Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement
Abstract
1. Introduction
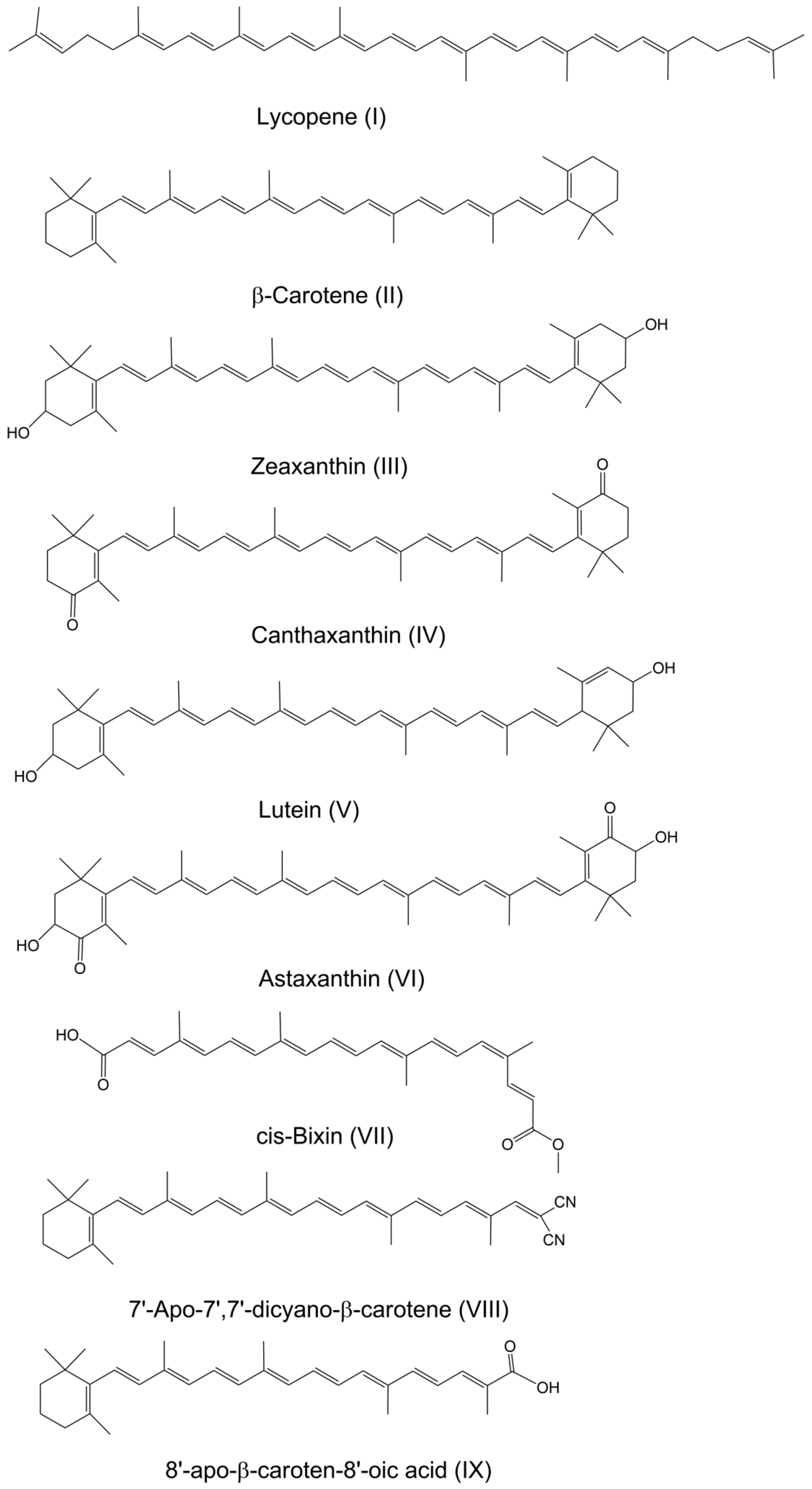
2. Inclusion Complexes
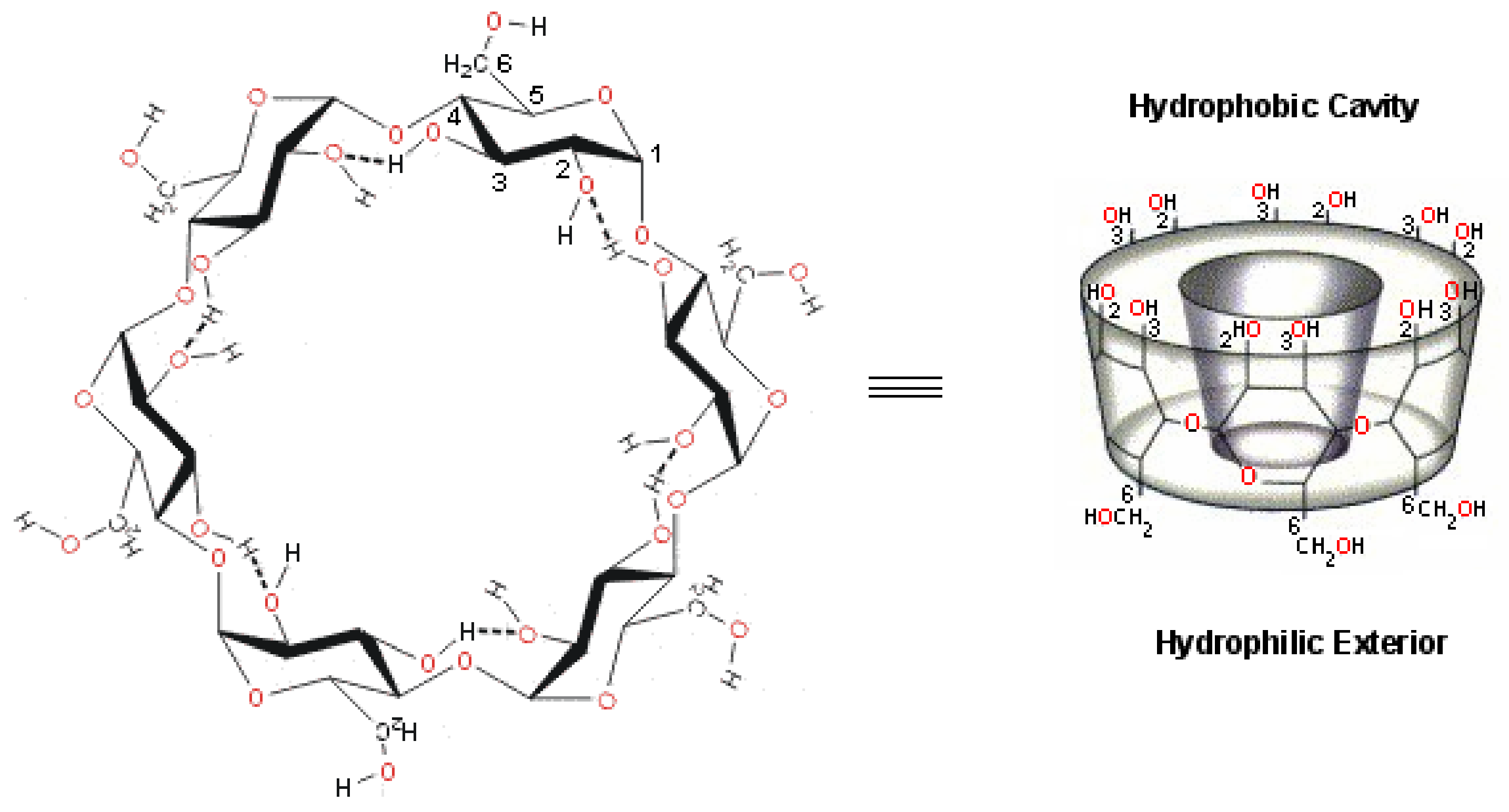
2.1. Cyclodextrins
2.2. Glycyrrhizic Acid
- (1)
- Carotenoids are able to form inclusion complexes with GA micelles at high concentrations (>1 mM of GA), as well as with pre-micellar GA aggregates (dimers) at low concentrations (1 µM–1 mM) [16]. These complexes are extremely stable. The stability constants of carotenoids-GA complexes are 1–2 orders higher than stability constants of carotenoids-CD complexes.
- (2)
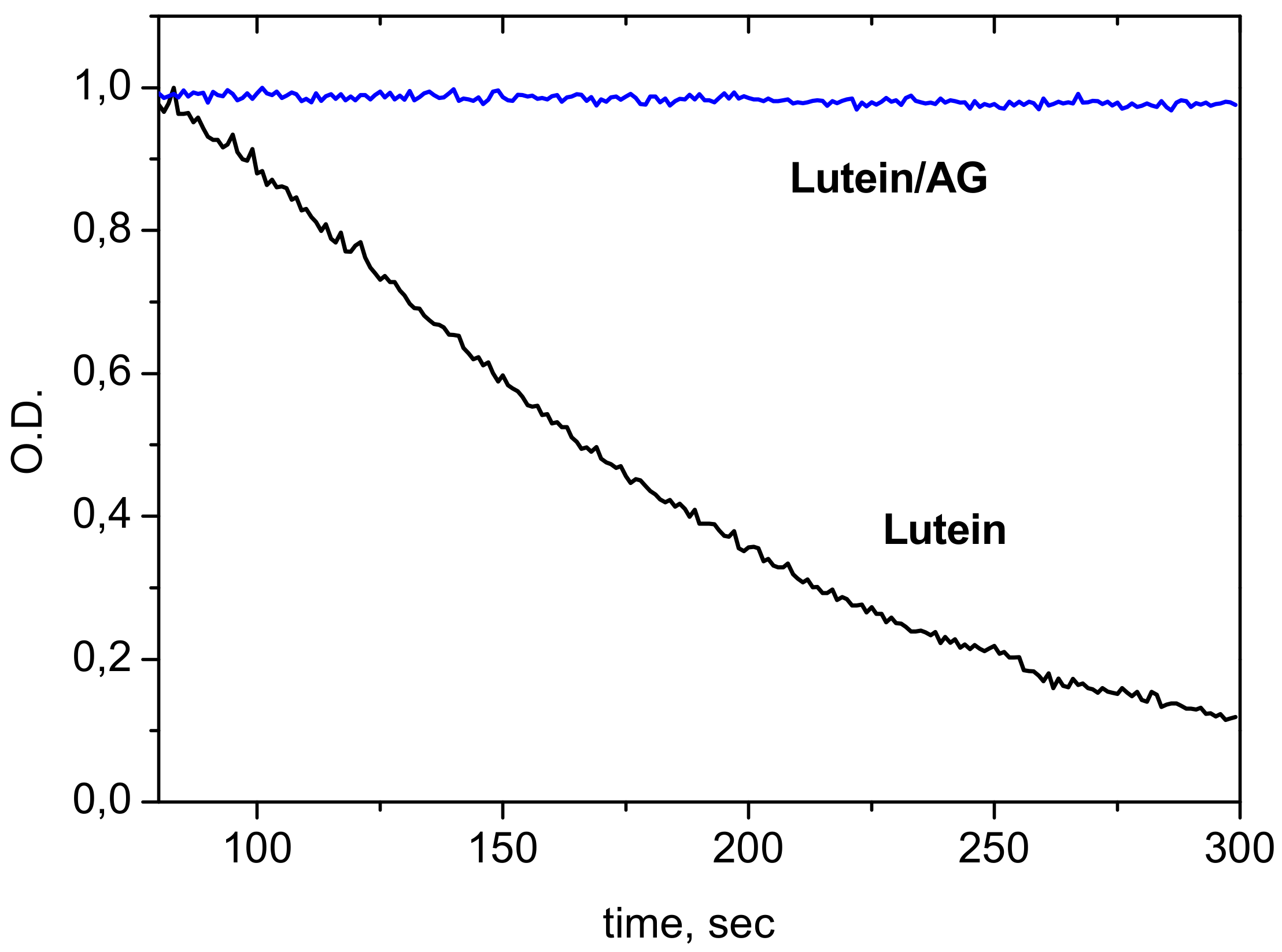
- Encapsulation of carotenoids lutein and zeaxanthin into GA micelles protects these carotenoids from oxidation by reactive oxygen species (O3 and OH radical) and metal ions [60]. For example, oxidation rate of lutein and zeaxanthin by ozone molecules in aqueous-ethanol solution decreased 10 times in the presence of 1 mM of GA. Similar effects were detected in the presence of disodium salt of GA: in the presence of 1 mM of Na2GA the oxidation rate of lutein and zeaxanthin by Fe3+ ions decreases by 10–20 times [60].
- (3)
- In contrast to most of water soluble oligosaccharides and polysaccharides [14,58], GA is able to form supramolecular complexes with carotenoids not only in aqueous solutions where GA complexes increased the carotenoid solubility more than 1000-fold [60], but also in non-aqueous organic solvents (alcohols, DMSO, acetonitrile) [14,16,58]. This fact is important for discussion the possibility of GA-assisted transport of carotenoid molecules through lipophilic cell membranes and their membrane protection properties.
- (4)
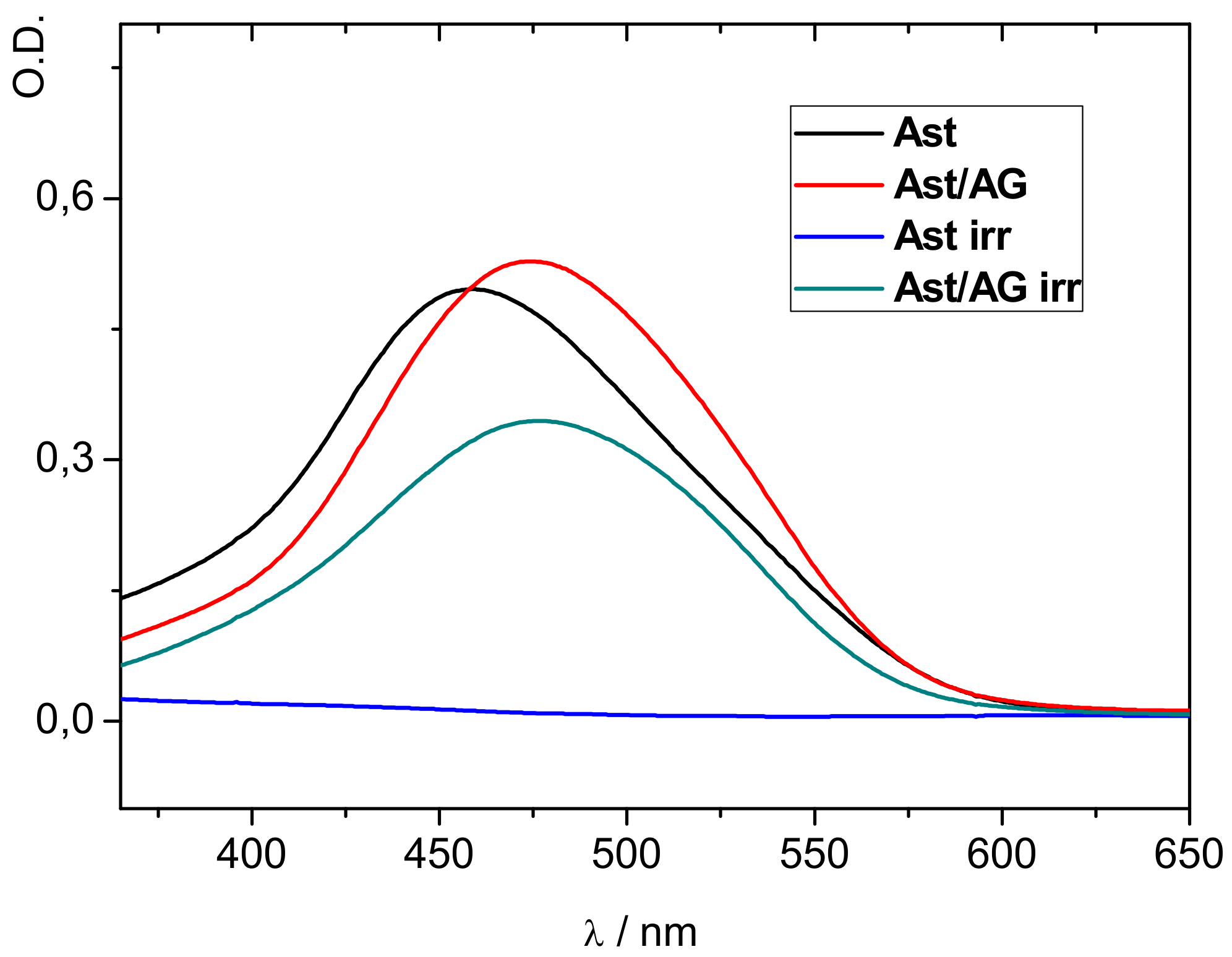
- In organic solvents GA is able to form supramolecular complexes not only with neutral carotenoid molecules, but also with their paramagnetic forms-radical cations and charge transfer complexes with electron donors [16]. Also, there is a significant increase of the lifetime of β-carotene radical cations (50-fold) in the presence of GA [16]. High stability of the carotenoid radical cations imbedded into GA host opens possibilities for the application of these complexes for the design of artificial light-harvesting, photoredox and catalytic systems.
- (5)
- One of the most important biological properties of carotenoids is their antioxidant activity. In aqueous environment as well as in lipid membranes carotenoids trap toxic oxygen radicals and thus prevent damage to living organism [62,63]. Some studies were performed to elucidate how the complexation with GA affects the ability of carotenoids to scavenge reactive oxygen radicals [17,59]. The antioxidant activity of carotenoid complexes was studied by the EPR spin-trapping technique. The details of this technique and effectiveness in the measurement of scavenging rates towards hydroperoxyl OOH radical are described in our earlier study [7]. Comparison of the scavenging rates of hydroperoxyl radicals by free carotenoids and their GA complexes in non-aqueous solution (DMSO) shows a strong dependence of the rate constants on the carotenoids structure and their oxidation potentials (see Table 1).
2.3. Arabinogalactan
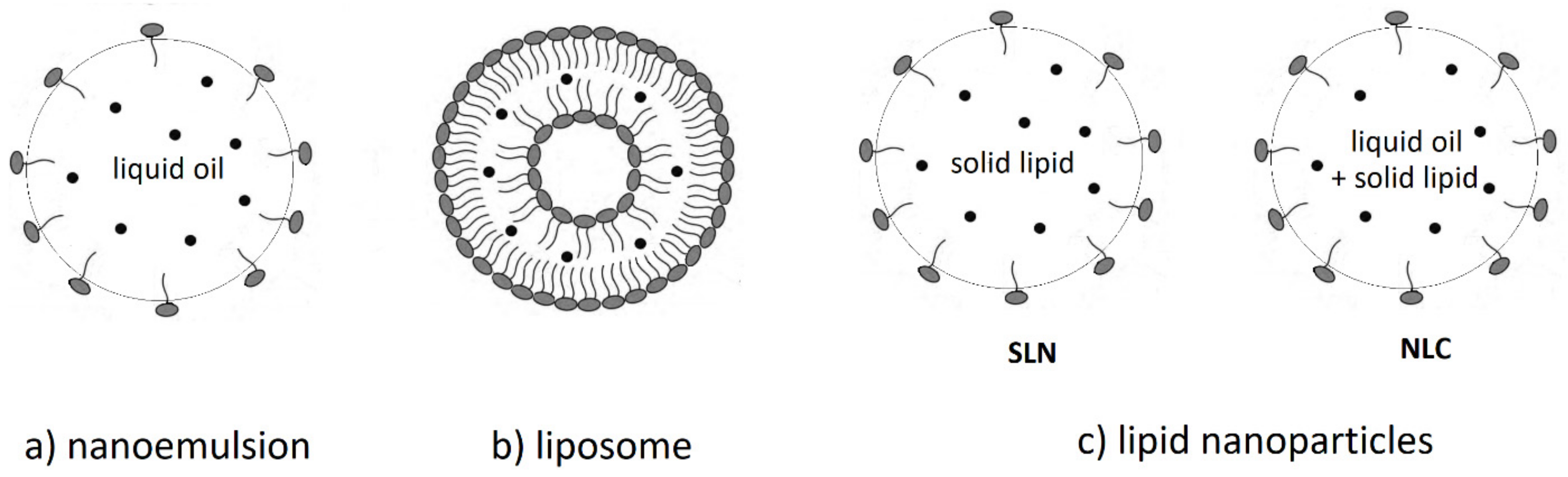
3. Biovailability of Carotenoids and Lipid-Based Nanocarriers
3.1. Nanoemulsions
3.2. Nanoliposomes
3.3. Lipid-Based Nanoparticles: Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
3.3.1. Solid Lipid Nanoparticles (SLNs)
3.3.2. Nanostructured Lipid Carriers (NLCs)
4. Biopolymeric Nanoparticles
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Green, A.S.; Fascetti, A.J. Vitamin A requirement: The efficacy and importance of β-carotene in animal species. Sci. World J. 2016, 7393620. [Google Scholar] [CrossRef]
- Yan, T.; Li, H.; Wu, J.; Niu, Q.; Duan, W.; Han, Q.; Ji, W.; Zhang, T.; Lv, W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol. Lett. 2017, 14, 5400–5408. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.-J.; Lee, B.J.; Joo, M.K.; Chun, H.J.; Lee, S.W.; Bak, Y.-T. Astaxanthin inhibits proliferation of human gastric cancer cell lines by interrupting cell cycle progression. Gut Liver 2016, 10, 369–374. [Google Scholar] [CrossRef] [PubMed]
- McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of astaxanthin on the proliferation and migration of breast cancer cells in vitro. Antioxidants 2018, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Sun, L.; Yu, H.-S.; Liang, L.-P.; Li, W.; Ding, H.; Song, X.-B.; Zhang, L.-J. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Tay-Agbozo, S.; Street, S.; Kispert, L. The carotenoid bixin found to exhibit the highest measured carotenoid oxidation potential to date consistent with its practical protective use in cosmetics, drugs and food. J. Photochem. Photobiol. B Biol. 2018, 186, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Kispert, L.D. Carotenoids as scavengers of free radicals in a Fenton reaction: Antioxidants or pro-oxidants? Free Radic. Biol. Med. 2001, 31, 398–404. [Google Scholar] [CrossRef]
- Tay-Agbozo, S.; Street, S.; Kispert, L.D. The carotenoid bixin: Optical studies of aggregation in polar/water solvents. J. Photochem. Photobiol. A Chem. 2018, 362, 31–39. [Google Scholar] [CrossRef]
- Husa, N.N.; Hamzah, F.; Said, H.M. Characterization and storage stability study of bixin extracted from Bixa orellana using organic solvent IOP. Conf. Ser. Mater. Sci. Eng. 2018, 358, 012035. [Google Scholar]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Padma Ishwarya, S. Rationale for encapsulation of carotenoids. In Spray Drying Techniques for Food Ingredient Encapsulation, 1st ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2015; p. 165. [Google Scholar]
- Magyar, A.; Bowman, M.K.; Molnár, P.; Kispert, L. Neutral carotenoid radicals in photoprotection of wild-type Arabidopsis thaliana. J. Phys. Chem. B 2013, 117, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, M.; Kowalczyk, S. A study of free radical chemistry: their role and pathophysiological significance. Acta Biochim. Pol. 2013, 60, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Meteleva, E.S.; Dushkin, A.V.; Konovalova, T.A.; Kispert, L.D. Water soluble complexes of carotenoids with arabinogalactan. J. Phys. Chem. B 2009, 113, 275–282. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Salakhutdinov, N.F.; Kispert, L.D. Host−guest complexes of carotenoids with β-glycyrrhizic acid. J. Phys. Chem. B 2006, 110, 6991–6998. [Google Scholar] [CrossRef]
- Polyakov, N.; Leshina, T.; Salakhutdinov, N.; Konovalova, T.; Kispert, L. Antioxidant and redox properties of supramolecular complexes of carotenoids with β-glycyrrhizic acid. Free. Radic. Biol. Med. 2006, 40, 1804–1809. [Google Scholar] [CrossRef]
- Kispert, L.D.; Polyakov, N.E. Carotenoid radicals: cryptochemistry of natural colorants. Chem. Lett. 2010, 39, 148–155. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Apanasenko, I.E.; Shilov, A.G.; Khalikov, S.S.; Polyakov, N.E. Effect of natural polysaccharides and oligosaccharides on the permeability of cell membranes. Russ. Chem. Bull. 2017, 66, 129–135. [Google Scholar] [CrossRef]
- Higashi, T. Cyclodextrin-based molecular accessories for drug discovery and drug delivery. Chem. Pharm. Bull. 2019, 67, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Denora, N. Nanoformulations for drug delivery: Safety, toxicity, and efficacy. In Computational Toxicology: Methods and Protocols; Nicolotti, O., Ed.; Springer: Berlin, Germany, 2018; Volume 1800, pp. 347–365. [Google Scholar] [CrossRef]
- Jafari, S. Nanoencapsulation Technologies for the Food and Nutraceutical Industries, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 187–261. [Google Scholar]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Mendichi, R.; Selva, A. Non-covalent associations of cyclomaltooligosaccharides (cyclodextrins) with trans-β-carotene in water: Evidence for the formation of large aggregates by light scattering and NMR spectroscopy. Carbohydr. Res. 1998, 310, 261–267. [Google Scholar] [CrossRef]
- Mele, A.; Mendichi, R.; Selva, A.; Molnar, P.; Tóth, G. Non-covalent associations of cyclomaltooligosaccharides (cyclodextrins) with carotenoids in water. A study on the α- and β-cyclodextrin/ψ,ψ-carotene (lycopene) systems by light scattering, ionspray ionization and tandem mass spectrometry. Carbohydr. Res. 2002, 337, 1129–1136. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Hand, E.O.; Kispert, L.D. Inclusion complexes of carotenoids with cyclodextrins: 1H NMR, EPR, and optical studies. Free Rad. Biol. Med. 2004, 36, 872–880. [Google Scholar] [CrossRef]
- Lobo, F.A.; Silva, V.; Domingues, J.; Rodrigues, S.; Costa, V.; Falcao, D.; de Lima Araujo, K.G. Inclusion complexes of yellow bell pepper pigments with β-cyclodextrin: preparation, characterisation and application as food natural colorant. J. Sci. Food Agric. 2018, 98, 2665–2671. [Google Scholar] [CrossRef]
- Fernández-García, E.; Pérez-Gálvez, A. Carotenoid:β-cyclodextrin stability is independent of pigment structure. Food Chem. 2017, 221, 1317–1321. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Orellano, L.A.A.; De Lazari, M.G.T.; Campos, P.P.; Cortes, M.E.; Sinisterra, R.D. Evidence of hypoglycemic, lipid-lowering and hepatoprotective effects of the bixin and bixin: β-CD inclusion compound in high-fat-fed obese mice. Biomed. Pharmacother. 2018, 106, 363–372. [Google Scholar] [CrossRef]
- Nalawade, P.; Gajjar, A. Assessment of in-vitro bio accessibility and characterization of spray dried complex of astaxanthin with methylated betacyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 63–75. [Google Scholar] [CrossRef]
- Shibata, S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. J. Pharm. Soc. Jpn. 2000, 120, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.; Polyakov, N. Glycyrrhizic acid as a multifunctional drug carrier—From physicochemical properties to biomedical applications: A modern insight on the ancient drug. Int. J. Pharm. 2019, 559, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.J.; Yin, A.C.Y. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013, 8, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wu, L.; Hu, M.; Dong, W.; Xu, M.; Zhang, P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomed. Pharmacother. 2017, 95, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Petrova, S.S.; Schlotgauer, A.A.; Kruppa, A.I.; Leshina, T.V. Self-association of glycyrrhizic acid. NMR Study. Z. Phys. Chem. 2016, 231, 1–17. [Google Scholar] [CrossRef]
- Borisenko, S.N.; Lekar, A.V.; Milov, A.A.; Vetrova, E.V.; Borisenko, N.I. Mass-spectrometry and quantum-chemical study of self-association processes of glycyrrhizinic acid molecules. Chemistry of plant materials, in Russian 2013, 2, 85–92. [Google Scholar] [CrossRef]
- Borisenko, S.N.; Lekar’, A.V.; Vetrova, E.V.; Filonova, O.V. A mass spectrometry study of the self-association of glycyrrhetinic acid molecules. Russ. J. Bioorganic Chem. 2016, 42, 716–720. [Google Scholar] [CrossRef]
- Baltina, L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003, 10, 155–171. [Google Scholar] [CrossRef]
- Tolstikova, T.; Khvostov, M.; Bryzgalov, A. The complexes of drugs with carbohydrate-containing plant metabolites as pharmacologically promising agents. Mini-Reviews Med. Chem. 2009, 9, 1317–1328. [Google Scholar] [CrossRef]
- Dushkin, A.V.; Meteleva, E.S.; Tolstikova, T.G.; Khvostov, M.V.; Dolgikh, M.P. Complexing of pharmacons with glycyrrhizic acid as a route to the development of the preparations with enhanced efficiency. Chem. Sustain. Dev. 2010, 18, 437–444. [Google Scholar]
- Zhang, Q.; Polyakov, N.E.; Chistyachenko, Y.S.; Khvostov, M.V.; Frolova, T.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018, 25, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Zhu, X.; Meteleva, E.S.; Chistyachenko, Y.S.; Suntsova, L.P.; Polyakov, N.E.; Khvostov, M.V.; Baev, D.S.; Tolstikova, T.G.; Yu, J.; et al. Enhanced solubility and bioavailability of simvastatin by mechanochemically obtained complexes. Int. J. Pharm. 2017, 534, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Sapra, B.; Jain, S.; Tiwary, A.K. Transdermal delivery of carvedilol containing glycyrrhizin and chitosan as permeation enhancers: Biochemical, biophysical, microscopic and pharmacodynamic evaluation. Drug Deliv. 2008, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Khan, V.K.; Taraban, M.B.; Leshina, T.V. Complex of calcium receptor blocker Nifedipine with glycyrrhizic acid. J. Phys. Chem. B 2008, 112, 4435–4440. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Khan, V.K.; Taraban, M.B.; Leshina, T.V.; Salakhutdinov, N.F.; Tolstikov, G.A. Complexation of lappaconitine with glycyrrhizinic acid. Structure, stability and reactivity studies. J. Phys. Chem. B 2005, 109, 24526–24530. [Google Scholar] [CrossRef] [PubMed]
- Kornievskaya, V.S.; Kruppa, A.I.; Polyakov, N.E.; Leshina, T.V. Effect of glycyrrhizic acid on lappaconitine phototransformation. J. Phys. Chem. B 2007, 111, 11447–11452. [Google Scholar] [CrossRef]
- Yang, F.-H.; Zhang, Q.; Liang, Q.-Y.; Wang, S.-Q.; Zhao, B.-X.; Wang, Y.-T.; Cai, Y.; Li, G.-F. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: Paclitaxel-loaded glycyrrhizic acid micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, B.; Wang, S.; Liang, Q.; Cai, Y.; Yang, F.; Li, G. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Deliv. 2016, 23, 1623–1635. [Google Scholar] [CrossRef]
- Meteleva, E.S.; Chistyachenko, Y.S.; Suntsova, L.P.; Tsyganov, M.A.; Vishnivetskaya, G.B.; Avgustinovich, D.F.; Khvostov, M.V.; Polyakov, N.E.; Tolstikova, T.G.; Mordvinov, V.A.; et al. Physicochemical properties and anti-opisthorchosis effect of mechanochemically synthesized solid compositions of praziquantel with glycyrrhizic acid disodium salt. Dokl. Biochem. Biophys. 2018, 481, 228–231. [Google Scholar] [CrossRef]
- Meteleva, E.S.; Chistyachenko, Y.S.; Suntsova, L.P.; Khvostov, M.V.; Polyakov, N.E.; Selyutina, O.Y.; Tolstikova, T.G.; Frolova, T.S.; Mordvinov, V.A.; Dushkin, A.V.; et al. Disodium salt of glycyrrhizic acid-a novel supramolecular delivery system for antihelmintic drug praziquantel. J. Drug Deliv. Sci. Tec. 2019, 50, 66–77. [Google Scholar] [CrossRef]
- Selyutina, O.; Apanasenko, I.; Kim, A.; Shelepova, E.; Khalikov, S.; Polyakov, N. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surfaces B Biointerfaces 2016, 147, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery. Drug Deliv. 2016, 23, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Apanasenko, I.E.; Polyakov, N.E. Membrane-modifying activity of glycyrrhizic acid. Russ. Chem. Bull. 2015, 64, 1555–1559. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Effect of glycyrrhizic acid on hemolysis of red blood cells and properties of cell membranes. Russ. Chem. Bull. 2014, 63, 1201–1204. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Kispert, L.D. Water soluble supramolecular complexes of β-carotene and other carotenoids. In Beta Carotene: Dietary Sources, Cancer and Cognition, 1st ed.; Haugen, L., Bjornson, T., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2019; pp. 191–230. [Google Scholar]
- Polyakov, N.E.; Magyar, A.; Kispert, L.D. Photochemical and optical properties of water-soluble xanthophyll antioxidants: Aggregation vs complexation. J. Phys. Chem. B 2013, 117, 10173–10182. [Google Scholar] [CrossRef]
- Apanasenko, I.E.; Selyutina, O.Y.; Polyakov, N.E.; Suntsova, L.P.; Meteleva, E.S.; Dushkin, A.V.; Vachali, P.; Bernstein, P.S. Solubilization and stabilization of macular carotenoids by water soluble oligosaccharides and polysaccharides. Arch. Biochem. Biophys. 2015, 572, 58–65. [Google Scholar] [CrossRef]
- Li, B.; Vachali, P.P.; Shen, Z.; Gorusupudi, A.; Nelson, K.; Besch, B.M.; Bartschi, A.; Longo, S.; Mattinson, T.; Shihab, S.; et al. Retinal accumulation of zeaxanthin, lutein, and β-carotene in mice deficient in carotenoid cleavage enzymes. Exp. Eye Res. 2017, 159, 123–131. [Google Scholar] [CrossRef]
- Sarialtin, S.Y.; Coban, T. An overview on the role of macular xanthophylls in ocular diseases. Rec. Nat. Prod. 2018, 12, 107–120. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Carotenoids and Human Health; Humana Press: New York, NY, USA, 2013. [Google Scholar]
- Tay-Agbozo, S.; Street, S.C.; Kispert, L.D. Diffuse-Reflectance Infrared Fourier Transform and Electron Nuclear Double Resonance study of the carotenoid bixin attached to irradiated TiO2. J. Phys. Chem. C 2018, 122, 19075–19081. [Google Scholar] [CrossRef]
- Focsan, A.L.; Bowman, M.K.; Shamshina, J.; Krzyaniak, M.D.; Magyar, A.; Polyakov, N.E.; Kispert, L.D. EPR study of the astaxanthin n-octanoic acid monoester and diester radicals on silica–alumina. J. Phys. Chem. B 2012, 116, 13200–13210. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Berg, C.J.; Hsu, C.-C.; Merrill, B.A.; Tauber, M.J. Characterization of carotenoid aggregates by steady-state optical spectroscopy. J. Phys. Chem. B 2012, 116, 10617–10630. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tauber, M.J. High-yield singlet fission in a carotenoid aggregate observed by picosecond resonance Raman spectroscopy. J. Am. Chem. Soc. 2010, 132, 13988–13991. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Welc, R.; Gruszecki, W.I. The effect of carotenoids on the concentration of singlet oxygen in lipid membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Markowska, E.; Sielewiesiuk, J. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim. Biophys. Acta Biomembr. 1991, 1068, 68–72. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Odonmazig, P. Structural and molecular properties of the arabinogalactan isolated from Mongolian larchwood (Larix dahurica L.). Carbohydr. Res. 1994, 252, 317–324. [Google Scholar] [CrossRef]
- Dushkin, A.V.; Tolstikova, T.G.; Khvostov, M.V.; Tolstikov, G.A. Complexes of polysaccharides and glycyrrhizic acid with drug molecules-Mechanochemical synthesis and pharmacological activity. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 573–602. [Google Scholar]
- Kong, R.; Zhu, X.; Meteleva, E.S.; Polyakov, N.E.; Khvostov, M.V.; Baev, D.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Atorvastatin calcium inclusion complexation with polysaccharide arabinogalactan and saponin disodium glycyrrhizate for increasing of solubility and bioavailability. Drug Deliv. Transl. Res. 2018, 8, 1200–1213. [Google Scholar] [CrossRef]
- Khvostov, M.V.; Borisov, S.A.; Tolstikova, T.G.; Dushkin, A.V.; Tsyrenova, B.D.; Chistyachenko, Y.S.; Polyakov, N.E.; Dultseva, G.G.; Onischuk, A.A.; An’kov, S.V. Supramolecular complex of Ibuprofen with larch polysaccharide arabinogalactan: Studies on bioavailability and pharmacokinetics. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 431–440. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Apanasenko, I.E.; Khalikov, S.S.; Polyakov, N.E. Natural poly- and oligosaccharides as novel delivery systems for plant protection compounds. J. Agric. Food Chem. 2017, 65, 6582–6587. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Meteleva, E.S.; Dushkin, A.V.; Konovalova, T.A.; Kispert, L.D. Enhancement of the photocatalytic activity of TiO2 nanoparticles by water-soluble complexes of carotenoids. J. Phys. Chem. B 2010, 114, 14200–14204. [Google Scholar] [CrossRef] [PubMed]
- Stancanelli, R.; Løjkner, L.D.; Larsen, K.L.; Guardo, M.; Cannavà, C.; Tommasini, S.; Ventura, C.A.; Calabrò, M.L.; Micali, N.; Villari, V.; et al. Structural and spectroscopic features of lutein/butanoyl-β-cyclodextrin nanoassemblies. J. Pharmaceut. Biomed. 2012, 71, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kispert, L.D. Reaction of carotenoids and ferric chloride: Equilibria, isomerisation, and products. J. Phys. Chem. B 2003, 107, 5333–5338. [Google Scholar] [CrossRef]
- Gao, Y.; Webb, S.; Kispert, L.D. Deprotonation of carotenoid radical cation and formation of a didehydrodimer. J. Phys. Chem. B 2003, 107, 13237–13240. [Google Scholar] [CrossRef]
- Gluschenko, O.Y.; Polyakov, N.E.; Leshina, T.V. NMR relaxation study of cholesterol binding with plant metabolites. Appl. Magn. Reson. 2011, 41, 283–294. [Google Scholar] [CrossRef]
- Henry, L.K.; Puspitasari-Nienaber, N.L.; Jarén-Galán, M.; Van Breemen, R.B.; Catignani, G.L.; Schwartz, S.J. Effects of ozone and oxygen on the degradation of carotenoids in an aqueous model system. J. Agric. Food Chem. 2000, 48, 5008–5013. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Mao, L.; Wang, D.; Liu, F.; Gao, Y. Emulsion design for the delivery of β-carotene in complex food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 770–784. [Google Scholar] [CrossRef]
- Jafari, S.M. Nanoencapsulation of Food Bioactive Ingredients: Principles and Applications; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Aboofazeli, R. Nanometric-Scaled Emulsions (Nanoemulsions). Iran. J. Pharm. Res.: IJPR 2010, 9, 325–326. [Google Scholar]
- Dos Santos, P.P.; Andrade, L.D.A.; Flôres, S.H.; Rios, A.D.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Martín-Belloso, O.; McClements, D.J. Excipient nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; McClements, D.J.; Xiao, H. Influence of lipid content in a corn oil preparation on the bioaccessibility of β-carotene: A comparison of low-fat and high-fat samples. J. Food Sci. 2017, 82, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Qian, C.; Martin-Belloso, O.; McClements, D. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef]
- Mashurabad, P.C.; Palika, R.; Jyrwa, Y.W.; Bhaskarachary, K.; Pullakhandam, R. Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits. J. Food Sci. Technol. 2017, 54, 333–341. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Wang, C.; Zhao, C.; Peng, Q.; Zhang, T.; Zhao, C. Stability and in vitro digestibility of beta-carotene in nanoemulsions fabricated with different carrier oils. Food Sci. Nutr. 2018, 6, 2537–2544. [Google Scholar] [CrossRef]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A.; Yildiz, F. Chemistry, encapsulation, and health benefits of β-carotene - A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion micro emulsion and nano emulsion: A review. Syst. Rev. Pharm. 2017, 8, 39–47. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Charleer, L.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stability during gastrointestinal conditions effects lipid digestion kinetics. Food Chem. 2018, 246, 179–191. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Attaie, R.; De González, M.N. Lutein-enriched emulsion-based delivery system: Impact of casein-phospholipid emulsifiers on chemical stability. In Progress in Carotenoid Research; IntechOpen: London, UK, 2018. [Google Scholar]
- Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Chemical and physical stability of astaxanthin-enriched emulsion-based delivery systems. Food Biophys. 2016, 11, 302–310. [Google Scholar] [CrossRef]
- Yi, J.; Li, Y.; Zhong, F.; Yokoyama, W. The physicochemical stability and in vitro bioaccessibility of beta-carotene in oil-in-water sodium caseinate emulsions. Food Hydrocoll. 2014, 35, 19–27. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, X.; Guo, M. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLOS ONE 2018, 13, e0192511. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Shu, G.; Holland, B.J.; Kobayashi, I.; Nakajima, M.; Barrow, C.J. Formulation and characterization of O/W nanoemulsions encapsulating high concentration of astaxanthin. Food Res. Int. 2017, 102, 364–371. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Shi, J.; Arunasalam, K.; Yeung, D.; Kakuda, Y.; Mittal, G.; Jiang, Y. Saponins from edible legumes: Chemistry, processing, and health benefits. J. Med. Food 2004, 7, 67–78. [Google Scholar] [CrossRef]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef]
- Shu, G.; Khalid, N.; Chen, Z.; Neves, M.A.; Barrow, C.J.; Nakajima, M. Formulation and characterization of astaxanthin-enriched nanoemulsions stabilized using ginseng saponins as natural emulsifiers. Food Chem. 2018, 255, 67–74. [Google Scholar] [CrossRef]
- Chen, Z.; Shu, G.; Taarji, N.; Barrow, C.J.; Nakajima, M.; Khalid, N.; Neves, M.A. Gypenosides as natural emulsifiers for oil-in-water nanoemulsions loaded with astaxanthin: Insights of formulation, stability and release properties. Food Chem. 2018, 261, 322–328. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Sheng, B.; Li, L.; Zhang, X.; Jiao, W.; Zhao, D.; Wang, X.; Wan, L.; Li, B.; Rong, H. Physicochemical properties and chemical stability of β-carotene bilayer emulsion coated with bovine serum albumin and Arabic gum compared to monolayer emulsions. Molecules 2018, 23, 495. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014, 5, 1232. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloids Surfaces B Biointerfaces 2014, 123, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposome as a delivery system for carotenoids: Comparative antioxidant activity of carotenoids as measured by ferric reducing antioxidant power, DPPH assay and lipid peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef]
- Stojiljkovic, N.; Ilić, S.; JakovljeviĆ, V.; Stojanović, N.; Stojnev, S.; Kocić, H.; Stojanovic, M.; Kocic, G. The encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxidative Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.; Lobo, J.S.; Silva, A. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Silva, P.B.; Rigon, R.B.; Sato, M.R.; Chorilli, M. Formulating SLN and NLC as innovative drug delivery systems for non-invasive routes of drug administration. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Pyo, S.-M.; Müller, R.H.; Keck, C.M. Encapsulation by nanostructured lipid carriers. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2017; pp. 114–137. [Google Scholar] [CrossRef]
- Poonia, N.; Kharb, R.; Lather, V.; Pandita, D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Futur. Sci. OA 2016, 2, FSO135. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomater. 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.S.; Ghoshal, G.; Katare, O.P. Beta-carotene-Encapsulated Solid Lipid Nanoparticles (BC-SLNs) as Promising Vehicle for Cancer: An Investigative Assessment. AAPS Pharm. Sci. Tech. 2019, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and physicochemical characterization of lycopene-loaded solid lipid nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.P.; Manjunath, K.; Bhagawati, S.T.; Thippeswamy, B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection—Preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014, 473, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.C.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Cellular uptake of β-carotene from protein stabilized solid lipid nanoparticles prepared by homogenization-evaporation method. J. Agric. Food Chem. 2014, 62, 1096–1104. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Mouhid, L.; Corzo-Martínez, M.; Torres, C.; Vázquez, L.; Reglero, G.; Fornari, T.; De Molina, A.R. Improving in vivo efficacy of bioactive molecules: An overview of potentially antitumor phytochemicals and currently available lipid-based delivery systems. J. Oncol. 2017, 2017, 1–34. [Google Scholar] [CrossRef]
- New Research on Astaxanthin and Cancer. Available online: https://www.lifeextension.com/Magazine/2017/12/Update-Astaxanthin/Page-01 (accessed on 31 October 2019).
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid based nanoformulation of lycopene improves oral delivery: Formulation optimization, ex vivo assessment and its efficacy against breast cancer. J. Microencapsul. 2017, 34, 416–429. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of astaxanthin-loaded nanostructured lipid carriers as affected by pH, ionic strength, heat treatment, simulated gastric juice and freeze–thawing. J. Food Sci. Technol. 2017, 54, 3132–3141. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of astaxanthin-loaded nanostructured lipid carriers in beverage systems. J. Sci. Food Agric. 2017, 98, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.A.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Venegas, J.B.; Pauthe, E.; et al. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Mol. 2018, 23, 2601. [Google Scholar] [CrossRef] [PubMed]
- Zirak, M.B.; Pezeshki, A. Effect of surfactant concentration on the particle size, stability and potential zeta of beta carotene nano lipid carrier. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 924–932. [Google Scholar]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Kundu, J.; Kundu, S.C. Biopolymeric nanoparticles. Sci. Technol. Adv. Mater. 2010, 11, 14104. [Google Scholar] [CrossRef]
- Egusquiaguirre, S.; Pedraz, J.; Hernandez, R.M.; Igartua, M. Nanotherapeutic platforms for cancer treatment: From preclinical development to clinical application. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Elsevier: Amsterdam, The Netherlands, 2016; pp. 813–869. [Google Scholar]
- Rahaiee, S.; Shojaosadati, S.A.; Hashemi, M.; Moini, S.; Razavi, S.H. Improvement of crocin stability by biodegradeble nanoparticles of chitosan-alginate. Int. J. Boil. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef]
- Hong, D.Y.; Lee, J.-S.; Lee, H.G. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int. J. Boil. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of astaxanthin-loaded core–shell nanoparticles consisting of chitosan oligosaccharides and poly(lactic-co-glycolic acid): Enhancement of water solubility, stability, and bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Crosslinked chitosan-dextran sulfate nanoparticle for improved topical ocular drug delivery. Mol. Vis. 2015, 21, 1224–1234. [Google Scholar]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017, 227, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Ferruzzi, M.; Jones, O.G. Fate of lutein-containing zein nanoparticles following simulated gastric and intestinal digestion. Food Hydrocoll. 2019, 87, 229–236. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Bodoki, E.; Vostinaru, O.; Samoila, O.; Dinte, E.; Bodoki, A.E.; Swetledge, S.; Astete, C.E.; Sabliov, C.M. Topical nanodelivery system of lutein for the prevention of selenite-induced cataract. Nanomedicine 2019, 15, 188–197. [Google Scholar] [CrossRef]
- Mirakabad, F.S.T.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Kushwah, V.; Ghoshal, G.; Jain, A.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Beta carotene-loaded zein nanoparticles to improve the biopharmaceutical attributes and to abolish the toxicity of methotrexate: A preclinical study for breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 402–412. [Google Scholar] [CrossRef]


| [GA], mM | β-Carotene | Canthaxanthin | 7-apo-7,7-Dicyano-β-carotene |
|---|---|---|---|
| (E1/2 = 0.634 V) | (E1/2 = 0.765 V) | (E1/2 = 0.825 V) | |
| 0 | 0.5 | 2 | 7 |
| 0.5 | 0.5 | 59 | 133 |
| Delivery System | Carotenoid | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| β-CD | lutein, zeaxanthin, α-cryptoxanthin, α-carotene and β-carotene | kneading method | better color protection for the beverage | [30] Lobo 2018 |
| β-CD | lycopene, lutein, capsanthin and capsorubin | mixture of methylene chloride solution of carotenoid with ethanol solution of the carrier. | more stable against oxidating agents as AAPH and H2O2 | [31] Fernández-García 2017 |
| β-CD | bixin | freeze-drying technique | more palatable and hepatoprotective effect | [32] Pinzón-García 2018 |
| methyl-β-CD | astaxanthin | spray drying method | improved solubility | [33] Nalawade 2015 |
| GA, AG | astaxanthin, lutein, zeaxanthin | mixture of ethanol solution of carotenoid with water solution of the carrier. | solubility enhancement, prevention of H-aggregates formation in ethanol/water mixture, 7 folds increase of photostability in solution. | [16] Polyakov 2006 [59] Polyakov 2013 |
| GA, Na2GA or AG | lutein, zeaxanthin | solid state mechanochemical method | 2000 fold solubility enhancement, more than 10 fold increase of carotenoids stability in solution towards oxidation by ozone and Fe ions | [60] Apanacenko 2015 |
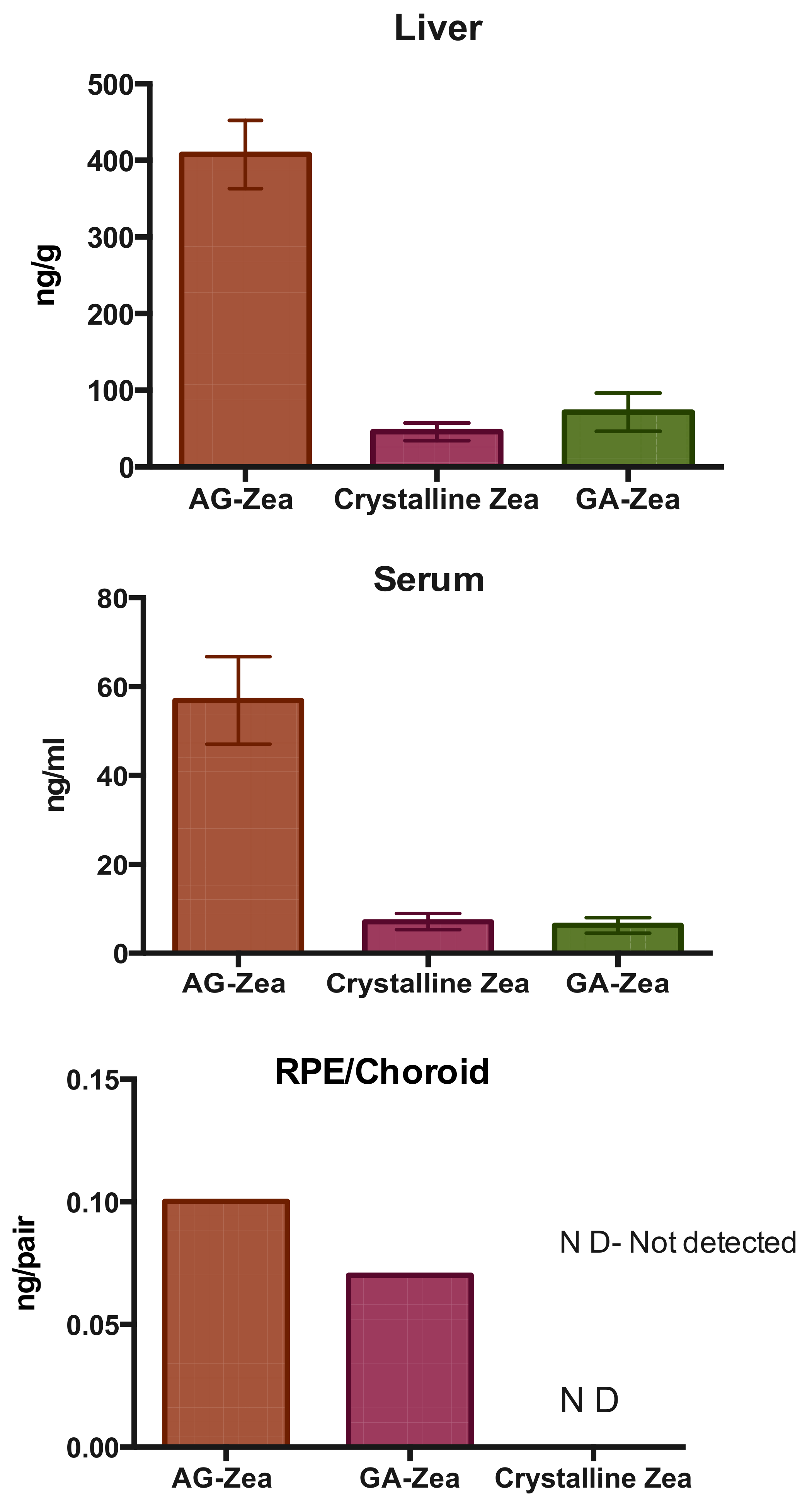
| GA and AG | lutein, zeaxanthin, and β-carotene | solid state mechanochemical method | solubility and bioavailability enhancement, increase of zeaxanthin level in the serum, liver and RPE/choroid of mice. | [61] Li 2017 |
| Delivery System | Carotenoid | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| Tween 20 stabilized O/W emulsion | β-carotene | high-pressure homogenization | higher bioaccessibility in low fat oil | [90] Xia 2017 |
| whey protein isolate (WPI) stabilized O/W nanoemulsion | β-carotene | high-pressure homogenization | higher bioaccessibility of β-carotene with palm oil | [93] Zhou 2018 |
| caseinate stabilized nanoemulsions | astaxanthin | pressure homogenization | emulsion stable at all incubation temperatures (5–70 °C) and chemical stability of astaxanthin against changes in pH, ionic strength and light exposure | [98] Liu 2016 |
| sodium caseinate stabilized O/Wnanoemulsions | β-carotene | homogenization at low and high pressures | very stable to coalescence or flocculation over 30 days | [99] Yi 2014 |
| Whey protein stabilized O/W nanoemulsion | lutein | ultrasonication | encapsulated lutein content was reduced by only 4% after four weeks storage at 4 °C | [100] Zhao 2019 |
| modified lecithin (ML) versus sodium caseinate (SC) stabilized O/W nanoemulsions | astaxanthin | high-pressure homogenization | good physical and chemical stability (>70%) after 30 days of storage for SC | [101] Khalid 2017 |
| Ginseng saponins stabilized O/W nanoemulsions | astaxanthin | high-pressure homogenization | excellent stability at 5 °C | [105] Shu 2018 |
| gypenosides stabilized O/W nanoemulsions | astaxanthin | high-pressure homogenization | stability during 30 days of storage at both 5 and 25 °C | [106] Chen 2018 |
| O/W nanoemulsion stabilized with bovine serum albumin (BSA) as the inner emulsifier and Arabic gum as outer emulsifier | β-carotene | high-pressure homogenization | better chemical stability under different environmental stresses when compared with monolayer emulsions studied | [108] Sheng 2018 |
| Delivery System | Carotenoid | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| soybean phosphatidyl choline nanoliposomes | astaxanthin | film dispersion-ultrasonic technique | enhanced thermal stability and water dispersibility of astaxanthin. | [109] Pan 2018 |
| nanoliposomes | lutein, β-carotene, lycopene, and canthaxanthin. | thin-film evaporation method | enhanced loading ability, bioaccesibility and antioxidant activity in this order: lutein> β-carotene> lycopene> canthaxanthin | [110,111,112] Tan 2014 |
| phospholipid nanoliposomes | lycopene | centrifugation | enhanced antioxidant activity, preventing reactive oxygen species-induced kidney tissue damage | [113] Stojiljkovic 2018 |
| Delivery System | Carotenoid | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| SLNs | β-carotene | hot homogenization | improved bioavailability and anticancer activity | [120] Jain 2019 |
| SLNs | lycopene | modified hot homogenization method | stable after 2 months in aqueous medium (4 °C) | [121] Nazemiyeh 2016 |
| soya and egg lecithin stabilized SLNs | bixin | hot homogenisation followed by ultrasonication technique | entrapment efficiency, loading efficiency and enhanced hepatoprotection | [122] Rao 2014 |
| Poloxamer 188 and lecithin stabilized SLNs | astaxanhin | solvent displacement method | neuroprotection from oxidative stress | [123] Bhatt 2016 |
| sodium caseinate (SC), whey protein isolate (WPI), or soy protein isolate (SPI) stabilized SLNs | β-carotene | homogenization-evaporation method | improved stability and uptake of betacarotene | [124] Yi 2014 |
| Delivery System | Carotenoid | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| Tween 80 and Poloxamer 188 stabilized NLCs | lycopene | ultrasonication | enhanced oral bioavailability of lycopene, increased cytotoxicity against the human breast tumour cells | [128] Singh 2017 |
| Tween 80 and lecithin stabilized NLCs | astaxanthin | melt-emulsification and ultrasonication technique | pH, ionic strength, heat and simulated gastric juice had no drastic effects on the chemical stability | [129] Tamjidi 2017 |
| Tween 80 stabilized NLCs | astaxanthin | melt-emulsification and ultrasonication technique | improved the physical stability in acidic beverage (solutions with 0 or 12% sucrose; pH 3–7) during 30–60 days storage at 6 or 20 °C. | [130] Tamjidi 2017 |
| Tween 80 and Poloxamer 407 stabilized NLCs | astaxanthin | hot homogenization (HH) method | loading capacity (90%) and enhanced antioxidant activity | [131] Rodriguez-Ruiz |
| Poloxamer 407 (3%) stabilized NLCs | β-carotene | hot-high shear homogenizer (Hot-HSH) method | stable after 60 days of storage at 25 °C | [132] Zirak 2015 |
| Eumulgin SG stabilized NLCs | lycopene | high pressure homogenization | high stability at different temp. (4, 30, 40 °C) for 120 days; no degradation at low temperatures. | [133] Okonogi 2015 |
| Delivery System | Carotenoid | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| chitosan/sodium alginate | crocin | modified ionic gelation method | better stability of nanoparticles during manipulation and storage. | [137] Rahaiee 2015 |
| chitosan/poly-glutamic acid | lutein | ionic gelation | 12 fold solubility enhancement | [138] Hong 2016 |
| chitosan oligosaccharides and poly(lactic-co-glycolic acid) | astaxanthin | spontaneous self-assembly | improved stability and prolonged release in simulated gastrointestinal juices | [139] Liu 2019 |
| chitosan- nanoparticle | lutein | topical ocular delivery | [140] Chaiyasan 2015 | |
| DNA/chitosan nanoparticles | astaxanthin | improved cellular uptake and antioxidation capability | [141] Wang 2017 |
| Delivery System | Carotenoid | Encapsulation Method | Results | Reference |
|---|---|---|---|---|
| zein nanoparticles | lutein | solvent-induced nanoprecipitation | improved lutein digestive stability but reduced micellarization | [142] Cheng 2018 |
| zein-caseinate nanoparticles | fucoxanthin | antisolvent precipitation | increased stability, bioaccessibility, antioxidant activity and antiproliferative activity | [143] Li 2018 |
| zein/ poly(lactic-co glycolic acid) nanoparticles | lutein | modified emulsion/evaporation method | enhanced stability and increased bioavailability of lutein in the rat eye when delivered topically | [144] Bodoki 2018 |
| zein nanoparticles with lecithin and pluronic F127 surfactants | lutein | liquid-liquid dispersion method | increased stability and controlled release | [146] Chuacharoen 2016 |
| zein with Tween 80 and pluronic F-68 surfactants | β-carotene | modified phase separation technique | Improved cellular uptake, cytotoxicity and oral biopharmaceutical performance | [147] Jain 2018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement. Molecules 2019, 24, 3947. https://doi.org/10.3390/molecules24213947
Focsan AL, Polyakov NE, Kispert LD. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement. Molecules. 2019; 24(21):3947. https://doi.org/10.3390/molecules24213947
Chicago/Turabian StyleFocsan, A. Ligia, Nikolay E. Polyakov, and Lowell D. Kispert. 2019. "Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement" Molecules 24, no. 21: 3947. https://doi.org/10.3390/molecules24213947
APA StyleFocsan, A. L., Polyakov, N. E., & Kispert, L. D. (2019). Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement. Molecules, 24(21), 3947. https://doi.org/10.3390/molecules24213947







