Determination of Urinary Hydroxyl PAHs Using Graphene Oxide@Diatomite Based Solid-Phase Extraction and High-Performance Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
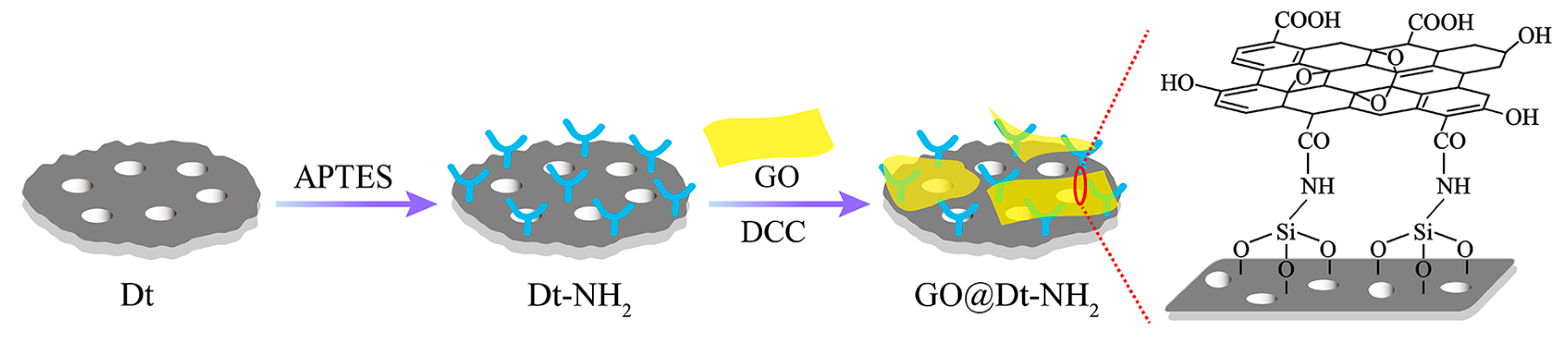
2.1. Fabrication and Characterization of the GO@Dt–NH2 Composite
2.2. Optimization of the SPE Conditions
2.2.1. The Loading Volume
2.2.2. Eluting Solvent and Eluting Volume
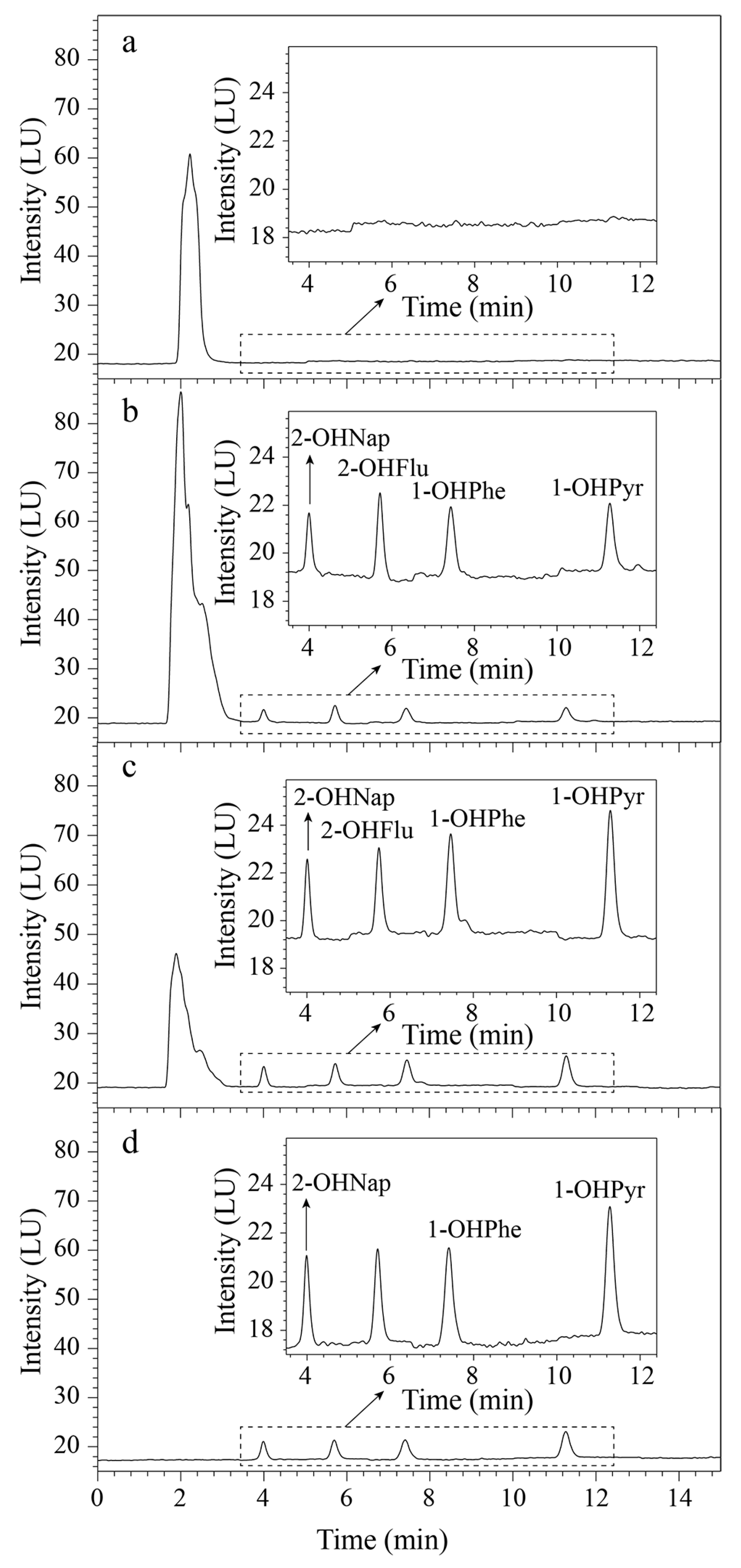
2.3. Validation of the GO@Dt–NH2 Based SPE HPLC-FLD Method
2.4. Urine Sample Analysis and Comparison with Commercial C18 Adsorbent
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Instruments
3.3. Preparation of GO@Dt–NH2 Composites
3.4. Urine Sample Collection and Preparation
3.5. SPE Procedure
3.6. HPLC-FLD Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ravindra, K.; Sokhi, R.; Grieken, R.V. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Boström, C.E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health. Perspect. 2002, 110, 451–489. [Google Scholar] [PubMed]
- Motorykin, O.; Matzke, M.M.; Waters, K.M.; Simonich, S.L.M. Association of carcinogenic polycyclic aromatic hydrocarbon emissions and smoking with lung cancer mortality rates on a global scale. Environ. Sci. Technol. 2013, 47, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M.; Lou, S.; Zelenyuk, A.; Easter, R.C.; Corley, R.A.; Thrall, B.D.; Rasch, P.J.; Fast, J.D.; Massey Simonich, S.L.; Shen, H.; et al. Global long-range transport and lung cancer risk from polycyclic aromatic hydrocarbons shielded by coatings of organic aerosol. Proc. Natl. Acad. Sci. USA 2017, 114, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, H.; Kearney, G.D.; Kan, H.; Sheps, D.S. Studying the effects of polycyclic aromatic hydrocarbons on peripheral arterial disease in the United States. Sci. Total Environ. 2013, 461–462, 341–347. [Google Scholar] [CrossRef]
- Stults, W.P.; Wei, Y. Ambient air emissions of polycyclic aromatic hydrocarbons and female breast cancer incidence in US. Med. Oncol. 2018, 35, 88. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Liao, L.M.; Strickland, P.T.; Shu, X.O.; Yang, G.; Ji, B.T.; Li, H.L.; Rothman, N.; Kamangar, F.; Gao, Y.T.; et al. Polycyclic aromatic hydrocarbons: Determinants of urinary 1-hydroxypyrene glucuronide concentration and risk of colorectal cancer in the Shanghai women’s health study. BMC Cancer 2013, 13, 282. [Google Scholar] [CrossRef]
- Santos, P.M.; Sánchez, M.D.N.; Pavόn, J.L.P.; Cordero, B.M. Determination of polycyclic aromatic hydrocarbons in human biological samples: A critical review. TrAC Trends Anal. Chem. 2019, 113, 194–209. [Google Scholar] [CrossRef]
- Woudneh, M.B.; Benskin, J.P.; Grace, R.; Hamilton, M.C.; Magee, B.H.; Hoeger, G.C.; Forsberg, N.D.; Cosgrove, J.R. Quantitative determination of hydroxy polycylic aromatic hydrocarbons as a biomarker of exposure to carcinogenic polycyclic aromatic hydrocarbons. J. Chromatogr. A 2016, 1454, 93–100. [Google Scholar] [CrossRef]
- Gaudreau, É.; Bérubé, R.; Bienvenu, J.F.; Fleury, N. Stability issues in the determination of 19 urinary (free and conjugated) monohydroxy polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem. 2016, 408, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Dugheri, S.; Bonari, A.; Gentili, M.; Cappelli, G.; Pompilio, I.; Bossi, C.; Arcangeli, G.; Campagna, M.; Mucci, N. High-throughput analysis of selected urinary hydroxy polycyclic aromatic hydrocarbons by an innovative automated solid-phase microextraction. Molecules 2018, 23, 1869. [Google Scholar]
- Semreen, M.H.; Shanableh, A.; Semerjian, L.; Alniss, H.; Mousa, M.; Bai, X.L.; Acharya, K. Simultaneous determination of pharmaceuticals by solid-phase extraction and liquid chromatography-tandem mass spectrometry: A case study from sharjah sewage treatment plant. Molecules 2019, 24, 633. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Juśkiewicz, J.; Wiczkowski, W. Using the SPE and micro-HPLC-MS/MS method for the analysis of betalains in rat plasma after red beet administration. Molecules 2017, 22, 2137. [Google Scholar] [CrossRef] [PubMed]
- Jornet-Martínez, N.; Ortega-Sierra, A.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Analysis of contact traces of cannabis by in-tube solid-phase microextraction coupled to nanoliquid chromatography. Molecules 2018, 23, 2359. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Fang, S.F.; Wan, X.J.; Luo, Y.; Zhou, J.Y.; Li, Y.; Li, Y.J.; Wang, F.; Huang, O.P. Quantification of monohydroxylated polycyclic aromatic hydrocarbons in human urine samples using solid-phase microextraction coupled with glass-capillary nanoelectrospray ionization mass spectrometry. Anal. Chim. Acta 2017, 973, 68–74. [Google Scholar] [CrossRef]
- Rahimia, M.; Bahara, S.; Heydarib, R.; Amininasaba, S.M. Determination of quercetin using a molecularly imprinted polymer as solid-phase microextraction sorbent and high-performance liquid chromatography. Microchem. J. 2019, 148, 433–441. [Google Scholar] [CrossRef]
- Mehdinia, A.; Khodaee, N.; Jabbari, A. Fabrication of graphene/Fe3O4@polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal. Chim. Acta 2015, 868, 1–9. [Google Scholar] [CrossRef]
- Duo, H.X.; Lu, X.F.; Wang, S.; Wang, L.C.; Guo, Y.; Liang, X.J. Synthesis of magnetic metal–organic framework composites, Fe3O4-NH2@MOF-235, for the magnetic solid-phase extraction of benzoylurea insecticides from honey, fruit juice and tap water samples. New J. Chem. 2019, 43, 12563–12569. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, X.Q.; Row, K.H. Magnetic solid-phase extraction with Fe3O4/molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids for the rapid purification of alkaloid isomers (theobromine and theophylline) from green tea. Molecules 2017, 22, 1061. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H. Electrospun nanofibers-based online micro-solid phase extraction for the determination of monohydroxy polycyclic aromatic hydrocarbons in human urine. J. Chromatogr. A 2017, 1521, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Meng, L.; Pittman, E.N.; Etheredge, A.; Hubbard, K.; Trinidad, D.A.; Kato, K.; Ye, X.; Calafat, A.M. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 931–937. [Google Scholar] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [PubMed]
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From conception to realization: An historial account of graphene and some perspectives for its future. Angew. Chem. Int. Edit. 2010, 49, 9336–9344. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Zeng, L.; Wang, T.; Cai, Y.; Jiang, G. Evaluation of graphene as an advantageous adsorbent for solid-phase extraction with chlorophenols as model analytes. J. Chromatogr. A 2011, 1218, 197–204. [Google Scholar] [CrossRef]
- Chen, X.; Hai, X.; Wang, J. Graphene/graphene oxide and their derivatives in the separation/isolation and preconcentration of protein species: A review. Anal. Chim. Acta 2016, 922, 1–10. [Google Scholar] [CrossRef]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. TrAC Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Cui, B.; Guo, B.; Wang, H.; Zhang, D.; Liu, H.; Bai, L.; Yan, H.; Han, D. Graphene oxide-based composite monolith as new sorbent for the on-line solid phase extraction and high performance liquid chromatography determination of β-sitosterol in food samples. Talanta 2018, 186, 200–205. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. TrAC Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Guan, W.; Li, Z.; Zhang, H.; Hong, H.; Rebeyev, N.; Ye, Y.; Ma, Y. Amine modified graphene as reversed-dispersive solid phase extraction materials combined with liquid chromatography-tandem mass spectrometry for pesticide multi-residue analysis in oil crops. J. Chromatogr. A 2013, 1286, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.A.W.; Nodeh, H.R.; Sanagi, M.M. Graphene-based materials as solid phase extraction sorbent for trace metal ions, organic compounds, and biological sample preparation. Crit. Rev. Anal. Chem. 2016, 46, 267–283. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Jiang, G. Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction. Angew. Chem. Int. Edit. 2011, 50, 5913–5917. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Yang, M.; Li, L.; Cheung, H.Y. Graphene/TiO2 nanocomposite based solid-phase extraction and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for lipidomic profiling of avocado (Persea americana Mill.). Adv. Mater. 2009, 21, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Kumeria, T.; Aw, M.S.; Losic, D. Diatom silica for biomedical application: Recent progress and advances. Adv. Healthc. Mater. 2018, 7, 1800552. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Diatomaceous lessons in nanotechnology and advanced materials. Adv. Mater. 2009, 21, 2947–2958. [Google Scholar] [CrossRef]
- Xu, L.; Gao, X.; Li, Z.; Gao, C. Removal of fluoride by nature diatomite from high-fluorine water: An appropriate pretreatment for nanofiltration process. Desalination 2015, 369, 97–104. [Google Scholar] [CrossRef]
- Liu, H.T.; Huang, L.P.; Chen, Y.X.; Guo, L.M.; Li, L.M.; Zhou, H.Y.; Luan, T.G. Simultaneous determination of polycyclic musks in blood and urine by solid supported liquid-liquid extraction and gas chromatography-tandem mass spectrometry. J. Chromatogr. B 2015, 992, 96–102. [Google Scholar] [CrossRef]
- Seo, J.E.; Park, J.E.; Lee, J.Y.; Kwon, H.J. Determination of seven N-nitrosamines in agricultural food matrices using GC-PCI-MS/MS. Food Anal. Methods 2016, 9, 1595–1605. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, P.; Liu, D.; Deng, L.; Zhou, J.; Yu, W.; Chen, F. A hierarchically porous diatomite/silicalite-1 composite for benzene adsorption/desorption fabricated via a facile pre-modification in situ synthesis route. Chem. Eng. J. 2016, 294, 333–342. [Google Scholar] [CrossRef]
- Jiang, T.; Kuila, T.; Kim, N.H.; Ku, B.C.; Lee, J.H. Enhanced mechanical properties of silanized silica nanoparticle attached graphene oxide/epoxy composites. Compos. Sci. Technol. 2013, 79, 115–125. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are all available from the authors. |


| Analyte | Structure | logP | Adsorption Capacity (mg/g) | |
|---|---|---|---|---|
| Dt–NH2 | GO@Dt–NH2 | |||
| 2-OHNap | 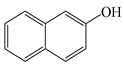 | 2.71 | 0.0 | 181.8 |
| 2-OHFlu | 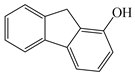 | 3.43 | 9.3 | 265.4 |
| 1-OHPhe | 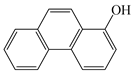 | 3.94 | 9.9 | 288.7 |
| 1-OHPyr |  | 4.29 | 17.8 | 409.6 |
| Analyte | Linear Range (ng/mL) | Regression Equation 1 (r) | LOD (ng/mL) | LOQ (ng/mL) | Spiked Level (ng/mL) | Recovery (%) | Precision (RSD, %, n = 3) | |
|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | |||||||
| 2-OHNap | 0.50–200 | y = 0.431x + 2.17 (0.999) | 0.15 | 0.50 | 0.5 | 95.5 | 6.4 | 11.8 |
| 1 | 90.6 | 2.9 | 3.0 | |||||
| 2 | 93.9 | 4.3 | 11.3 | |||||
| 2-OHFlu | 0.30–150 | y = 1.23x + 5.65 (0.999) | 0.10 | 0.30 | 0.5 | 93.0 | 3.3 | 9.6 |
| 1 | 95.0 | 4.3 | 5.7 | |||||
| 2 | 93.1 | 2.9 | 6.7 | |||||
| 1-OHPhe | 0.30–150 | y = 2.92x + 7.76 (0.999) | 0.10 | 0.30 | 0.5 | 93.2 | 6.1 | 8.6 |
| 1 | 96.2 | 2.4 | 2.7 | |||||
| 2 | 100 | 5.6 | 3.7 | |||||
| 1-OHPyr | 0.40–200 | y = 22.7x − 31.2 (0.999) | 0.12 | 0.40 | 0.5 | 93.8 | 5.5 | 5.9 |
| 1 | 95.5 | 2.7 | 7.9 | |||||
| 2 | 94.6 | 1.8 | 9.0 | |||||
| Sample | OH-PAHs | Found ± SD (ng/mL) | Recovery 1 (%) | RSD (%, n = 3) | Sample | OH-PAHs | Found ± SD (ng/mL) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2-OHNap | 2.16 ± 0.07 | 102 | 9.7 | 6 | 2-OHNap | N.D. | 93.1 | 8.5 |
| 2-OHFlu | 1.04 ± 0.07 | 99.3 | 2.0 | 2-OHFlu | N.D. | 95.3 | 3.1 | ||
| 1-OHPhe | N.D. 2 | 93.3 | 7.7 | 1-OHPhe | N.D. | 94.8 | 4.6 | ||
| 1-OHPyr | 4.60 ± 0.06 | 103 | 7.9 | 1-OHPyr | 4.07 ± 0.15 | 96.2 | 7.5 | ||
| 2 | 2-OHNap | 2.65 ± 0.26 | 96.6 | 2.1 | 7 | 2-OHNap | 2.41 ± 0.07 | 98.1 | 10.7 |
| 2-OHFlu | N.D. | 97.7 | 4.4 | 2-OHFlu | 1.14 ± 0.05 | 94.6 | 2.0 | ||
| 1-OHPhe | N.D. | 93.8 | 2.6 | 1-OHPhe | N.D. | 93.8 | 5.6 | ||
| 1-OHPyr | 1.23 ± 0.15 | 95.0 | 6.0 | 1-OHPyr | 4.47 ± 0.04 | 93.6 | 6.3 | ||
| 3 | 2-OHNap | 0.91 ± 0.26 | 105 | 5.1 | 8 | 2-OHNap | 2.78 ± 0.09 | 97.4 | 7.2 |
| 2-OHFlu | N.D. | 94.8 | 3.7 | 2-OHFlu | N.D. | 96.9 | 4.3 | ||
| 1-OHPhe | N.D. | 100 | 2.5 | 1-OHPhe | N.D. | 92.8 | 6.5 | ||
| 1-OHPyr | 2.02 ± 0.06 | 103 | 9.1 | 1-OHPyr | 4.80 ± 0.01 | 86.4 | 7.6 | ||
| 4 | 2-OHNap | 3.66 ± 0.15 | 105 | 5.6 | 9 | 2-OHNap | 3.71 ± 0.11 | 100 | 5.7 |
| 2-OHFlu | 3.18 ± 0.04 | 88.0 | 3.3 | 2-OHFlu | N.D. | 96.1 | 3.4 | ||
| 1-OHPhe | N.D. | 89.6 | 3.1 | 1-OHPhe | N.D. | 96.1 | 3.6 | ||
| 1-OHPyr | N.D. | 92.9 | 4.7 | 1-OHPyr | 2.70 ± 0.03 | 88.3 | 5.1 | ||
| 5 | 2-OHNap | 4.02 ± 0.08 | 96.2 | 6.7 | 10 | 2-OHNap | 2.88 ± 0.14 | 108 | 8.0 |
| 2-OHFlu | 1.33 ± 0.09 | 89.1 | 3.4 | 2-OHFlu | 1.50 ± 0.11 | 87.0 | 4.0 | ||
| 1-OHPhe | 1.37 ± 0.08 | 101 | 3.8 | 1-OHPhe | N.D. | 98.0 | 4.2 | ||
| 1-OHPyr | 2.22 ± 0.07 | 96.8 | 2.7 | 1-OHPyr | 3.66 ± 0.09 | 90.1 | 3.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Z.; Zhang, Z.; Zhao, T.; Wang, M.; Wang, X. Determination of Urinary Hydroxyl PAHs Using Graphene Oxide@Diatomite Based Solid-Phase Extraction and High-Performance Liquid Chromatography. Molecules 2019, 24, 4186. https://doi.org/10.3390/molecules24224186
Liu Y, Li Z, Zhang Z, Zhao T, Wang M, Wang X. Determination of Urinary Hydroxyl PAHs Using Graphene Oxide@Diatomite Based Solid-Phase Extraction and High-Performance Liquid Chromatography. Molecules. 2019; 24(22):4186. https://doi.org/10.3390/molecules24224186
Chicago/Turabian StyleLiu, Yuanman, Ziling Li, Ziyang Zhang, Tengwen Zhao, Manman Wang, and Xuesheng Wang. 2019. "Determination of Urinary Hydroxyl PAHs Using Graphene Oxide@Diatomite Based Solid-Phase Extraction and High-Performance Liquid Chromatography" Molecules 24, no. 22: 4186. https://doi.org/10.3390/molecules24224186
APA StyleLiu, Y., Li, Z., Zhang, Z., Zhao, T., Wang, M., & Wang, X. (2019). Determination of Urinary Hydroxyl PAHs Using Graphene Oxide@Diatomite Based Solid-Phase Extraction and High-Performance Liquid Chromatography. Molecules, 24(22), 4186. https://doi.org/10.3390/molecules24224186





