Transformation of Psoralen and Isopsoralen by Human Intestinal Microbial In Vitro, and the Biological Activities of Its Metabolites
Abstract
1. Introduction
2. Results and Discussion
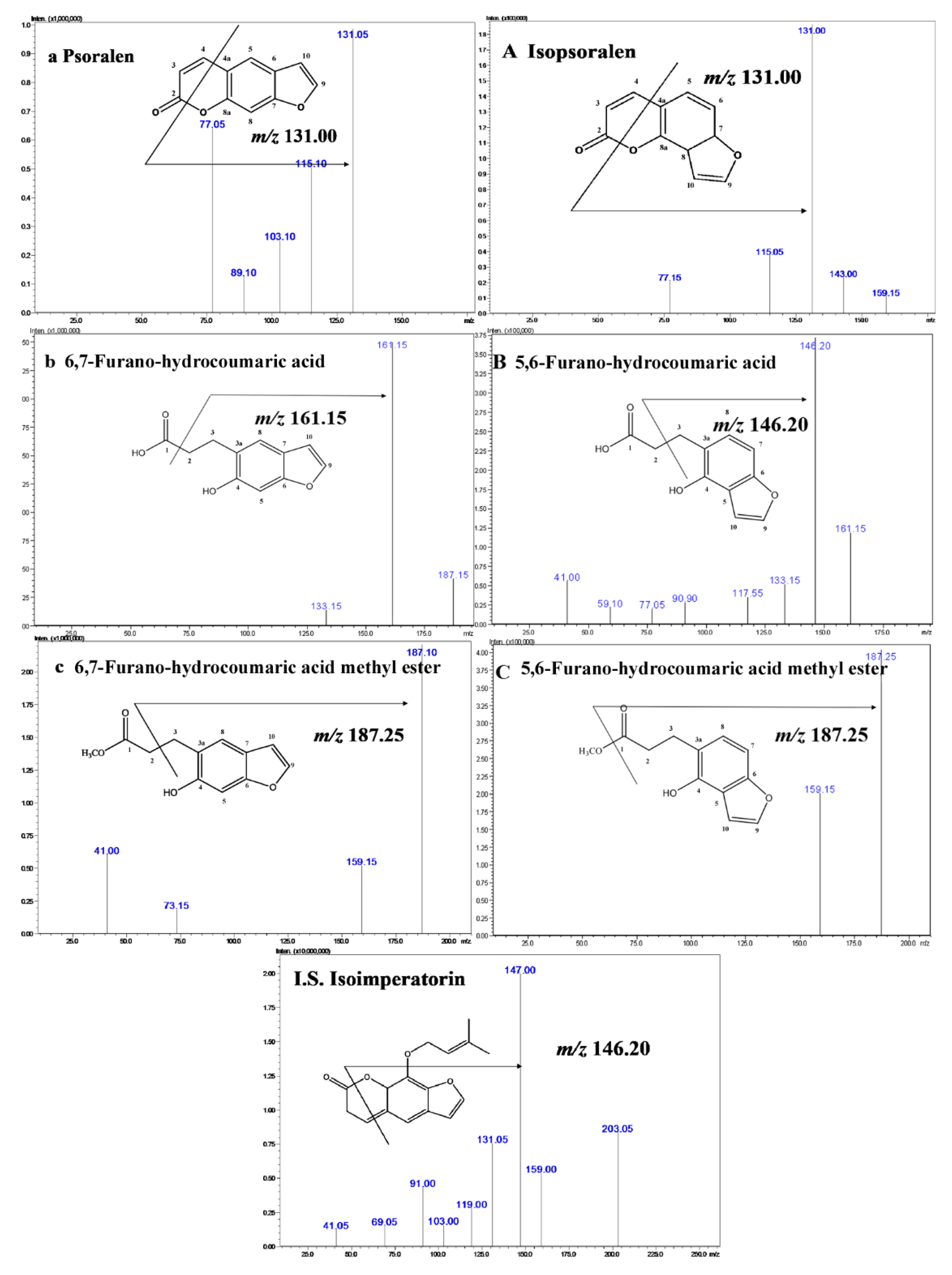
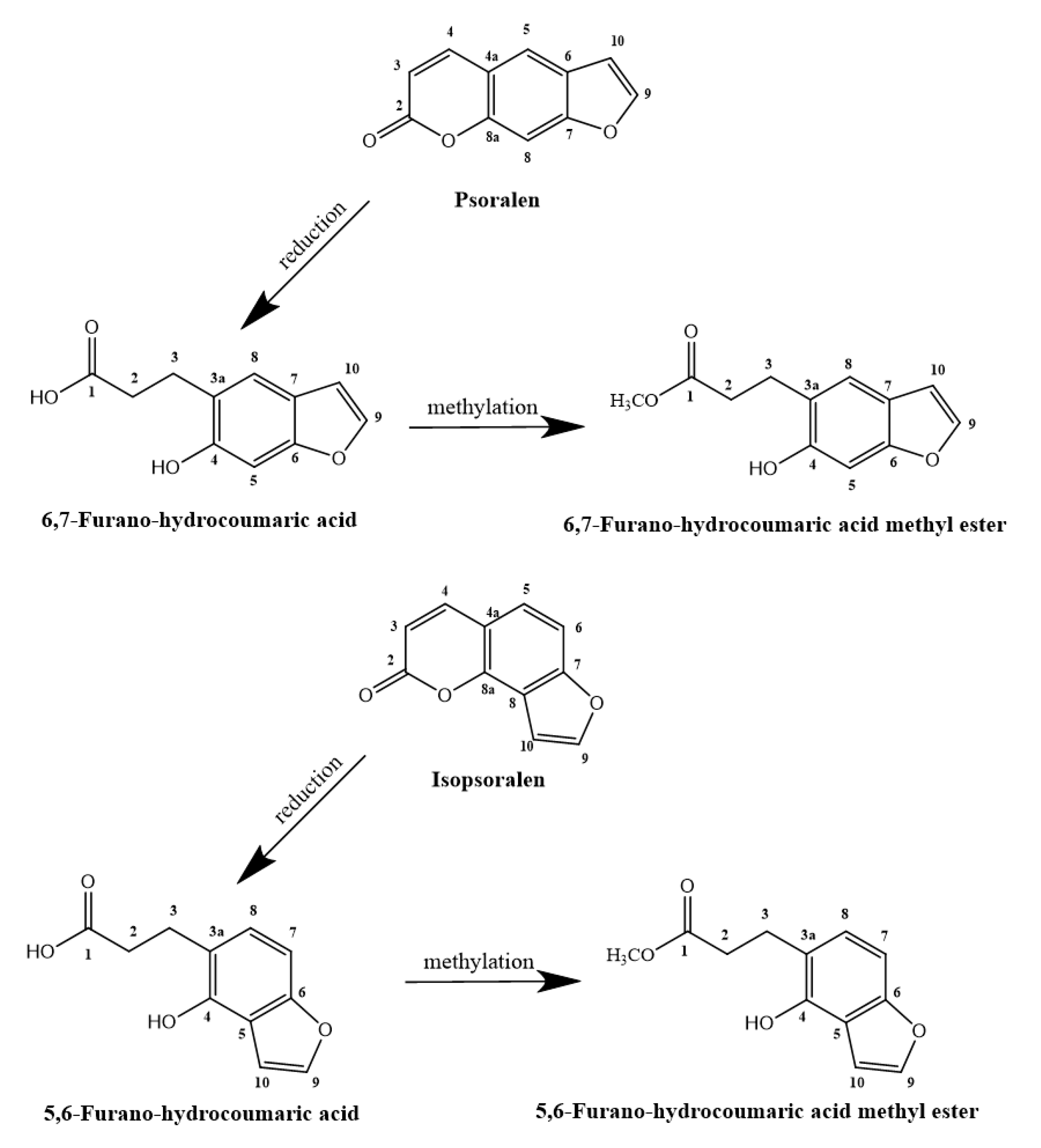
2.1. Metabolic Processing of P and IP by Human Intestinal Bacteria
2.2. Method Validation of the Archetype and Metabolites
2.2.1. UPLC-MS/MS Condition Optimization
2.2.2. The Assay Validation
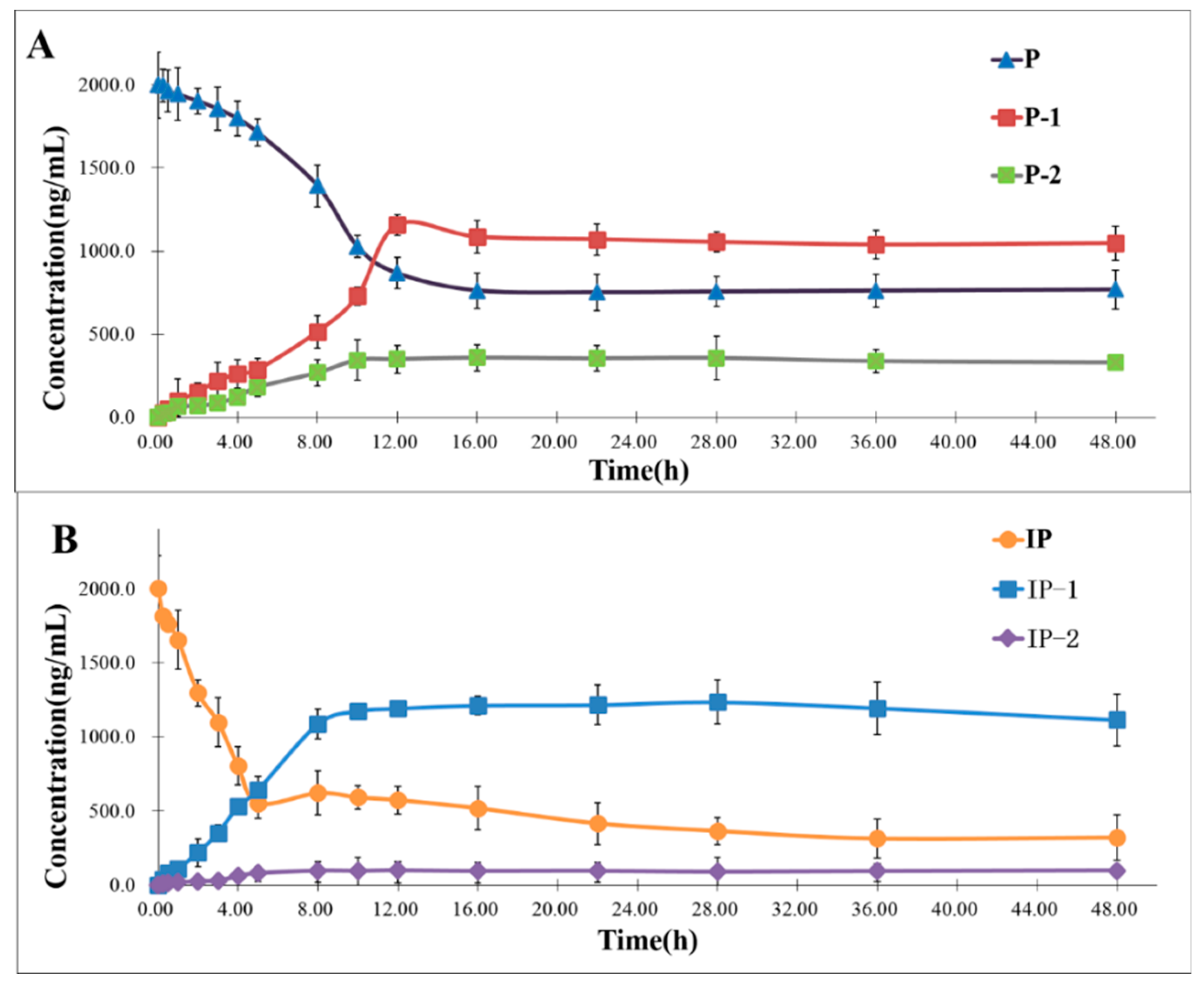
2.3. Time Course of the Biotransformation of P and IP by Human Intestinal Flora
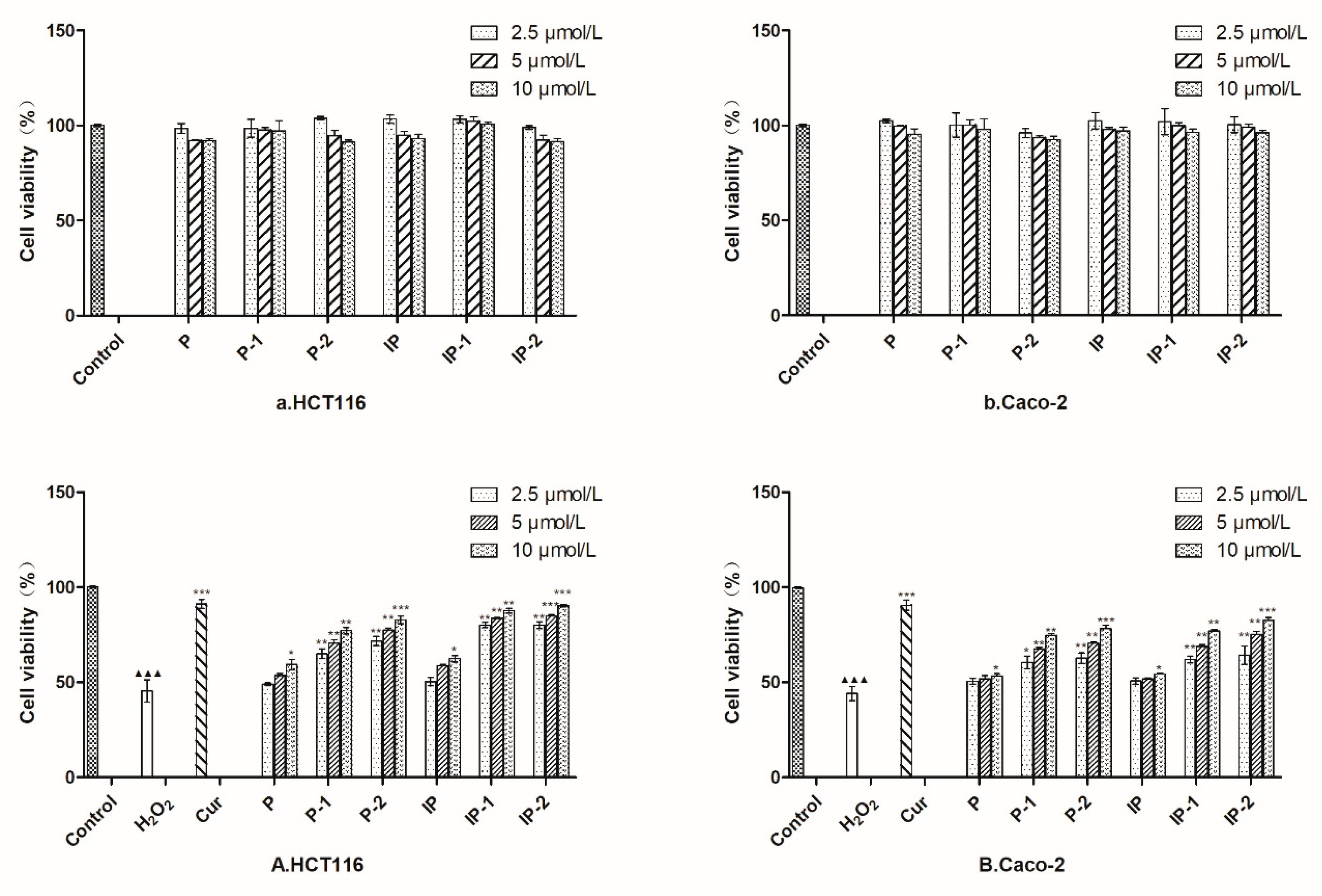
2.4. MTT Assay
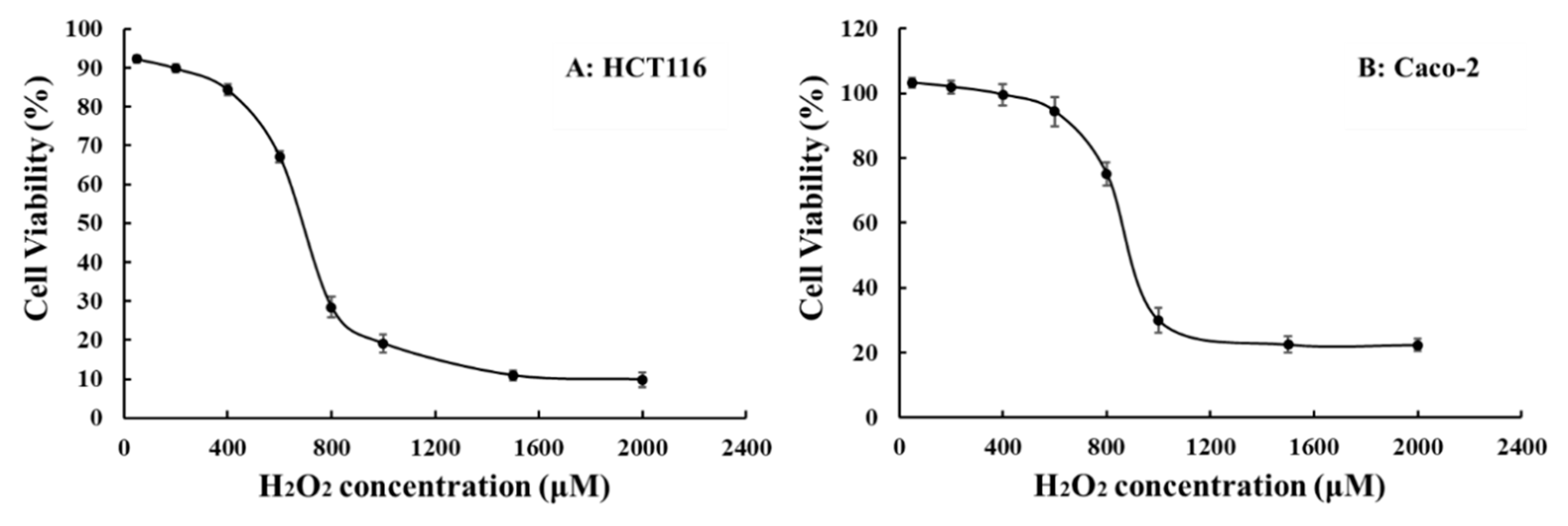
2.5. Compounds Alleviate the Decreased Viability of HCT116 and Caco-2 Cells Induced by H2O2
2.6. Discussion
3. Experimental Section
3.1. Material and Conditions
3.1.1. Material and Reagents
3.1.2. Instrumental and UPLC-MS/MS Conditions
3.2. Preparation of Representative Human Gut Bacteria
3.3. Elucidation of the Biotransformation Products from P and IP
3.4. Time Courses of the Biotransformation of P and IP by Human Intestinal Flora
3.5. Preparation of Standards and Quality Control Samples
3.6. Evaluation of Cytotoxicity
3.7. Alleviating Oxidative Stress Caused by H2O2 in HCT116 and Caco-2 cells
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Uikey, S.; Yadav, A.S.; Sharma, A.K.; Rai, A.K.; Raghuwanshi, D.K.; Badkhane, Y. The botany, chemistry, pharmacological and therapeutic application of Psoralea corylifolia L.—A Review. Int. J. Phytomedicine 2010, 2, 100–107. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People′s Republic of China; Medical Science and Technology Press: Beijing, China, 2010; Volume 1, p. 174. [Google Scholar]
- Li, C.C.; Wang, T.L.; Zhang, Z.Q.; Yang, W.Q.; Wang, Y.F.; Chai, X.; Wang, C.H.; Li, Z. Phytochemical and pharmacological studies on the genus Psoralea: A Mini Review. Evid Based Complement. Alternat Med. 2016, 2016, 1–17. [Google Scholar]
- Arya, P.; Gothalwal, R. Seed dormancy testing and germination frequency determination of Psoralea corylifolia L., an endangered medicinal plant. Tropical Plant. Res. 2017, 4, 49–54. [Google Scholar] [CrossRef]
- Nabi, N.G.; Shrivastava, M.; Dhar, R.S. Endangered medicinal plant Psoralea corylifolia: Traditional, phytochemical, therapeutic properties and micropropagation. UK J. Pharm. Biosci. 2017, 5, 40–46. [Google Scholar] [CrossRef]
- Kiran, B.; Lalitha, V.; Raveesha, K.A. Antibacterial activity of bioactive compound isolated from seeds of Psoralea corylifolia L. against Gram positive and Gram negative bacterial species. J. Pharmacy Res. 2012, 5, 144–146. [Google Scholar]
- Seo, E.; Kang, H.; Oh, Y.S.; Jun, H.S. Psoralea corylifolia L. seed extract attenuates diabetic nephropathy by inhibiting renal fibrosis and apoptosis in streptozotocin-induced diabetic mice. Nutrients 2017, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Li, Y.C.; Pan, Y.; Li, J.M.; Xu, Q.; Mo, S.F.; Qiao, C.F.; Jiang, F.X.; Xu, H.X.; Lu, X.B.; et al. Antidepressant-like effects of psoralidin isolated from the seeds of Psoralea corylifolia in the forced swimming test in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Parween, T.; Siddiqi, T.O. Mahmooduzzafar, anti-oxidant modulation in response to gamma radiation induced oxidative stress in developing seedlings of Psoralea corylifolia L. J. Environ. Radioact. 2012, 113, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kiran, B.; Lalitha, V.; Raveesha, K.A. Antifungal activity of aqueous and solvent extracts of seeds of Psoralea corylifolia L. against seed borne fungi of maize. Int. J. Pharm. Life Sci. 2011, 2, 1133–1136. [Google Scholar]
- Chen, C.H.; Hwang, T.L.; Chen, L.C.; Chang, T.H.; Wei, C.S.; Chen, J.J. Isoflavones and anti-inflammatory constituents from the fruits of Psoralea corylifolia. Phytochemistry 2017, 143, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Song, J.C. Research progress of the chemical constituents and bioactivity of Psoralea corylifolia. Pract. Pharm. Clin. Rem. 2016, 19, 1184–1188. [Google Scholar]
- Im, A.R.; Chae, S.W.; Zhang, G.J.; Lee, M.Y. Neuroprotective effects of Psoralea corylifolia Linn seed extracts on mitochondrial dysfunction induced by 3-nitropropionic acid. BMC Complement. Altern. 2014, 14, 370. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.R.; Chang, C.L.; Hsieh, P.Y.; Lin, L.W.; Ching, H. Psoralen and isopsoralen, two coumarins of Psoraleae Fructus, can alleviate scopolamine-induced amnesia in rats. Planta. Medica. 2007, 73, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.X.; Zhao, P.W.; Niu, J.Z.; Wang, J.F. Effect of psoralen on proliferation of human breast carcinoma cells. Chin. Pharmacol. Bull. 2007, 23, 1448–1451. [Google Scholar]
- Li, X.M.; Yang, Q.; Li, X.B.; Cheng, Q.; Zhang, K.; Han, J.; Zhao, J.N.; Liu, G.; Zhao, M.G. Estrogen-like neuroprotection of isopsoralen against spinal cord injury through estrogen receptor ERα. Metab. Brain Dis. 2017, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, C.; Zhou, C.; Xu, D.; Qu, H.B. Screening antitumor compounds psoralen and isopsoralen from Psoralea corylifolia L. seeds. Evid. Based Complement. Alternat. Med. 2011, 2011, 363052. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, J.C.; Jang, K.S.; Choi, G.J. Anti-oomycete activity of furanocoumarins from seeds of Psoralea corylifolia against Phytophthora infestans. Plant. Pathol. J. 2009, 25, 103–107. [Google Scholar] [CrossRef]
- Wu, C.; Sun, Z.; Ye, Y.; Han, X.; Song, X.; Liu, S. Psoralen inhibits bone metastasis of breast cancer in mice. Fitoterapia 2013, 91, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Oh, K.Y.; Kim, J.Y.; Ryu, H.W.; Jeong, T.S.; Park, K.H. Polyphenols displaying tyrosinase inhibition from the seed of Psoralea corylifolia. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 427–432. [Google Scholar] [CrossRef]
- Xiao, G.D.; Li, G.W.; Chen, L.; Zhang, Z.J.; Yin, J.J.; Wu, T.; Cheng, Z.H.; Wei, X.H.; Wang, Z.T. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J. Chromatogr. A 2010, 1217, 5470–5476. [Google Scholar] [CrossRef] [PubMed]
- Carini, F.; Tomasello, G.; Jurjus, A.; Geagea, A.; Kattar, S.; Damiani, P.; Sinagra, E.; Rappa, F.; David, S.; Cappello, F.; et al. Colorectal cancer and inflammatory bowel diseases: Effects of diet and antioxidants. J. Biol. Regul. Homeost. Agents 2017, 31, 791–795. [Google Scholar] [PubMed]
- Chen, X.M.; Yang, Y.F.; Zhang, Y.T. Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro. FEBS Lett. 2013, 587, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.X.; Hu, Y.; Li, G.Y.; Xu, W.; Zhang, Y.T.; Yang, X.W. Multi-target anti-alzheimer activities of four prenylated compounds from Psoralea Fructus. Molecules 2018, 23, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Yang, X.W.; Zhang, Y.B.; Zhai, Y.Y.; Xu, W.; Zhao, B.; Liu, D.L.; Yu, H.J. Biotransformation of phlorizin by human intestinal flora and inhibition of biotransformation products on tyrosinase activity. Food Chemistry 2012, 132, 936–942. [Google Scholar] [CrossRef]
- Feng, L.; Wang, L.; Jiang, X. Pharmacokinetics, tissue distribution and excretion of coumarin components from Psoralea corylifolia L. in rats. Arch. Pharm. Res. 2010, 33, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Zhang, Y.B.; Chen, Z.J.; Zhang, Y.T.; Yang, X.W. Plasma pharmacokinetics and cerebral nuclei distribution of major constituents of Psoraleae fructus in rats after oral administration. Phytomedicine 2018, 38, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.M.; Chang, Y.X.; Wang, Y.F.; Liu, E.W.; Li, J.; Kang, L.Y.; Gao, X.M. In vivo pharmacokinetics of bakuchiol after oral administration of bakuchiol extraction in rat plasma. J. Ethnopharmacol. 2010, 128, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, S.; Miyazawa, M. Microbial reduction of coumarin, psoralen, and xanthyletin by Glomerella cingulata. Tetrahedron 2011, 67, 495–500. [Google Scholar] [CrossRef]
- Orabi, K.Y.; Sayed, K.A.E. Biocatalytic studies of the furanocoumarins angelicin and chalepensin. Nat. Prod. Commun. 2007, 2, 565–569. [Google Scholar] [CrossRef]
- Kim, L.; Kim, Y.; Kwon, O.; Ji, Y.K. Antioxidant activities of ethanolic and acidic ethanolic extracts of astringent persimmon in H2O2 -stimulated Caco-2 human colonic epithelial cells. Food Sci. Biotechnol. 2017, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, P.; Fuccelli, R.; Sepporta, M.V.; Fabiani, R. In vitro chemo-preventive activities of hydroxytyrosol: The main phenolic compound present in extra-virgin olive oil. Food Funct. 2016, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.N.; Weng, X.C.; Hou, W.; Li, Q.H.; Bi, K.S. Antioxidants from a Chinese medicinal herb—Psoralea corylifolia L. Food Chem. 2005, 91, 287–292. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, Y.S.; Ko, C.N.; Cho, K.H.; Bae, H.S.; Lee, K.S.; Kim, J.J.; Park, E.K.; Kim, D.H. Fecal metabolic activities of herbal components to bioactive compounds. Arch. Pharmacal Res. 2002, 25, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2009, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bai, Y.J.; Chen, Y.Y.; Zhao, Y.Y. Studies on chemical constituents from seed of Psoralea corylifolia. Zhongguo Zhong Yao Za Zhi 2008, 33, 1410–1412. (In Chinese) [Google Scholar] [PubMed]
- Deng, G.G.; Yang, X.W.; Zhang, Y.B.; Wei, X.U.; Wei, W.; Li, Z.G. Chemical constituents from lipophilic parts in roots of Angelica dahurica cv.Yubaizhi. Zhongguo Zhong Yao Za Zhi 2017, 42, 2102–2109. (In Chinese) [Google Scholar] [PubMed]
- Yang, X.W.; Xu, W. Establishment of model and standard operation procedure for biotransformation of chemical constituents of traditional Chinese medicine by human intestinal bacteria. Zhongguo Zhong Yao Za Zhi 2011, 36, 19–26. (In Chinese) [Google Scholar] [PubMed]
- Carmichael, J.; Mitchell, J.B.; Degraff, W.G.; Gamson, J.; Gazdar, A.F.; Johnson, B.E.; Glatstein, E.; Minna, J.D. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer 1985, 57, 540–547. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
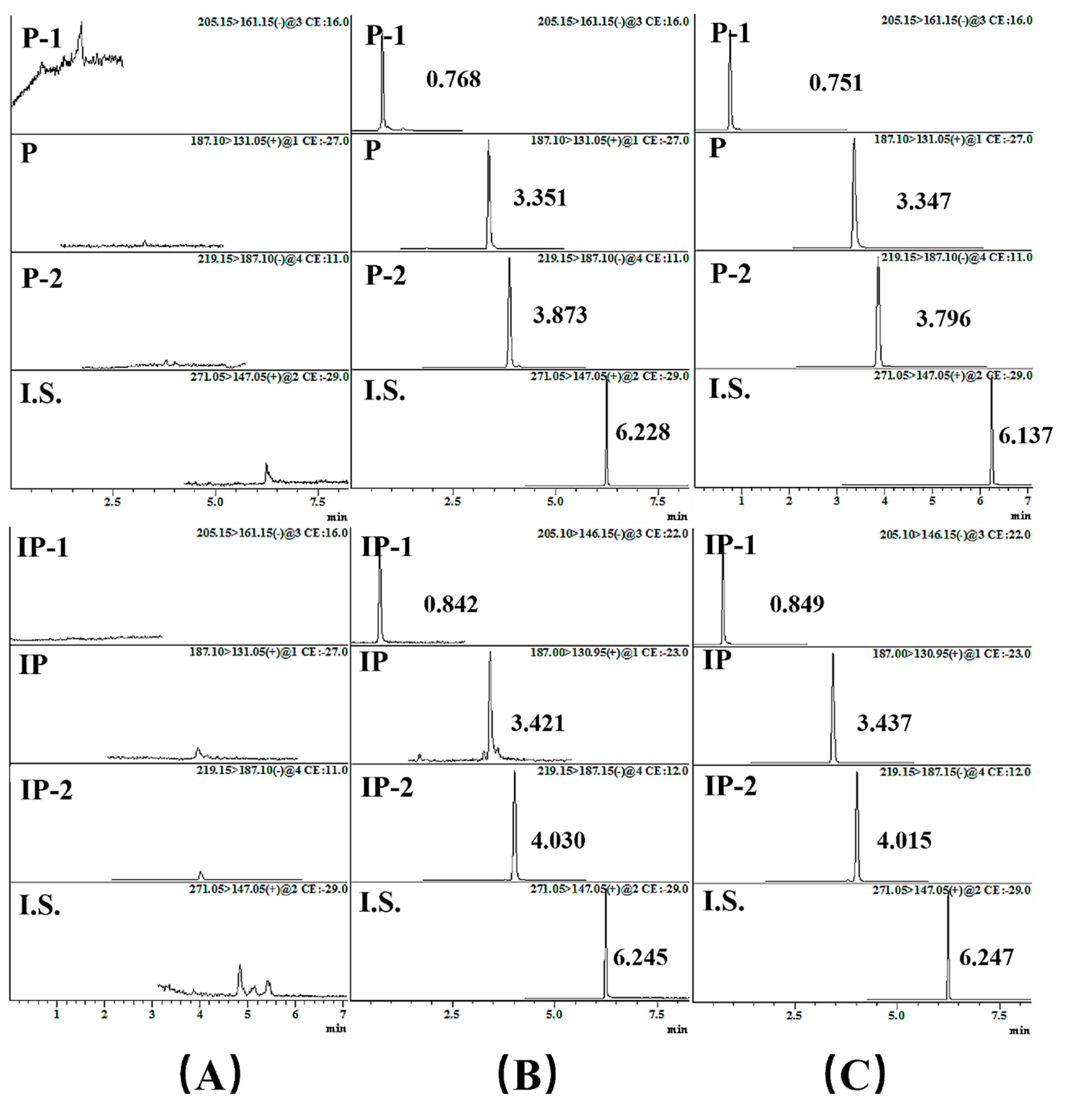
| Analyte | MRM | Precursor Ion–Product Ion (m/z) | Q1 Pre-Bias * (V) | Collision Energy (V) | Q3 Pre-Bias * (V) | Dwell Time (msec) | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| P | MRM (+) | 187.10–131.05 | −14.0 | −27.0 | −23.0 | 100.0 | 3.351 |
| P-1 | MRM (−) | 205.15–161.15 | 23.0 | 16.0 | 16.0 | 100.0 | 0.768 |
| P-2 | MRM (−) | 219.15–187.10 | 16.0 | 11.0 | 18.0 | 100.0 | 3.873 |
| IP | MRM (+) | 187.00–131.95 | −14.0 | −23.0 | −22.0 | 100.0 | 3.421 |
| IP-1 | MRM (−) | 205.10–146.15 | 15.0 | 22.0 | 28.0 | 100.0 | 0.842 |
| IP-2 | MRM (−) | 219.15–187.15 | 16.0 | 12.0 | 19.0 | 100.0 | 4.030 |
| I.S. | MRM (+) | 271.05–147.05 | −30.0 | −29.0 | −27.0 | 44.0 | 6.245 |
| Analytes | Regression Equation | r2 | Linear Range * | LLOQ * | LLOD * |
|---|---|---|---|---|---|
| P | y = 46.4226x + 198.909 | 0.9977 | 4.88–2440 | 4.88 | 2.44 |
| P-1 | y = 318.924x + 316.47 | 0.9921 | 5.30–2650 | 5.30 | 2.65 |
| P-2 | y = 48.9102x + 1577.72 | 0.9931 | 2.02–1010 | 2.02 | 1.01 |
| IP | y = 227.105x − 2813.5 | 0.9939 | 5.06–2530 | 5.06 | 2.53 |
| IP-1 | y = 226.079x − 1952.0 | 0.9906 | 4.46–2230 | 4.46 | 2.23 |
| IP-2 | y = 854.015x − 2543.2 | 0.9957 | 2.14–1070 | 2.14 | 1.07 |
| Compounds | Spiked Concentration (ng/mL) | Measured Concentration (ng/mL) | Accuracy (%) | Precision (%) | |
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||
| P | 4.880 | 4.80 ± 0.50 | −1.40 | 4.80 | 9.50 |
| 12.20 | 13.22 ± 1.70 | 8.39 | 12.72 | 13.69 | |
| 610.0 | 611.0 ± 75.17 | 0.16 | 13.05 | 3.04 | |
| 1952 | 2067 ± 144.0 | 5.89 | 7.14 | 5.47 | |
| P-1 | 5.300 | 5.140 ± 0.330 | −3.00 | 7.80 | 5.20 |
| 13.25 | 13.07 ± 1.530 | −1.36 | 12.35 | 3.63 | |
| 662.5 | 594.5 ± 60.34 | −10.26 | 10.11 | 10.45 | |
| 2120 | 2204 ± 203.0 | 3.96 | 9.19 | 9.38 | |
| P-2 | 2.020 | 2.240 ± 0.156 | 10.80 | 3.50 | 6.30 |
| 5.050 | 5.680 ± 0.590 | 12.43 | 10.56 | 9.82 | |
| 252.5 | 250.8 ± 29.33 | −0.69 | 12.00 | 9.08 | |
| 808.0 | 847.3 ± 34.10 | 4.86 | 4.25 | 1.54 | |
| IP | 5.060 | 5.140 ± 0.321 | 1.60 | 4.40 | 5.60 |
| 12.65 | 12.65 ± 0.460 | 0.12 | 3.23 | 5.99 | |
| 632.5 | 556.7 ± 63.88 | −0.14 | 11.54 | 10.96 | |
| 2024 | 1794 ± 110.1 | 0.57 | 8.45 | 2.78 | |
| IP-1 | 4.460 | 4.471 ± 0.407 | 8.20 | 9.60 | 7.00 |
| 11.15 | 12.36 ± 1.041 | −2.12 | 8.00 | 11.10 | |
| 557.5 | 528.0 ± 45.88 | −5.30 | 8.65 | 9.01 | |
| 1784 | 1820 ± 86.62.3 | 2.02 | 6.49 | 7.85 | |
| IP-2 | 2.140 | 2.401 ± 0.321 | 12.20 | 9.20 | 11.80 |
| 5.350 | 5.030 ± 0.430 | −5.90 | 8.81 | 4.86 | |
| 267.5 | 242.2 ± 25.68 | −7.75 | 10.93 | 7.79 | |
| 856.0 | 839.2 ± 65.35 | −2.02 | 9.29 | 13.04 | |
| Compounds | Spiked Concentration (ng/mL) | Matrix Effect | Extraction Recovery | ||
|---|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | ||
| P | 12.20 | 86.87 | 5.68 | 98.24 | 12.54 |
| 610.0 | 88.37 | 7.94 | 95.99 | 5.57 | |
| 1952 | 98.49 | 11.52 | 91.86 | 5.12 | |
| P-1 | 13.25 | 89.77 | 3.54 | 85.82 | 8.49 |
| 662.5 | 101.14 | 9.56 | 94.81 | 4.10 | |
| 2120 | 99.26 | 10.55 | 89.49 | 13.53 | |
| P-2 | 5.05 | 110.48 | 10.38 | 97.17 | 6.51 |
| 252.5 | 96.99 | 4.52 | 96.02 | 4.84 | |
| 808 | 86.87 | 4.77 | 90.48 | 6.39 | |
| IP | 12.63 | 91.15 | 10.80 | 95.39 | 4.67 |
| 632.5 | 95.51 | 3.37 | 91.31 | 4.72 | |
| 2024 | 92.37 | 4.18 | 89.94 | 2.75 | |
| IP-1 | 11.15 | 94.00 | 7.38 | 89.65 | 3.87 |
| 557.5 | 93.53 | 7.86 | 87.95 | 9.78 | |
| 1784 | 98.19 | 8.75 | 82.04 | 8.58 | |
| IP-2 | 5.35 | 98.55 | 11.08 | 97.28 | 13.34 |
| 267.5 | 95.74 | 14.34 | 85.71 | 12.70 | |
| 856.0 | 83.88 | 14.10 | 94.07 | 14.67 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, L.; Cui, Z.-X.; Liu, X.-Y.; Xu, W.; Yang, X.-W. Transformation of Psoralen and Isopsoralen by Human Intestinal Microbial In Vitro, and the Biological Activities of Its Metabolites. Molecules 2019, 24, 4080. https://doi.org/10.3390/molecules24224080
Liu L, Zhang L, Cui Z-X, Liu X-Y, Xu W, Yang X-W. Transformation of Psoralen and Isopsoralen by Human Intestinal Microbial In Vitro, and the Biological Activities of Its Metabolites. Molecules. 2019; 24(22):4080. https://doi.org/10.3390/molecules24224080
Chicago/Turabian StyleLiu, Lu, Lei Zhang, Ze-Xu Cui, Xiao-Yan Liu, Wei Xu, and Xiu-Wei Yang. 2019. "Transformation of Psoralen and Isopsoralen by Human Intestinal Microbial In Vitro, and the Biological Activities of Its Metabolites" Molecules 24, no. 22: 4080. https://doi.org/10.3390/molecules24224080
APA StyleLiu, L., Zhang, L., Cui, Z.-X., Liu, X.-Y., Xu, W., & Yang, X.-W. (2019). Transformation of Psoralen and Isopsoralen by Human Intestinal Microbial In Vitro, and the Biological Activities of Its Metabolites. Molecules, 24(22), 4080. https://doi.org/10.3390/molecules24224080





