Application of N-Dodecyl l-Peptide to Enhance Serum Stability while Maintaining Inhibitory Effects on Myometrial Contractions Ex Vivo
Abstract
1. Introduction
2. Results
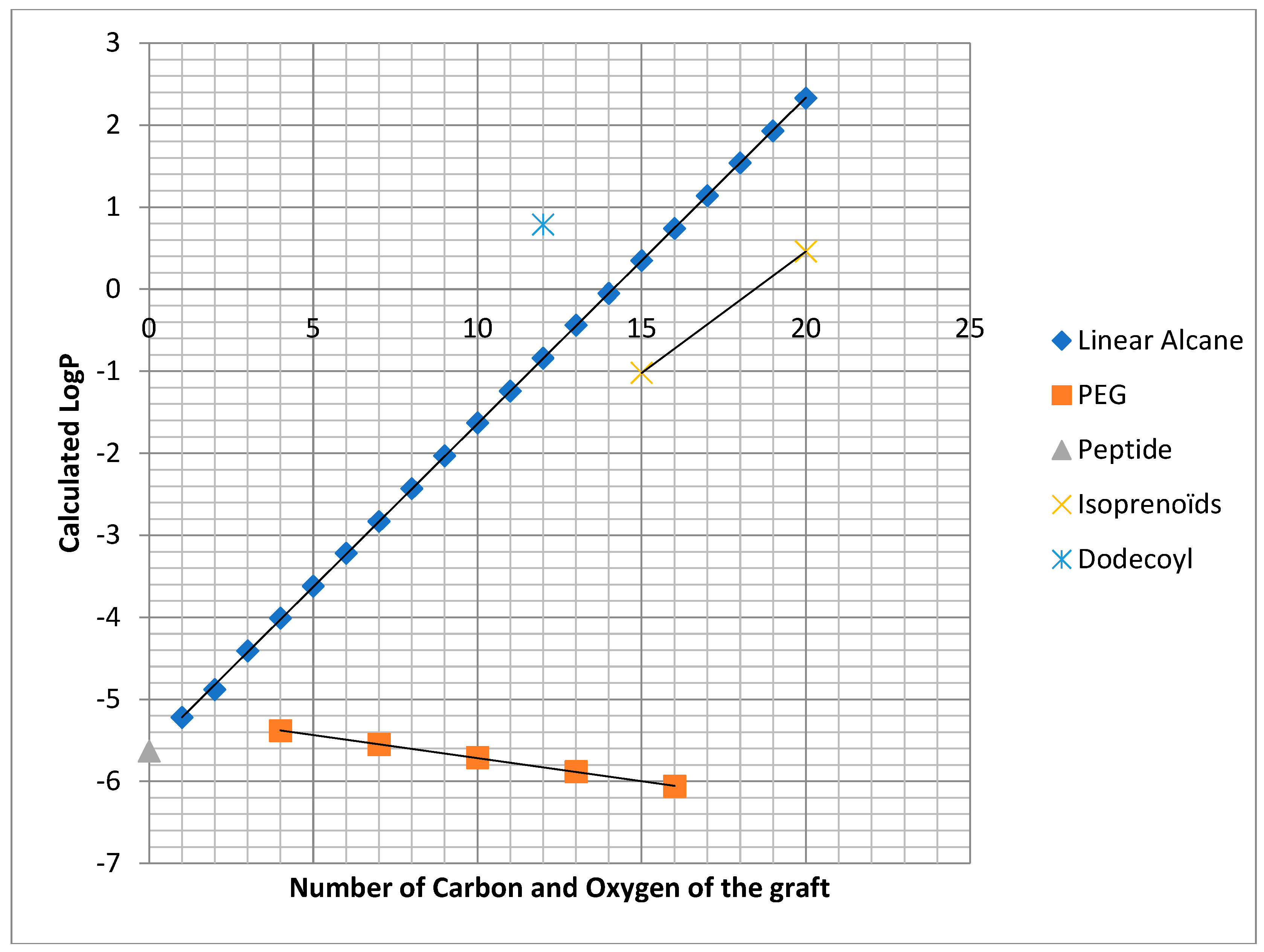
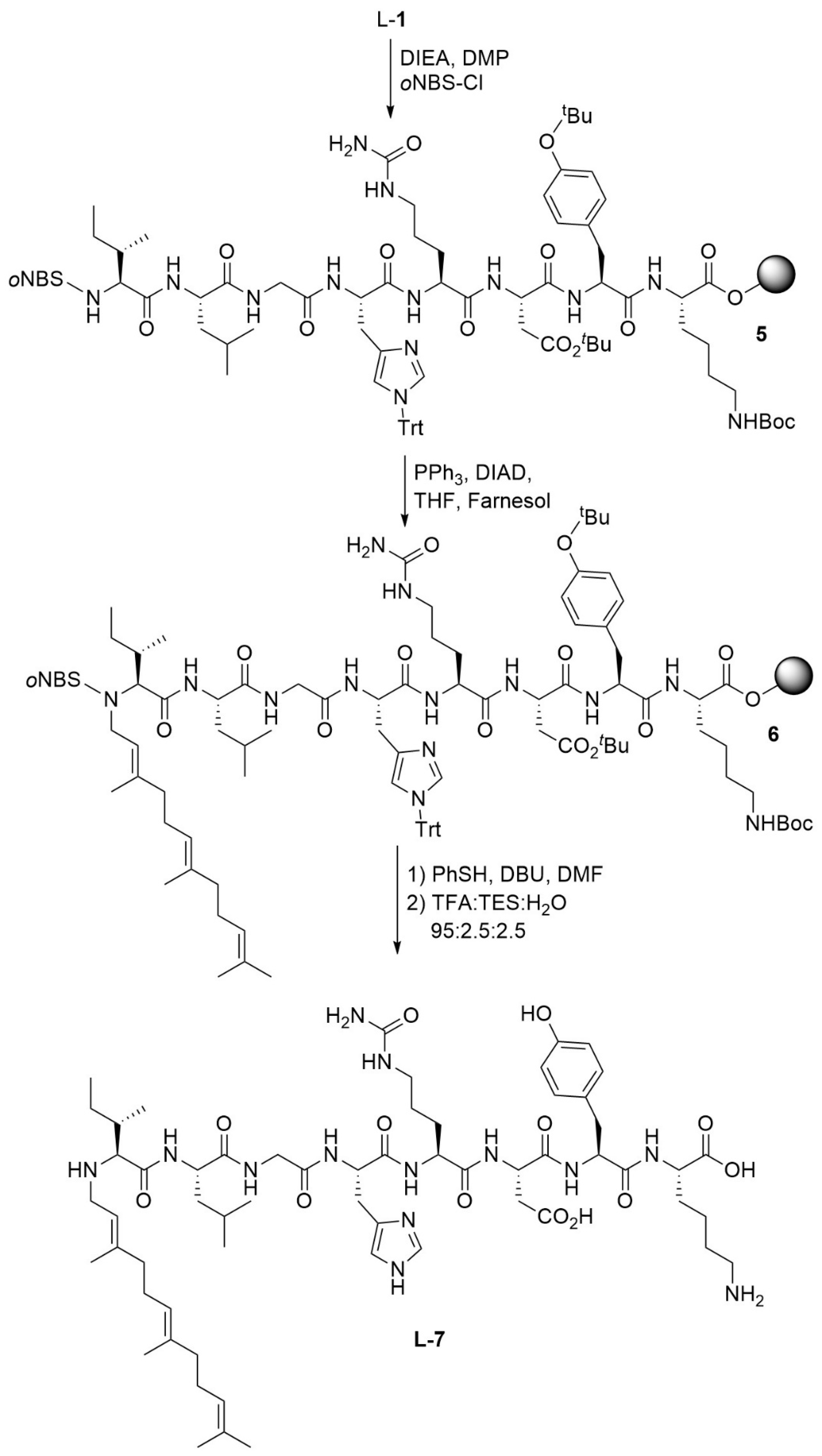
2.1. Chemistry
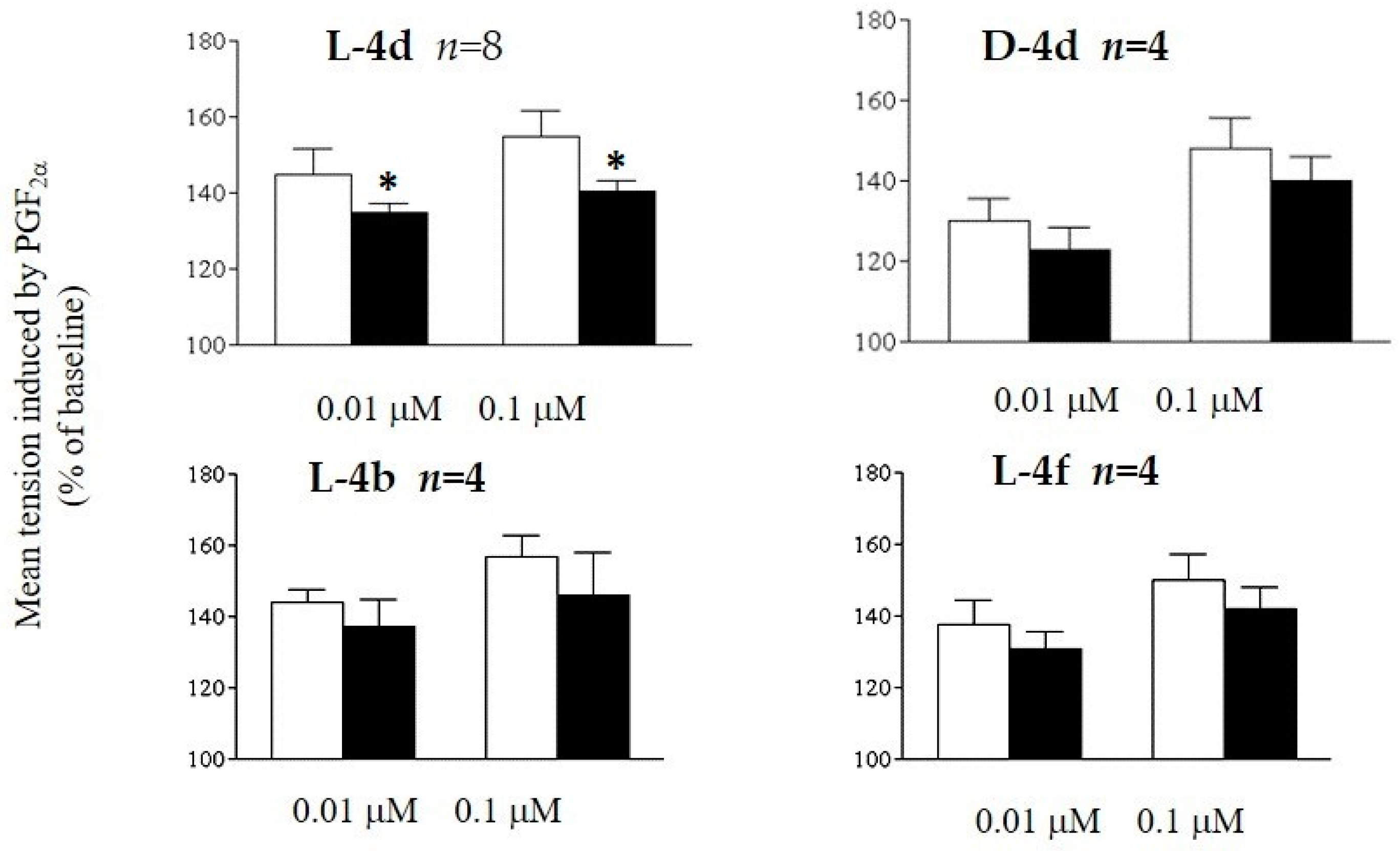
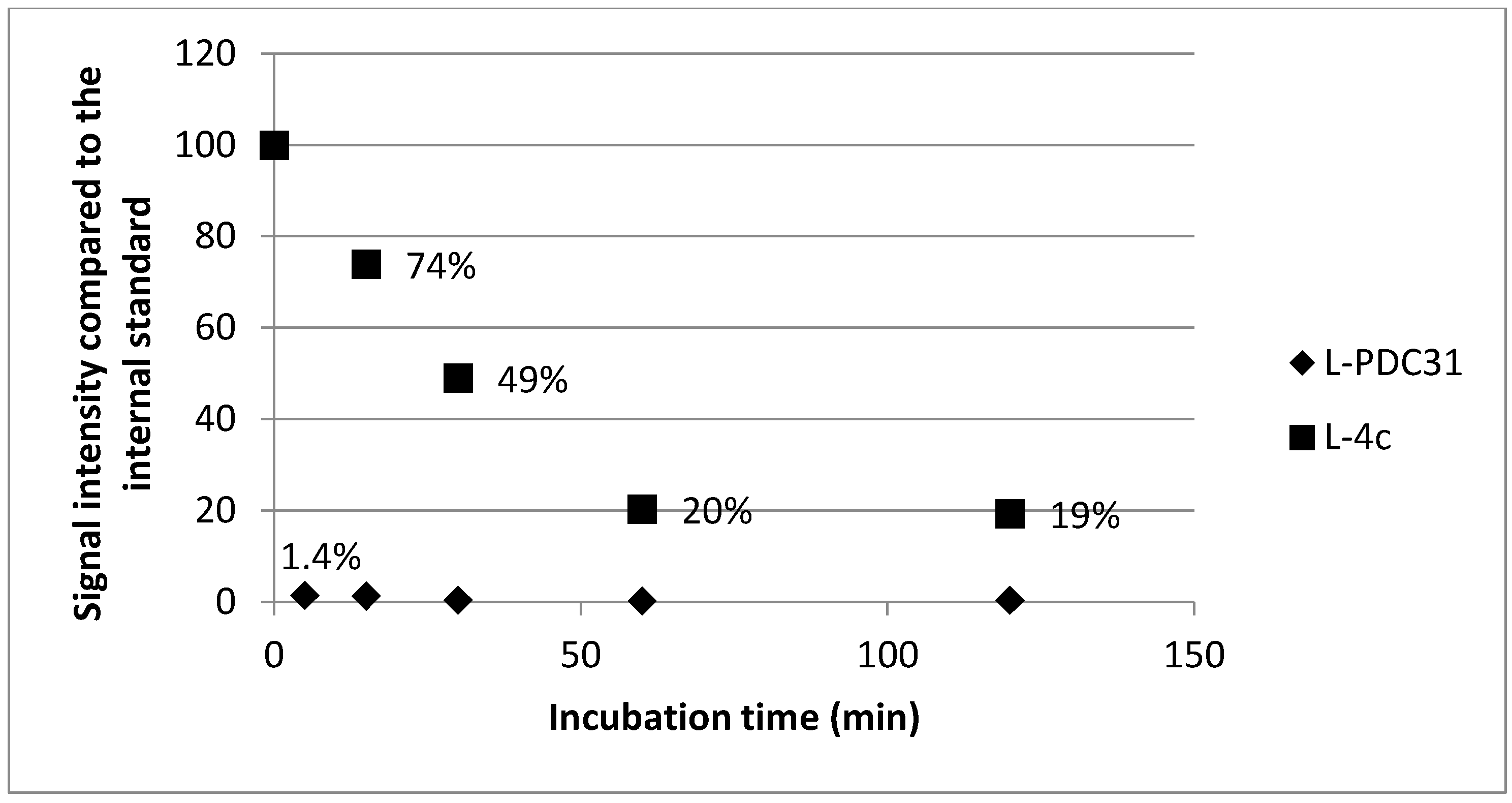
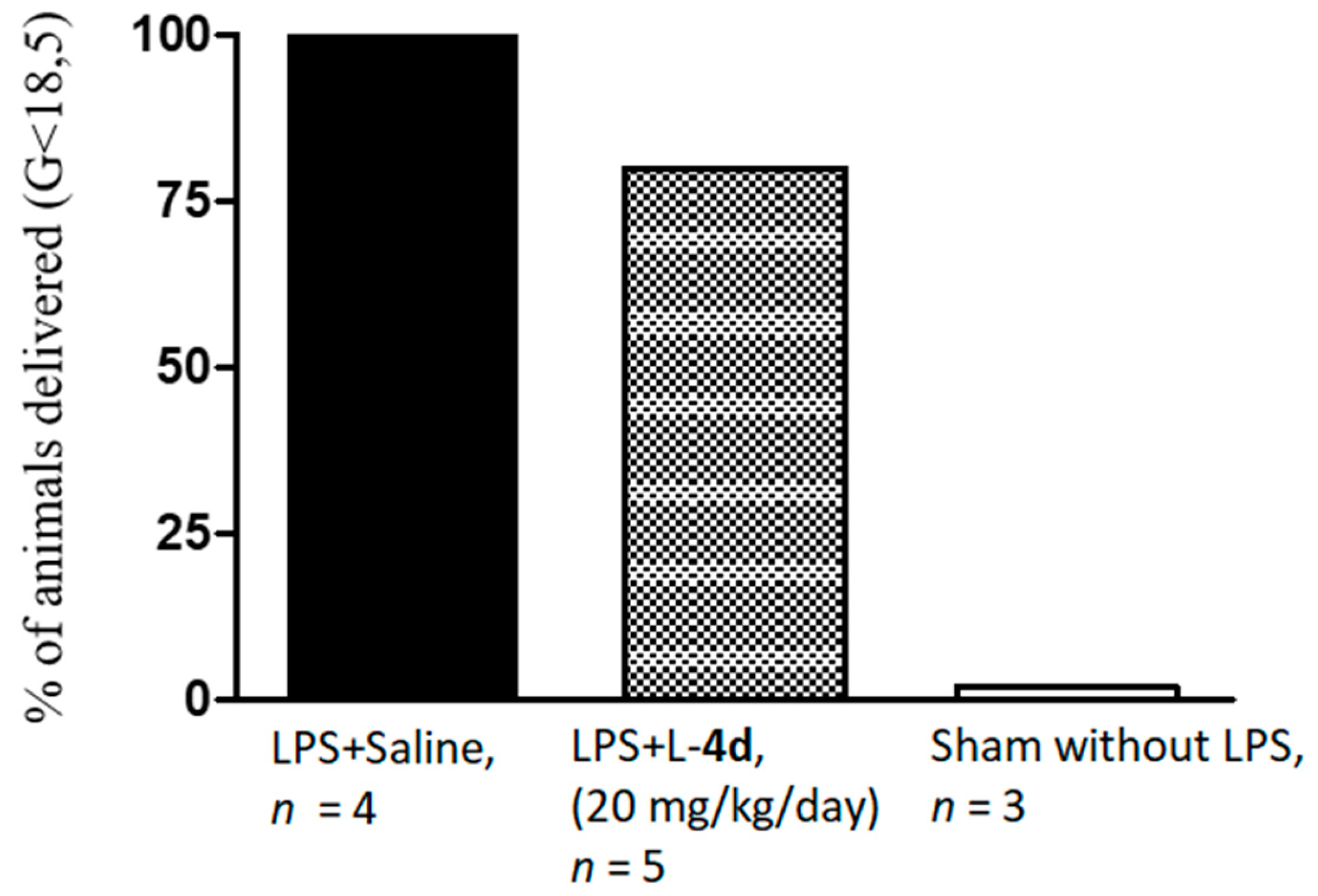
2.2. Biology
3. Discussion
4. Materials and Methods
4.1. General
4.2. Chemistry
4.2.1. Resin Loading and Capping
4.2.2. Automated Peptide Synthesis
4.2.3. Peptide Cleavage and Purification
4.3. Biology
4.3.1. Serum Stability Study
4.3.2. Ex Vivo Study
4.3.3. In Vivo Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slattery, M.M.; Morrison, J.J. Preterm delivery. Lancet 2002, 360, 1489–1497. [Google Scholar] [CrossRef]
- Russell, R.B.; Green, N.S.; Steiner, C.A.; Meikle, S.; Howse, J.L.; Poschman, K.; Dias, T.; Potetz, L.; Davidoff, M.J.; Damus, K.; et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics 2007, 120, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.; Flannery, D.J.; Schluchter, M.; Cartar, L.; Borawski, E.; Klein, N. Outcomes in Young Adulthood for Very-Low-Birth-Weight Infants. N. Engl. J. Med. 2002, 346, 149–157. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.R.W.; David, C.M.; Fielder, A.R. Ophthalmological problems associated with preterm birth. Eye 2007, 21, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Marlow, M.W.; Wolke, D.; Bracelwell, M.A.; Samara, M. Neurologic and developmental disability at six years of age after extremely preterm birth. New Engl. J. Med. Chem. 2005, 352, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Stampfer, M.J.; Manson, J.E.; Rosner, B.; Hankinson, S.E.; Colditz, G.A.; Hennekens, C.H.; Willet, W.C. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 1997, 315, 396–400. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Oestergaard, M.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional and worldwide estimates of preterm birth. Lancet 2012, 379, 2126–2172. [Google Scholar]
- Papatsonis, D.; Flenady, V.; Cole, S.; Liley, H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst. Rev. 2005. [Google Scholar]
- King, F.J.; Flenady, V.; Papatosnis, D.; Dekker, G.; Carbonne, B. Calcium channel blockers for inhibiting preterm labour; a systematic review of the evidence and a protocol for administration of nifedipine. Aust. N. Z. J. Obstet. Gynaecol. 2003, 43, 192–198. [Google Scholar] [CrossRef]
- Meidahl Petersen, K.; Jimenez-Solem, E.; Andersen, J.T.; Petersen, M.; Brødbæk, K.; Køber, L.; Torp-Pedersen, C.; Poulsen, H.E. β-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open 2012, 2, e001185. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hillier, J.E.; Doyle, L.W. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst. Rev. 2002. [Google Scholar]
- Loudon, J.A.Z.; Groom, K.A.; Bennett, R. Prostaglandin inhibitors in preterm labour. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 731–744. [Google Scholar] [CrossRef]
- Haas, D.M. Tocolytic therapy: a meta-analysis and decision analysis. Obstet. Gynecol. 2009, 113, 585–594. [Google Scholar]
- Simhan, H.N.; Caritis, S.N. Prevention of preterm delivery. N. Engl. J. Med. 2007, 357, 477–487. [Google Scholar] [CrossRef]
- Olson, D.M. The role of prostaglandins in the initiation of parturition. Best Pr. Res. Clin. Obs. Gynaecol. 2003, 17, 717–730. [Google Scholar] [CrossRef]
- Olson, D.M.; Zaragoza, D.B.; Shallow, M.C.; Cook, J.L.; Mitchell, B.F.; Grigsby, P.; Hirst, J. Myometrial activation and preterm labour: evidence supporting a role for the prostaglandin F receptor—a review. Placenta 2003, 24, 47–54. [Google Scholar] [CrossRef][Green Version]
- Sugimoto, Y.; Yamasaki, A.; Segi, E.; Tsuboi, K.; Aze, Y.; Nishimura, T.; Oida, H.; Yoshida, N.; Tanaka, T.; Katsuyama, M.; et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science 1997, 277, 681–683. [Google Scholar] [CrossRef]
- Peri, K.G.; Quiniou, C.; Hou, X.; Abran, D.; Varma, D.R.; Lubell, W.D.; Chemtob, S. THG113: a novel selective FP antagonist that delays preterm labor. Semin. Perinatol. 2002, 26, 389–397. [Google Scholar] [CrossRef]
- Peri, K.; Polyak, F.; Lubell, W.D.; Thouin, E.; Chemtob, S. Peptides and peptidomimetics useful for inhibiting the activity of prostaglandin F2a receptor. WO 2003104266A2, 18 December 2002. [Google Scholar]
- Böttcher, B.; Laterza, R.M.; Wildt, L.; Seufert, R.J.; Buhling, K.J.; Singer, C.F.; Hill, W.; Griffin, P.; Jilma, B.; Schulz, M.; et al. A first-in-human study of PDC31 (prostaglandin F2α receptor inhibitor) in primary dysmenorrhea. Hum. Rreprod. 2014, 29, 2465–2473. [Google Scholar] [CrossRef]
- Presland, J. Identifying novel modulators of G protein-coupled receptors via interaction at allosteric sites. Curr. Opin. Drug Discovery Dev. 2005, 8, 567–576. [Google Scholar]
- Gokarn, Y.R.; McLean, M.; Laue, T.M. Effect of PEGylation on protein hydrodynamics. Mol. Pharm. 2012, 9, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Moosmann, A.B.; Blath, J.; Lindner, R.; Muller, E.; Bottinger, H. Aldehyde PEGylation kinetics: A standard protein versus a pharmaceutically relevant single chain variable fragment. Bioconjug. Chem. 2011, 22, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.B.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Del. Rev. 2002, 54, 459–476. [Google Scholar] [CrossRef]
- Ryan, S.M.; Mantovani, G.; Wang, X.; Haddleton, D.M.; Brayden, D.J. Advances in PEGylation of important biotech molecules: delivery aspects. Exoert Opin. Drug Deliv. 2008, 5, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Bailon, P.; Palleroni, A.; Schaffer, C.A.; Spence, C.L.; Fung, W.J.; Porter, J.E.; Ehrlich, G.K.; Pan, W.; Xu, Z.X.; Modi, M.W.; et al. Rational design of a potent, long lasting form of interferon: a 40kDa branched poly-ethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug. Chem. 2001, 12, 195–202. [Google Scholar] [CrossRef]
- Trainer, J.; Drake, W.M.; Katznelson, L.; Freda, P.U.; Herman-Bonert, V.; van der Lely, A.J.; Dimaraki, E.V.; Stewart, P.M.; Friend, K.E.; Vance, M.L.; et al. Treatment of Acromegaly with the Growth Hormone-Receptor Antagonist Pegvisomant. N. Engl. J. Med. 2000, 342, 1171–1177. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes. Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef]
- Wang, J.H.; Hogenkam, D.J.; Tran, M.; Li, W.Y.; Yoshimura, R.F.; Johnstone, T.B.C.; Shen, W.C.; Gee, K.W. Reversible Lipidization for the Oral Delivery of leu-enkephalin. J. Drug Target. 2006, 14, 127–136. [Google Scholar] [CrossRef]
- Al-Obeidi, F.; Hruby, V.; Yaghoubi, N.; Marwan, M.M.; Hadley, M.E. Synthesis and biological activities of fatty acid conjugates of a cyclic lactam alpha-melanotropin. J. Med. Chem. 1992, 35, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.P.; Ottaway, N.L.; Perez-Tilve, D.; Ma, D.; Gelfanov, V.M.; Tschöp, M.H.; Dimarchi, R.D. Peptide lipidation stabilizes structure to enhance biological function. Mol. Metab. 2013, 2, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H.; Miao, V.; Wrigley, S.K. Natural products to drugs, daptomycin and related lipopeptide antibiotics. Nat. Proc. Re 2005, 22, 717–741. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.; Hruby, V.J. Design of cyclic and other templates for potent and selective peptide α-MSH analogues. Curr. Opini. Chem. Biol. 2005, 9, 352–358. [Google Scholar] [CrossRef]
- Hadley, M.E.; al-Obeidi, F.; Hruby, V.J.; Weinrach, J.C.; Freedberg, D.; Jiang, J.W.; Stover, R.S. Biological Activities of Melanotropic Peptide Fatty Acid Conjugates. Pigment Cell Res. 1991, 4, 180–185. [Google Scholar] [CrossRef]
- Wright, T.H.; Brooks, A.E.; Didsbury, A.J.; Williams, G.M.; Harris, W.; Dunbar, R.; Brimble, M.A. Direct peptide lipidation through thiol-ene coupling enables rapid synthesis and evaluation of self-adjuvanting vaccine candidates. Angew. Chem. Int. Ed. Engl. 2013, 52, 10616–10619. [Google Scholar] [CrossRef]
- Rezler, E.M.; Khan, D.R.; Lauer-Fields, J.; Cudic, M.; Baronas-Lowell, D.; Fields, G.B. Targeted Drug Delivery Utilizing Protein-Like Molecular Architecture. J. Am. Chem. Soc. 2007, 129, 4961–4972. [Google Scholar] [CrossRef]
- Versluis, F.; Voskuhl, J.; van Kolck, B.; Zope, H.; Bremmer, M.; Albregtse, T.; Kros, A. In situ modification of plain liposomes with lipidated coiled coil forming peptides induces membrane fusion. J. Am. Chem. Soc. 2013, 135, 8057–8062. [Google Scholar] [CrossRef]
- Johannessen, L.; Remsberg, J.; Gaponenko, V.; Adams, K.M.; Barchi, J.J., Jr.; Tarasov, S.G.; Jiang, S.; Tarasova, N.I. Peptide structure stabilization by membrane anchoring and its general applicability to the development of potent cell-permeable inhibitors. Chembiochem. 2011, 12, 914–921. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.D. Protein prenylation, more than just glue? Curr. Opin. Cell Biol. 1992, 4, 1008–1016. [Google Scholar] [CrossRef]
- Lane, K.T.; Beese, L.S. Thematic review series, lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J. Lipid Res. 2006, 47, 681–699. [Google Scholar] [PubMed]
- London, N.; Lamphear, C.L.; Hougland, J.L.; Fierke, C.A.; Schueler-Furman, O. Identification of a novel class of farnesylation targets by structure-based modeling of binding specificity. PLOS Comput. Biol. 2011, 7, e1002170. [Google Scholar] [CrossRef] [PubMed]
- Marshell, C.J. Protein prenylation: a mediator of protein-protein interactions. Science 1993, 259, 1865–1866. [Google Scholar] [CrossRef] [PubMed]
- Ochocki, J.D.; Igbavboa, U.; Gibson Wood, W.; Wattenberg, E.V.; Distefano, M.D. Enlarging the scope of cell-penetrating prenylated peptides to include farnesylated ‘CAAX’ box sequences and diverse cell types. Chem. Biol. Drug Des. 2010, 76, 107–115. [Google Scholar] [CrossRef][Green Version]
- Shen, G.; Huhman, D.; Lei, Z.; Snyder, J.; Sumner, L.W.; Dixon, R.A. Characterization of an isoflavonoid-specific prenyltransferase from Lupinus albus. Plant Physiol. 2012, 159, 70–80. [Google Scholar] [CrossRef]
- Benfenati, E.; Gini, G.; Piclin, N.; Roncaglioni, A.; Varì, M.R. Predicting logP of pesticides using different software. Chemosphere 2003, 53, 1155–1164. [Google Scholar] [CrossRef]
- Medic-Saric, M.; Ana Mornar, A.; Badovinac-Črnjević, T.; Jasprica, I. Experimental and Calculation Procedures for Molecular Lipophilicity: A Comparative Study for 3,3’-(2-Methoxybenzylidene)bis(4-hydroxycoumarin). Croatica Chemica Acta. 2004, 77, 367–370. [Google Scholar]
- Veronese, F.M. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001, 22, 405–417. [Google Scholar] [CrossRef]
- Lubell, W.D.; Blankenship, J.W.; Fridkin, G.; Kaul, R. “Peptides,” in Science of Synthesis; Weinreb, S.M., Ed.; Thieme: Stuttgart, Germany, 2005; pp. 713–809. [Google Scholar]
- Arya, W.; Barnes, C.Q.; Daroswska, M.L. A Solid Phase Library Synthesis of Hydroxyindoline-Derived Tricyclic Derivatives by Mitsunobu Approach. J. Comb. Chem. 2004, 6, 65–72. [Google Scholar] [CrossRef]
- Bisegger, P.; Manov, N.; Bienz, S. Solid-phase synthesis of cyclic polyamines. Tetrahedron 2008, 64, 7531–7536. [Google Scholar] [CrossRef]
- Lencina, C.L.; Dassonville-Klimpt, A.; Sonnet, P. New efficient enantioselective synthesis of 2-oxopiperazines: a practical access to chiral 3-substituted 2-oxopiperazines. Tet. Asymmetry 2008, 19, 1689–1697. [Google Scholar] [CrossRef]
- Tumkevicius, S.; Masevicius, V.; Petraityte, G. 4-Amino-5-(arylaminomethyl)-2-(methylthio)furo [2,3-d]pyrimidines via Mitsunobu Reaction of 4-Amino-5-(hydroxymethyl)-2-(methylthio)furo [2,3-d]pyrimidine with N-Mesyl- and N-Nosylarylamines. Synthesis 2012, 44, 1329–1338. [Google Scholar] [CrossRef]
- Mir, F.M.; Atmuri, N.D.P.; Bourguet, C.B.; Fores, J.R.; Hou, X.; Chemtob, S.; Lubell, W.D. Paired Utility of Aza-Amino Acyl Proline and Indolizidinone Amino Acid Residues for Peptide Mimicry: Conception of Prostaglandin F2α Receptor Allosteric Modulators That Delay Preterm Birth. J. Med. Chem. 2019, 62, 4500–4525. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Tanebe, K.; Sasaki, Y.; Momma, K.; Yoneda, S.; Saito, S. Evaluation of the tocolytic effect of a selective cyclooxygenase-2 inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Mol. Hum. Reprod. 2001, 7, 595–602. [Google Scholar] [CrossRef]
- Tahara, M.; Kawagishi, R.; Sawada, K.; Morishige, K.; Sakata, M.; Tasaka, K.; Murata, Y. Tocolytic effect of a Rho-kinase inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Am. J. Obstet. Gynecol. 2005, 192, 903–908. [Google Scholar] [CrossRef]
- Goupil, E.; Tassy, D.; Bourguet, C.; Quiniou, C.; Wisehart, V.; Pétrin, D.; Le Gouill, C.; Devost, D.; Zingg, H.H.; Bouvier, M.; et al. A novel biased allosteric compound inhibitor of parturition selectively impedes the prostaglandin F2alpha-mediated Rho/ROCK signaling pathway. J. Biol. Chem. 2010, 285, 25624–25636. [Google Scholar] [CrossRef]
- O’Brien, M.; Morrison, J.J.; Smith, T.J. Expression of prothrombin and protease activated receptors in human myometrium during pregnancy and labor. Biol. Reprod. 2008, 78, 20–26. [Google Scholar] [CrossRef]
- Pham, W.; Kircher, M.F.; Weissleder, R.; Tung, C.H. Enhancing membrane permeability by fatty acylation of oligoarginine peptides. Chembiochem. 2004, 5, 1148–1151. [Google Scholar] [CrossRef]
- Friedrich, H.B.; Singh, N. The very efficient oxidation of alcohols by poly (4-vinylpyridine)-supported sodium ruthenate. Tet. Lett. 2000, 41, 3971–3974. [Google Scholar] [CrossRef]
- Vidal, D.M.; Fávaro, C.F.; Guimarães, M.M.; Zarbin, P.H.G. Identification and synthesis of the male-produced sex pheromone of the soldier beetle Chauliognathus fallax (Coleoptera: Cantharidae). J. Brazilian Chem. Soc. 2016, 27, 1678–4790. [Google Scholar]
- Han, X.; Dong, L.; Geng, C.; Jiao, P. Catalytic Asymmetric Synthesis of Isoxazolines from Silyl Nitronates. Org. Lett. 2015, 17, 3194–3197. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, I.; Leniewski, A.; Roszkowski, P.; Czarnocki, Z. Synthesis of a novel class of fatty acids-derived isoquinolines. Chem. Phys. Lipids 2005, 135, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Shi, W.; Li, J.; Su, D.; Wang, X.; Zhang, L.; Pan, L.; Wu, X.; Wu, H. Organocatalytic and Scalable Syntheses of Unsymmetrical 1, 2, 4, 5-Tetrazines by Thiol-Containing Promotors. Angew. Chem Int. Ed. 2019, 58, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Eissler, S.; Kley, M.; Bächle, D.; Loidl, G.; Meier, T.; Samson, D. Substitution determination of Fmoc-substituted resins at different wavelengths. J. Peptide Sci. 2017, 23, 757–762. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples not available. |

| Peptide | R-ILGHCitDYK R = | Retentin Time MeCN (min) | Retentin Time MeOH (min) | Purity (%) | [M + H]+ Calc. | [M + H]+ Found | TotalYield (%) |
|---|---|---|---|---|---|---|---|
| l-PDC31 | H- | 10.8 a | 9.9 b | >97 | 1002.5367 | 1002.5366 | 7.7 |
| 4b | H3C(CH2)5- | 9.8 a | 9.7 b | >92 | 1086.6306 | 1086.633 | 0.3 |
| 4c | H3C(CH2)10- | 11.1 a | 11.9 b | >89 | 1158.7089 | 1158.7034 | 1.4 |
| l-4d | H3C(CH2)11- | 12.6 c | 13.4 d | >95 | 1170.7245 | 1170.7251 | 0.7 |
| d-4d | H3C(CH2)11- | 13.0 a | 13.1 b | >94 | 1170.7245 | 1170.7238 | 1.2 |
| 4e | H3C(CH2)12- | 13.9 a | 14.2 b | >99 | 1184.7402 | 1084.7387 | 0.4 |
| 4f | H3C(CH2)15- | 15.0 a | 15.1 b | >94 | 627.9128 ([M + 2H]2+) | 627.9105 ([M + 2H]2+) | 0.3 |
| 4g | H3C[O(CH2)2]4- | 10.0 a | 9.6 b | >86 | 1192.6572 | 1192.6605 | 2.9 |
| 7 | Farnesyl- | 10.0 a | 13.3 b | >89 | 1206.7245 | 1206.7263 | 2.5 |
| 8 | H3C(CH2)10CO- | 7.1c | 10.2 d | >99 | 1184.7038 | 1184.7061 | 15.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poupart, J.; Hou, X.; Chemtob, S.; Lubell, W.D. Application of N-Dodecyl l-Peptide to Enhance Serum Stability while Maintaining Inhibitory Effects on Myometrial Contractions Ex Vivo. Molecules 2019, 24, 4141. https://doi.org/10.3390/molecules24224141
Poupart J, Hou X, Chemtob S, Lubell WD. Application of N-Dodecyl l-Peptide to Enhance Serum Stability while Maintaining Inhibitory Effects on Myometrial Contractions Ex Vivo. Molecules. 2019; 24(22):4141. https://doi.org/10.3390/molecules24224141
Chicago/Turabian StylePoupart, Julien, Xin Hou, Sylvain Chemtob, and William D. Lubell. 2019. "Application of N-Dodecyl l-Peptide to Enhance Serum Stability while Maintaining Inhibitory Effects on Myometrial Contractions Ex Vivo" Molecules 24, no. 22: 4141. https://doi.org/10.3390/molecules24224141
APA StylePoupart, J., Hou, X., Chemtob, S., & Lubell, W. D. (2019). Application of N-Dodecyl l-Peptide to Enhance Serum Stability while Maintaining Inhibitory Effects on Myometrial Contractions Ex Vivo. Molecules, 24(22), 4141. https://doi.org/10.3390/molecules24224141








