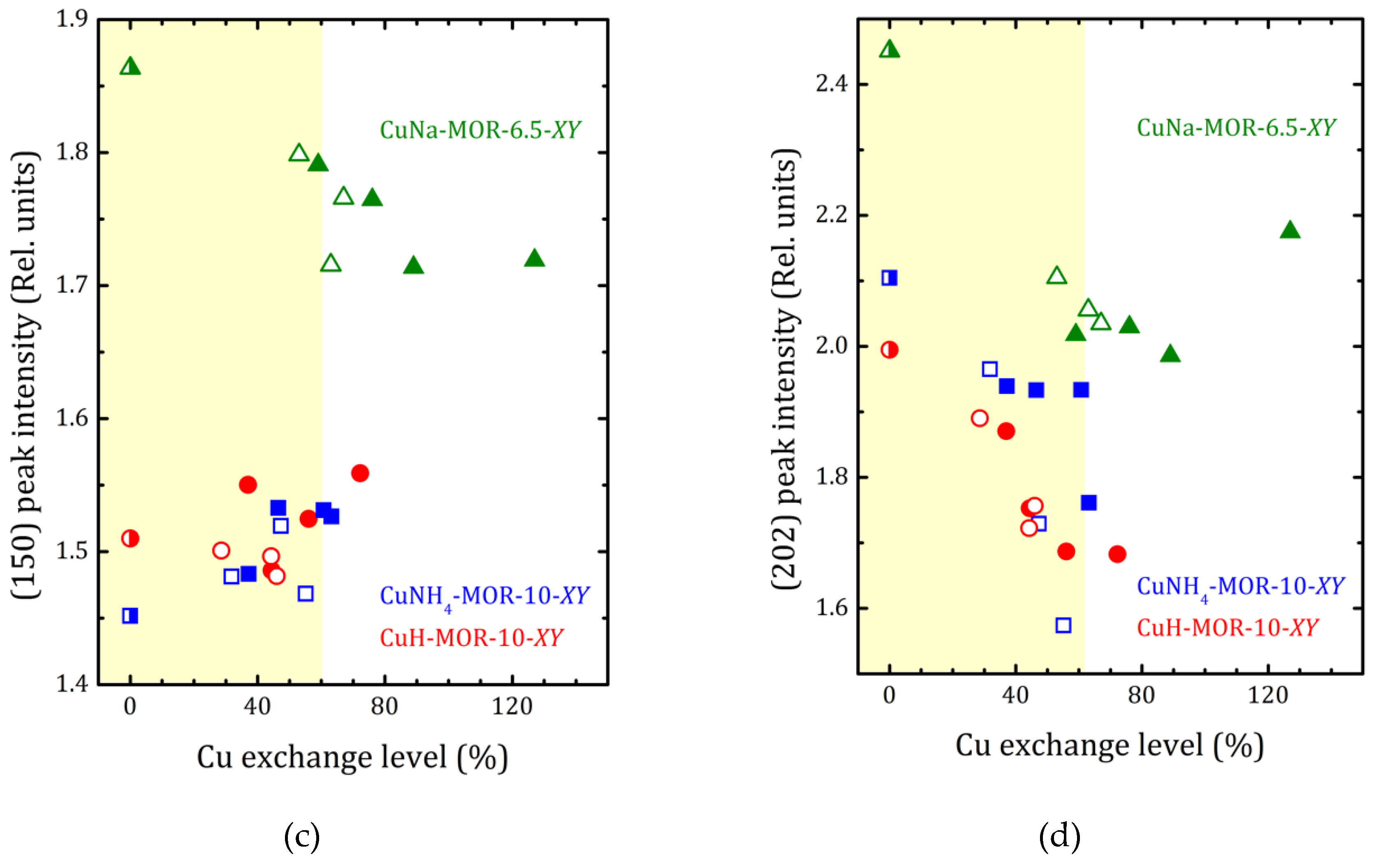
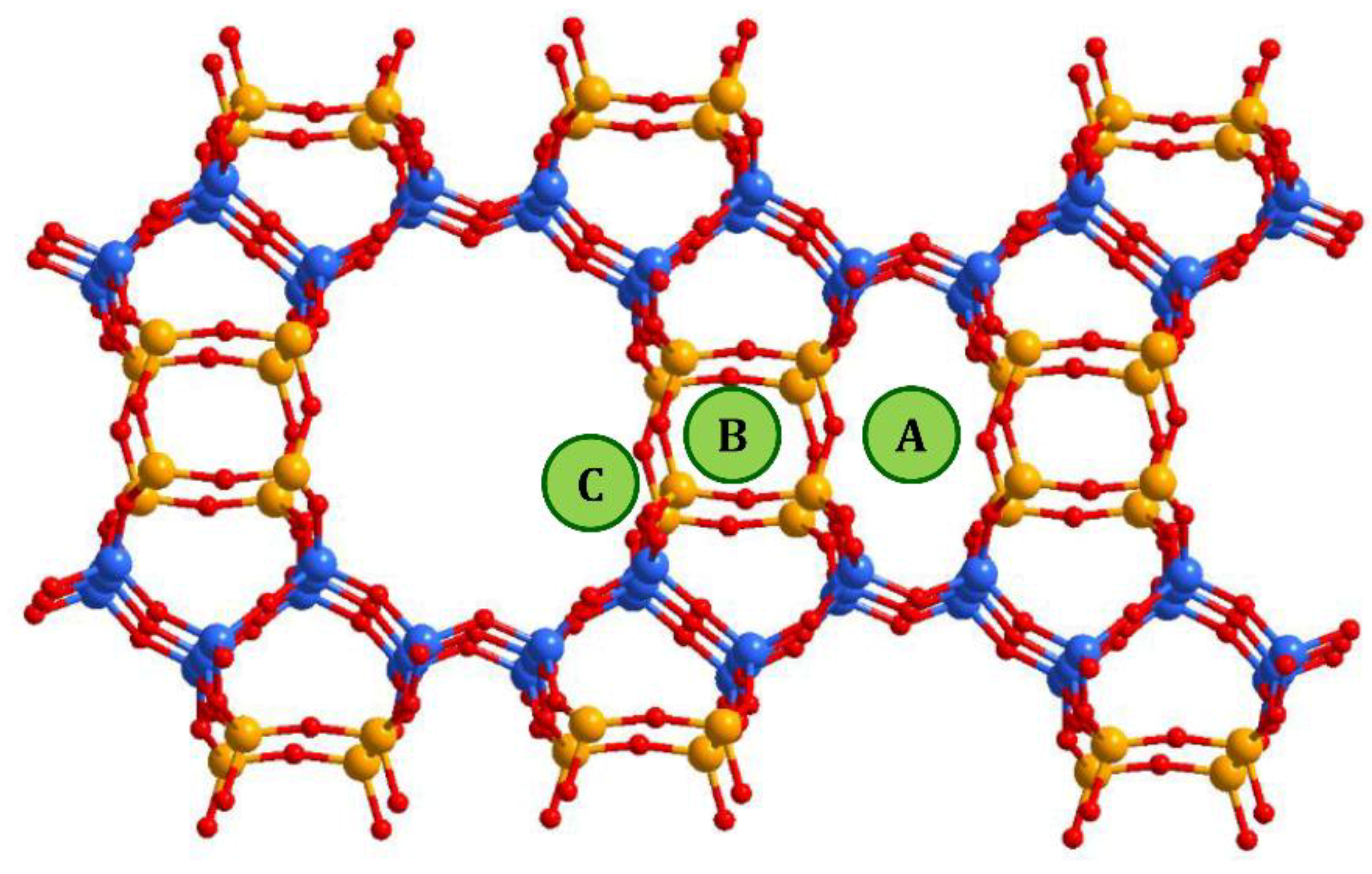
2.2. Structural Analysis
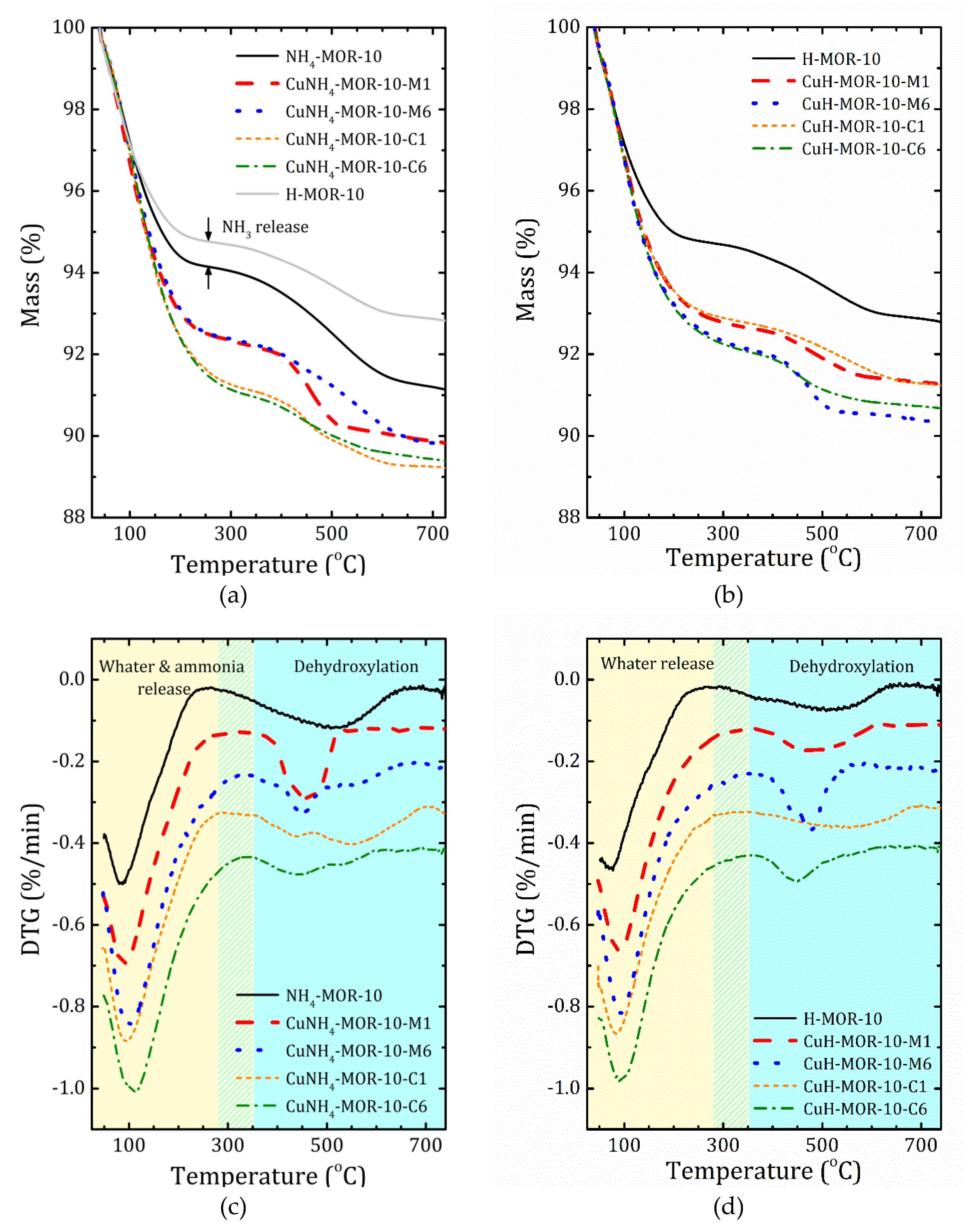
2.3. Thermal Analysis
2.4. Cu2p X-ray Photoelectron Spectroscopy (XPS) Analysis
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Speybroeck, V.; Hemelsoet, K.; Joos, L.; Waroquier, M.; Bell, R.G.; Catlow, C.R.A. Advances in theory and their application within the field of zeolite chemistry. Chem. Soc. Rev. 2015, 44, 7044–7111. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic materials for deNOx selective catalytic reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Shan, W.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, D.; Chen, F.; Zhan, X. NO decomposition and selective catalytic reduction of NO over Cu-ZSM-5 zeolite. Prog. Chem. 2014, 26, 248–258. [Google Scholar]
- Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Halgeri, A.B.; Shanbhag, G.V. Metal ion-exchanged zeolites as solid acid catalysts for the green synthesis of nopol from Prins reaction. Catal. Sci. Technol. 2014, 4, 4065–4074. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Halgeri, A.B. Metal ion-exchanged zeolites as highly active solid acid catalysts for the green synthesis of glycerol carbonate from glycerol. RSC Adv. 2015, 5, 14286–14293. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Whiting, G.T.; Dutta Chowdhury, A.; Weckhuysen, B.M. Zeolites and zeotypes for oil and gas conversion. In Advances in Catalysis; Jentoft, F.C., Ed.; Academic Press (Elsevier): Amsterdam, The Netherlands, 2015; Volume 58, pp. 143–314. [Google Scholar]
- Vanelderen, P.; Vancauwenbergh, J.; Sels, B.F.; Schoonheydt, R.A. Coordination chemistry and reactivity of copper in zeolites. Coord. Chem. Rev. 2013, 257, 483–494. [Google Scholar] [CrossRef]
- Schoonheydt, R.A. Transition metal ions in zeolites: Siting and energetics of Cu2+. Catal. Rev. Sci. Eng. 1993, 35, 129–168. [Google Scholar] [CrossRef]
- Iwamoto, M.; Hamada, H. Removal of nitrogen monoxide from exhaust gases through novel catalytic processes. Catal. Today 1991, 10, 57–71. [Google Scholar] [CrossRef]
- Dyballa, M.; Pappas, D.K.; Kvande, K.; Arstad, B.; Beato, P.; Olsbye, U.; Svelle, S. On how copper mordenite properties govern the framework stability and activity in the methane-to-methanol conversion. ACS Catal. 2019, 9, 365–375. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Jacob, J.; Chia, L.H.L.; Boey, F.Y.C. Thermal and non-thermal interaction of microwave radiation with materials. J. Mater. Sci. 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- Kim, J.; Mun, S.C.; Ko, H.U.; Kim, K.B.; Khondoker, M.A.H.; Zhai, L. Review of microwave assisted manufacturing technologies. Int. J. Precis. Eng. Manuf. 2012, 13, 2263–2272. [Google Scholar] [CrossRef]
- Kuroda, Y.; Okamoto, T.; Kumashiro, R.; Yoshikawa, Y.; Nagao, M. Microwave-assisted simple ion-exchange of ZSM-5-type zeolites with copper ions and their specific adsorption properties for N2 molecules at room temperature. Chem. Commun. (Camb). 2002, 1758–1759. [Google Scholar] [CrossRef]
- Bergada, O.; Gebretsadik, F.B.; Dolores, G.M.; Granados-Reyes, J.; Perez, E.; Sanchez, T.; Vicente, I.; Salagre, P.; Cesteros, Y. Microwave engineering for synthesizing clays and modifying properties in zeolites. In Microwave Engineering of Nanomaterials: From Mesoscale to Nanoscale; Guenin, E., Ed.; Pan Stanford Publishing: Singapore, 2016; pp. 163–194. [Google Scholar]
- Sapawe, N.; Jalil, A.A.; Triwahyono, S.; Shah, M.I.A.; Jusoh, R.; Salleh, N.F.M.; Hameed, B.H.; Karim, A.H. Cost-effective microwave rapid synthesis of zeolite NaA for removal of methylene blue. Chem. Eng. J. 2013, 229, 388–398. [Google Scholar] [CrossRef]
- Ou, X.; Xu, S.; Warnett, J.M.; Holmes, S.M.; Zaheer, A.; Garforth, A.A.; Williams, M.A.; Jiao, Y.; Fan, X. Creating hierarchies promptly : Microwave-accelerated synthesis of ZSM-5 zeolites on macrocellular silicon carbide (SiC) foams. Chem. Eng. J. 2017, 312, 1–9. [Google Scholar] [CrossRef]
- Bergadà, O.; Boix, E.; Salagre, P.; Cesteros, Y.; Medina, F.; Sueiras, J.E. Acidity properties of Ni-exchanged mordenites prepared with and without microwaves. Appl. Catal. A Gen. 2009, 368, 163–169. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Salagre, P. Effect of microwaves on the surface and acidic properties of dealuminated zeolites. Phys. Procedia 2010, 8, 104–108. [Google Scholar] [CrossRef][Green Version]
- Xu, M.; Wang, J.; Yu, T.; Wang, J.; Shen, M. New insight into Cu/SAPO-34 preparation procedure: Impact of NH4-SAPO-34 on the structure and Cu distribution in Cu-SAPO-34 NH3-SCR catalysts. Appl. Catal. B Environ. 2018, 220, 161–170. [Google Scholar] [CrossRef]
- Zhukov, Y.M.; Efimov, A.Y.; Shelyapina, M.G.; Petranovskii, V.; Zhizhin, E.V.; Burovikhina, A.; Zvereva, I.A. Effect of preparation method on the valence state and encirclement of copper exchange ions in mordenites. Microporous Mesoporous Mater. 2016, 224, 415–419. [Google Scholar] [CrossRef]
- Zhukov, Y.M.; Kovalyov, A.N.; Kultaeva, A.Y.; Shelyapina, M.G.; Petranovskii, V. A comparative analysis of the protonated and copper exchanged mordenites with SiO2/Al2O3 molar ratio equal to 10. Int. J. Nanotechnol. 2016, 13, 136–146. [Google Scholar] [CrossRef]
- Krylova, E.A.; Shelyapina, M.G.; Nowak, P.; Harańczyk, H.; Chislov, M.; Zvereva, I.A.; Privalov, A.F.; Becker, M.; Vogel, M.; Petranovskii, V. Mobility of water molecules in sodium- and copper-exchanged mordenites: Thermal analysis and 1H NMR. Microporous Mesoporous Mater. 2018, 265, 132–142. [Google Scholar] [CrossRef]
- Zhukov, Y.M.; Shelyapina, M.G.; Zvereva, I.A.; Efimov, A.Y.; Petranovskii, V. Microwave assisted versus convention Cu2+ exchange in mordenite. Microporous Mesoporous Mater. 2018, 259, 220–228. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Zvereva, I.A.; Yafarova, L.V.; Bogdanov, D.S.; Sukharzhevskii, S.M.; Zhukov, Y.M.; Petranovskii, V. Thermal analysis and EPR study of copper species in mordenites prepared by conventional and microwave-assisted methods. J. Therm. Anal. Calorim. 2018, 134, 71–79. [Google Scholar] [CrossRef]
- Barrer, R.; Klinowski, J. Influence of framework charge density on ion-exchange properties of zeolite. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1972, 68, 1956–1963. [Google Scholar] [CrossRef]
- Kuronen, M.; Harjula, R.; Jernström, J.; Vesteniusa, M.; Lehto, J. Effect of the framework charge density on zeolite ion exchange selectivitie. Phys. Chem. Chem. Phys. 2000, 2, 2655–2659. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Antúnez-García, J.; Petranovskii, V.; Fuentes-Moyado, S.; Galván, D.H.; Petranovski, V.; Chávez-Rivas, F. Analysis of theoretical and experimental X-ray diffraction patterns for distinct mordenite frameworks. J. Mater. Sci. 2019, 54, 7745–7757. [Google Scholar] [CrossRef]
- Wichterlová, B.; Dêdeček, J.; Sobalík, Z. Cu coordination in high silica zeolites. Effect of the framework Al local siting. Stud. Surf. Sci. Catal. 1995, 94, 641–648. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Sample | ICP-OES | ||
|---|---|---|---|
| Cu/Al | Si/Al | Cu-Exchange Level (%) | |
| NH4-MOR-10 | – | 8.5(1) | – |
| CuNH4-MOR-10-M1 | 0.19(1) | 8.4(1) | 37.2(1) |
| CuNH4-MOR-10-M2 | 0.23(1) | 8.3(1) | 46.5(1) |
| CuNH4-MOR-10-M3 | 0.32(1) | 8.2(1) | 63.2(1) |
| CuNH4-MOR-10-M6 | 0.30(1) | 8.0(1) | 60.7(1) |
| CuNH4-MOR-10-C1 | 0.16(1) | 8.3(1) | 31.8(1) |
| CuNH4-MOR-10-C3 | 0.24(1) | 8.1(1) | 47.3(1) |
| CuNH4-MOR-10-C6 | 0.27(1) | 8.2(1) | 55.1(1) |
| H-MOR-10 | – | 8.6(1) | – |
| CuH-MOR-10-M1 | 0.18(1) | 8.6(1) | 37.0(1) |
| CuH-MOR-10-M2 | 0.22(1) | 8.5(1) | 44.4(1) |
| CuH-MOR-10-M3 | 0.28(1) | 8.5(1) | 56.0(1) |
| CuH-MOR-10-M6 | 0.36(2) | 8.4(1) | 72.2(1) |
| CuH-MOR-10-C1 | 0.14(1) | 8.5(1) | 28.6(1) |
| CuH-MOR-10-C3 | 0.22(1) | 8.3(1) | 44.3(1) |
| CuH-MOR-10-C6 | 0.23(1) | 8.5(1) | 46.0(1) |
| Sample | Lattice Parameters (Å) | Unit Cell Volume (Å3) | K = a/b | ||
|---|---|---|---|---|---|
| a | b | c | |||
| NH4-MOR-10 | 18.167(2) | 20.371(1) | 7.4941(4) | 2773(2) | 0.8918(1) |
| CuNH4-MOR-10-M1 | 18.204(2) | 20.396(1) | 7.5020(4) | 2785(2) | 0.8925(1) |
| CuNH4-MOR-10-M2 | 18.186(2) | 20.360(1) | 7.4956(4) | 2775(2) | 0.8932(1) |
| CuNH4-MOR-10-M3 | 18.185(2) | 20.357(1) | 7.4944(4) | 2774(2) | 0.8933(1) |
| CuNH4-MOR-10-M6 | 18.209(2) | 20.381(1) | 7.5001(4) | 2783(2) | 0.8934(1) |
| CuNH4-MOR-10-C1 | 18.183(2) | 20.369(1) | 7.4959(4) | 2776(2) | 0.8927(1) |
| CuNH4-MOR-10-C3 | 18.189(2) | 20.362(1) | 7.4976(4) | 2777(2) | 0.8933(1) |
| CuNH4-MOR-10-C6 | 18.192(2) | 20.359(1) | 7.4979(4) | 2777(2) | 0.8936(1) |
| H-MOR-10 | 18.166(2) | 20.364(1) | 7.4917(4) | 2771(2) | 0.8921(1) |
| CuH-MOR-10-M1 | 18.196(2) | 20.387(1) | 7.4985(4) | 2782(2) | 0.8925(1) |
| CuH-MOR-10-M2 | 18.171(2) | 20.356(1) | 7.4929(4) | 2772(2) | 0.8927(1) |
| CuH-MOR-10-M3 | 18.184(2) | 20.363(1) | 7.4957(4) | 2776(2) | 0.8930(1) |
| CuH-MOR-10-M6 | 18.194(2) | 20.365(1) | 7.4948(4) | 2777(2) | 0.8934(1) |
| CuH-MOR-10-C1 | 18.169(2) | 20.355(1) | 7.4918(4) | 2771(2) | 0.8926(1) |
| CuH-MOR-10-C3 | 18.181(2) | 20.358(1) | 7.4937(4) | 2774(2) | 0.8931(1) |
| CuH-MOR-10-C6 | 18.180(2) | 20.358(1) | 7.4938(4) | 2774(2) | 0.8930(1) |
| Sample | Mass Loss (%) in the Temperature Range: | T0 (°C) | |
|---|---|---|---|
| 40 °C T T0 | T0T 740 °C | ||
| NH4-MOR-10 | 5.86 | 3.02 | 258 |
| CuNH4-MOR-10-M1 | 7.64 | 2.57 | 350 |
| CuNH4-MOR-10-M2 | 8.63 | 2.02 | 356 |
| CuNH4-MOR-10-M3 | 11.10 | 1.15 | 364 |
| CuNH4-MOR-10-M6 | 8.77 | 2.04 | 307 |
| CuNH4-MOR-10-C1 | 7.81 | 2.42 | 283 |
| CuNH4-MOR-10-C3 | 8.44 | 1.93 | 323 |
| CuNH4-MOR-10-C6 | 8.97 | 1.65 | 325 |
| H-MOR-10 | 5.26 | 1.95 | 270 |
| CuH-MOR-10-M1 | 7.28 | 1.46 | 325 |
| CuH-MOR-10-M2 | 7.15 | 1.44 | 336 |
| CuH-MOR-10-M3 | 7.64 | 1.59 | 331 |
| CuH-MOR-10-M6 | 7.76 | 1.93 | 322 |
| CuH-MOR-10-C1 | 7.19 | 1.58 | 331 |
| CuH-MOR-10-C3 | 7.04 | 1.51 | 340 |
| CuH-MOR-10-C6 | 7.82 | 1.53 | 355 |
| Sample Name | Initial Cation Form | Pretreatment | Ion Exchange Procedure |
|---|---|---|---|
| NH4-MOR-10 | [NH4]+ | No | No |
| H-MOR-10 | H+ | 120 min at 300 °C | No |
| CuNH4-MOR-10-MY | [NH4]+ | No | MW at 100 °C |
| CuNH4-MOR-10-CY | [NH4]+ | No | conventional at 20 °C |
| CuH-MOR-10-MY | H+ | 120 min at 300 °C | MW at 100 °C |
| CuH-MOR-10-CY | H+ | 120 min at 300 °C | conventional at 20 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelyapina, M.G.; Krylova, E.A.; Zhukov, Y.M.; Zvereva, I.A.; Rodriguez-Iznaga, I.; Petranovskii, V.; Fuentes-Moyado, S. Comprehensive Analysis of the Copper Exchange Implemented in Ammonia and Protonated Forms of Mordenite Using Microwave and Conventional Methods. Molecules 2019, 24, 4216. https://doi.org/10.3390/molecules24234216
Shelyapina MG, Krylova EA, Zhukov YM, Zvereva IA, Rodriguez-Iznaga I, Petranovskii V, Fuentes-Moyado S. Comprehensive Analysis of the Copper Exchange Implemented in Ammonia and Protonated Forms of Mordenite Using Microwave and Conventional Methods. Molecules. 2019; 24(23):4216. https://doi.org/10.3390/molecules24234216
Chicago/Turabian StyleShelyapina, Marina G., Ekaterina A. Krylova, Yurii M. Zhukov, Irina A. Zvereva, Inocente Rodriguez-Iznaga, Vitalii Petranovskii, and Sergio Fuentes-Moyado. 2019. "Comprehensive Analysis of the Copper Exchange Implemented in Ammonia and Protonated Forms of Mordenite Using Microwave and Conventional Methods" Molecules 24, no. 23: 4216. https://doi.org/10.3390/molecules24234216
APA StyleShelyapina, M. G., Krylova, E. A., Zhukov, Y. M., Zvereva, I. A., Rodriguez-Iznaga, I., Petranovskii, V., & Fuentes-Moyado, S. (2019). Comprehensive Analysis of the Copper Exchange Implemented in Ammonia and Protonated Forms of Mordenite Using Microwave and Conventional Methods. Molecules, 24(23), 4216. https://doi.org/10.3390/molecules24234216