In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria
Abstract
1. Introduction
2. Results
2.1. Organic Acids and Their Derivatives
2.2. Organic Acids and Their Derivatives Against G− Bacteria
2.3. Organic Acids and Their Derivatives Against G+ Bacteria
3. Discussion
4. Materials and Methods
4.1. Organic Acids and Their Derivatives
4.2. Tested Bacterial Strains
4.3. MIC Assays of G- Bacteria
4.4. MIC Assays of G+ Bacteria
4.5. Assay of Bacterial Susceptibility to Antimicrobial Drugs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.; Liu, Y.; Calsmiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 46, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). CVM GFI #213 New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209. 2013. Center for Veterinary Medicine. Available online: https://www.regulations.gov/docket?D=FDA-2011-D-0889 (accessed on 6 January 2019).
- Papatsiros, V.G.; Billinis, C. The prophylactic use of acidifiers as antibacterial agents in swine. In Antimicrobial Agents; Bobbarala, V., Ed.; IntechOpen: London, UK, 2012; pp. 295–310. [Google Scholar]
- Cherrington, C.A.; Hinton, M.; Chopra, I. Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. J. Appl. Microbiol. 1990, 68, 69–74. [Google Scholar]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Zentek, J.; Ferrara, F.; Pieper, R.; Tedin, L.; Meyer, W.; Vahjen, W. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J. Anim. Sci. 2013, 91, 3200–3210. [Google Scholar] [CrossRef]
- Goepfert, J.M.; Hicks, R. Effect of volatile fatty acids on Salmonella typhimurium. J. Bacteriol. 1969, 97, 956–958. [Google Scholar]
- Hsiao, C.-P.; Siebert, K. Modeling the inhibitory effects on organic acids on bacteria. Int. J. Food Microbiol. 1999, 47, 189–201. [Google Scholar] [CrossRef]
- Long, S.F.; Xu, Y.T.; Pan, L.; Wang, Q.Q.; Wang, C.L.; Wu, Y.J.; Wu, Y.Y.; Han, Y.M.; Yun, C.H.; Piao, X.S. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. Review. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Kashket, E.R. Bioenergetics of lactic acid bacteria: Cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 1987, 46, 233–244. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86, 245–269. [Google Scholar] [CrossRef] [PubMed]
- Salsali, H.R.; Parker, W.J.; Sattar, S.A. The effect of volatile fatty acids on the inactivation of Clostridium perfingens in anaerobic digestion. World J. Microbiol. Biotechnol. 2008, 24, 659–665. [Google Scholar] [CrossRef]
- Piva, A.; Morlacchini, M.; Casadei, G.; Gatta, P.P.; Biagi, G.; Prandini, A. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Italian J Anim. Sci. 2002, 1, 35–41. [Google Scholar] [CrossRef]
- Pituch, A.; Walkowiak, J.; Banaszkiewicz, A. Butyric acid in functional constipation. Prz. Gastroenterol. 2013, 8, 295–298. [Google Scholar] [CrossRef]
- Temelli, F.; King, J.W.; List, G.R. Conversion of oils to monoglyecrides by glycerolysis in supercritical carbon dioxide media. JAOCS 1996, 73, 699–706. [Google Scholar] [CrossRef]
- Machinsky, T.G.; Kessler, M.; Ribeiro, A.M.L.; Moraes, L.; Mello da Silva, I.C.; Mayorga Cortes, M.E. Nutrient digestibility and Ca and P balance in pigs receiving butyric acid, phytase and different calcium levels. Ciencia Rural. 2015, 40, 2350–2355. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.-M.; Rabenhorst, J. Applied biocatalysis for the synthesis of natural flavor compounds – current industrial processed and future prospects. Biotechnol. Lett. 2004, 26, 463–472. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2009, 85, 1629–1642. [Google Scholar] [CrossRef]
- Mallo, J.J.; Balfagón, A.; Gracia, M.I.; Honrubia, P.; Puyalto, M. Evaluation of different protections of butyric acid aiming for release in the last part of the gastrointestinal tract of piglets. J. Anim. Sci. 2012, 90, 227–229. [Google Scholar] [CrossRef]
- Stecher, B.; Hardt, W.-D. Mechanisms controlling pathogen colonization of the gut. Curr. Opin. Microbiol. 2011, 14, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Turner, S.J.; Lewis, G.D.; Stanley, R.A.; Robertson, A.M. The effect of pH on the inhibition of bacterial growth by physiological concentrations of butyric acid: Implications for neonates fed on suckled milk. Chem.-Biol. Interact. 1999, 113, 117–131. [Google Scholar] [CrossRef]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial activity of butyrate glycerides toward Salmonella Typhimurium and Clostridium perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Mondot, S.; Kang, S.; Furet, J.P.; de Carcer, A.; McSweeney, P.; Morrison, M.; Marteau, P.; Doré, J.; Leclerc, M. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm. Bowel Dis. 2011, 17, 185–192. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Thompson, J.L.; Hinton, M. Antibacterial activity of formic and propionic acids in the diets of hens on Salmonellas in the crop. Br. Poult. Sci. 1997, 38, 59–65. [Google Scholar] [CrossRef]
- Raftari, M.; Azizi Jalilian, F.; Abdulamir, A.S.; Son, R.; Sekawi, Z.; Fatimah, A.B. Effect of organic acid on Escherichia coli O157:H7 and Staphylococcus aureus contaminated meat. Open Microbiol. J. 2009, 3, 121–127. [Google Scholar] [CrossRef]
- Huang, C.B.; Altimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Beier, R.C.; Harvey, R.B.; Hernandez, C.A.; Hume, M.E.; Andrews, K.; Droleskey, R.E.; Davidson, M.K.; Bodeis-Jones, S.; Young, S.; Duke, S.E.; et al. Interactions of organic acids with Campylobacter coli from swine. PLoS ONE 2018, 13, e0202100. [Google Scholar] [CrossRef]
- M’Sadeq, S.A.; Wu, S.; Swick, R.A.; Choct, M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-put worldwide. Anim. Nutr. 2015, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Anacarso, I.; Quartieri, A.; De Leo, R.; Pulvirenti, A. Evaluation of the antimicrobial activity of a blend of monoglycerides against Escherichia coli and Enterococci with multiple drug resistance. Arch. Microbiol. 2017, 200, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.W.; Batema, R.P.; Ford, L.L. Susceptibility of Heliobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 1996, 40, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, A.; Novick, R.P. Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus. J. Bacteriol. 2000, 182, 2668–2671. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.L.; Schlievert, P.M. Glycerol monolaurate inhibits the effects of Gram-positive select agents on eukaryotic cells. Biochemistry 2006, 45, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Enig, M.; Brook, I.; Elliot, T.B. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol. Cell. Biochem. 2005, 272, 29–34. [Google Scholar] [CrossRef]
- Bergsson, G.; Hilmarsson, H.; Thormar, H. Antibacterial, antiviral and antifungal activities of lipids. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 47–80. [Google Scholar]
- Schlievert, P.M.; Peterson, M.L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef]
- Strandberg, K.L.; Peterson, M.L.; Lin, Y.-C.; Pack, M.C.; Chase, D.J.; Schlievert, P.M. Glycerol monolaurate inhibits Candida and Gardnerella vaginalis in vitro and in vivo but not Lactobacillus. Am. Soc. Microbiol. 2010, 54, 597–601. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T.; Tsvetkova, I.V.; Najdenski, H.M. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: Individual effects and synergistic relationships. Pol. J. Microbiol. 2009, 58, 43–47. [Google Scholar]
- Hancock, R.E. The bacterial outer membrane as a drug carrier. Trends Microbiol. 1997, 5, 37–42. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perpect Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Preston, A.; Mandrell, R.E.; Gibson, B.W.; Apicella, M.A. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 1996, 22, 139–180. [Google Scholar] [CrossRef] [PubMed]
- Projan, S.J.; Brown-Skrobot, S.; Schlievert, P.M.; Vandenesch, F.; Novick, R.P. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 1994, 176, 4204–4209. [Google Scholar] [CrossRef]
- APHIS Info Sheet. Escherichia coli on U.S. Swine sites: Prevalence and antimicrobial drug susceptibility. Available online: https://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2012/Swine2012_is_Ecoli.pdf (accessed on 9 September 2019).
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999, 30, 259–284. [Google Scholar] [PubMed]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Caly, D.L.; D’Inca, R.; Auclair, E.; Drider, D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: A microbiologist’s perspective. Front. Microbiol. 2015, 6, 1336–1347. [Google Scholar] [CrossRef]
- McDevitt, R.M.; Brooker, J.D.; Acamovic, T.; Sparks, N.H.C. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult. Sci. J. 2006, 62, 221–247. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Løvland, A. The economical impact of Clostridium perfringens is greater than anticipated. World Poult. 2000, 16, 50–51. [Google Scholar]
- Lee, K.W.; Lillehoj, H.S.; Jeong, W.; Jeoung, H.Y.; An, D.J. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011, 90, 1381–1390. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Schneitz, C.; Hofshagen, M.; Skjerve, E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacterial from adult fowl. Avian Dis. 2001, 45, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Onrust, L.; Driessche, K.V.; Ducatelle, R.; Schwarzer, K.; Haesebrouck, F.; Immerseel, F.V. Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic enteritis. Poult. Sci. 2018, 97, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, R.A.; Atwill, E.R.; Gulland, F.M.; Miller, M.A.; Dabritz, H.A.; Paradies, D.M.; Worcester, K.R.; Jang, S.; Lawrence, J.; Byrne, B.A.; et al. Risk factors for infection with pathogenic and antimicrobial-resistant fecal bacteria in northern elephant seals in California. Public Health Rep. 2008, 123, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Atwill, E.R.; Antaki, E.; Applegate, O.; Bergamaschi, B.; Bond, R.F.; Chase, J.; Ransom, K.M.; Samuels, W.; Watanabe, N.; et al. Fecal indicator and pathogenic bacteria and their antibiotic resistance in alluvial groundwater of an irrigated agricultural region with dairies. J. Environ. Qual. 2015, 44, 1435–1447. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, N.; Xiao, C.; Harter, T.; McCowan, B.; Liu, Y.; Atwill, E.R. Antibiotic-resistant E. coli in surface water and groundwater in dairy operations in Northern California. Environ. Monit. Assess. 2014, 186, 1253–1260. [Google Scholar] [CrossRef]
- CLSI Performance Standards for Antimicrobial Susceptibility Testing; 26th ed.; CLSI supplement M100S. Wayne, PA: CLSI. 2016. Available online: http://ljzx.cqrmhospital.com/upfiles/201601/20160112155335884.pdf (accessed on 9 September 2019).
Sample Availability: Not available. |
| Compound | Form | Chemical Formula | Chemical Structure | Gravity, g/mL | Tested Concentration, mg/L |
|---|---|---|---|---|---|
| Butyric acid | Liquid | C4H8O2 | 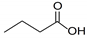 | 0.94 | 10, 250, 500, 1000, 2000, 2500, 3000, 3500 |
| Valeric acid | Liquid | C5H10O2 | 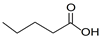 | 0.92 | 10, 250, 500, 1000, 2000, 2500, 3000, 3500 |
| Sodium formate | Solid | NaHCOO | 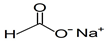 | ND 2 | 500, 1000, 2000, 4000, 8000, 10,000, 12,500, 15,000, 17,500, 20,000, 25,000 |
| ProPhorce 1 | Liquid | NaHCOO CH2O2 | - | 1.36 | 10, 250, 500, 1000, 2000, 4000, 8000, 10,000 |
| Propionate glycerides 3 | Liquid | C6H12O4 | 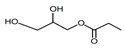 | 1.30 | 500, 1000, 2000, 2500, 3000, 3500, 5000, 7500, 10,000, 25,000 |
| Butyrate glycerides 3 | Liquid | C7H14O4 | 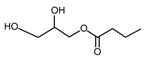 | 1.03 | 10, 250, 500, 1000, 2000, 2500, 3000, 3500, 5000, 10,000, 15,000, 20,000, 25,000, 30,000, 40,000, 50,000 |
| Valerate glycerides 3 | Liquid | C8H16O4 | 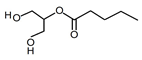 | 1.01 | 10, 250, 500, 1000, 2000, 2500, 3000, 3500, 5000, 10,000, 15,000, 20,000, 25,000 |
| Monolaurin | Solid | C15H30O4 | 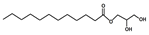 | ND | 10, 250, 500, 1000, 2000, 2500, 3000, 3500, 5000, 10,000, 15,000, 20,000, 25,000 |
| Escherichia coli | Escherichia coli | Salmonella enterica Typhimurium | Salmonella enterica Typhimurium | Campylobacter jejuni | Campylobacter jejuni | |
|---|---|---|---|---|---|---|
| Compound | ATCC 25922 | F18 | ATCC 14028 | ID# 4286 | ATCC 33560 | Campy 8DLIS D12-1 |
| Butyric acid | 2300 (±250) | 2500 (±0) | 2500 (±0) | 2300 (±250) | 800 (±300) | 500 (±0) |
| Valeric acid | 2700 (±400) | 2800 (±400) | 2700 (±300) | 2600 (±200) | 500 (±0) | 700 (±300) |
| Sodium formate | >20,000 | >20,000 | >20,000 | >20,000 | 2000 (±0) | 2000 (±0) |
| ProPhorce 1 | 2000 (±0) | 2200 (±700) | 2200 (±700) | 2200 (±700) | 700 (±300) | 1000 (±0) |
| Propionate glycerides | >10,000 | >10,000 | >10,000 | ≥10,000 | ≥10,000 | >10,000 |
| Butyrate glycerides | 15,000 (±0) | 10,000 (±0) | 11,700 (±2400) | 10,000 (±0) | >50,000 a | 10,000 (±0) |
| Valerate glycerides | 6700 (±2400) | 5000 (±0) | 10,000 (±5000) | 15,000 (±0) | 2500 (±1300) | 3700 (±900) |
| Monolaurin | 10,000 (±0) | 10,000 (±0) | 10,000 (±0) | 10,000 (±0) | 600 (±100) | 5000 (±0) |
| Antimicrobial Drug | Range of Concentrations (mg/L) | Escherichia coli | Escherichia coli | Salmonella enterica Typhimurium | Salmonella enterica Typhimurium | Campylobacter jejuni | Campylobacter jejuni |
|---|---|---|---|---|---|---|---|
| ATCC 25922 | F18 | ATCC 14028 | ID# 4286 | ATCC 33560 | Campy 8DLIS D12-1 | ||
| Amikacin | 8–32 | ORC | S | ORC | ORC | NA | NA |
| Piperacillin/tazobactam constant 4 | 8/4–128/4 | R | S | R | R | NA | NA |
| Tigecycline | 1–8 | NA | NA | NA | NA | NA | NA |
| Ticarcillin/clavulanic acid constant 2 | 8/2–64/2 | IR | S | ORC | IR | NA | NA |
| Levofloxacin | 1–8 | R | R | R | R | NA | NA |
| Nitrofurantoin | 32–64 | ORC | S | ORC | ORC | NA | NA |
| Tetracycline | 4–8 | ORC | ORC | ORC | ORC | R | R |
| Doripenem | 0.5–4 | R | S | R | R | NA | NA |
| Minocycline | 1–8 | ORC | IR | ORC | ORC | NA | NA |
| Ertapenem | 0.25–8 | R | IR | R | R | NA | NA |
| Trimethoprim/sulfamethoxazole | 2/38–4/76 | R | R | R | R | NA | NA |
| Imipenem | 0.5–8 | R | IR | R | R | NA | NA |
| Piperacillin | 16–64 | ORC | IR | ORC | ORC | NA | NA |
| Meropenem | 0.5–8 | R | S | R | R | NA | NA |
| Gentamicin | 2–8 | ORC | S | ORC | ORC | R | R |
| Cefazolin | 1–16 | R | R | R | R | NA | NA |
| Tobramycin | 2–8 | ORC | S | ORC | ORC | NA | NA |
| Ceftazidime | 1–16 | R | R | R | R | NA | NA |
| Ampicillin/sulbactam 2:1 ratio | 4/2–16/8 | ORC | IR | ORC | IR | NA | NA |
| Aztreonam | 1–16 | NA | NA | NA | NA | NA | NA |
| Ampicillin | 8–16 | ORC | ORC | ORC | IR | NA | NA |
| Cefepime | 4–32 | R | SSD | R | R | NA | NA |
| Ciprofloxacin | 0.5–2 | ORC | S | R | R | R | R |
| Ceftriaxone | 0.5–32 | R | R | R | R | NA | NA |
| Enterococcus faecalis | Clostridium perfringens | Streptococcus pneumoniae | Streptococcus suis | |
|---|---|---|---|---|
| Compound | ATCC 29212 | ATCC 12915 | ATCC 49619 | ATCC 43765 |
| Butyric acid | 2000 (±0) | 1200 (±400) | 1000 (±0) | 700 (±2400) |
| Valeric acid | 2000 (±0) | 1300 (±700) | 1000 (±0) | 1000 (±0) |
| Sodium formate | >20,000 | 18,800 (±7100) | 15,800 (±24,00) | 11,000 (±7100) |
| ProPhorce 1 | 1000 (±0) | 1000 (±0) | 1000 (±0) | 1900 (±3400) |
| Propionate glycerides | >10,000 | 11,300 (±6400) | >25,000 | >25,000 |
| Butyrate glycerides | 10,000 (±0) | 2600 (±1300) | 7700 (±2900) | 7800 (±2500) |
| Valerate glycerides | 10,000 (±0) | 3100 (±1200) | 2400 (±400) | 2000 (±700) |
| Monolaurin | 500 (±0) | 300 (±400) | 10 (±0) | 400 (±800) |
| Antimicrobial Drugs | Range of Concentrations (mg/L) | Enterococcus faecalis | Clostridium perfringens | Streptococcus pneumoniae | Streptococcus suis |
|---|---|---|---|---|---|
| ATCC 29212 | ATCC 12915 | ATCC 49619 | ATCC 43765 | ||
| Tigecycline | 0.015–0.5 | NA | NA | NA | NA |
| Erythromycin | 0.25–8 | IR | NA | S | S |
| Tetracycline | 1–32 | R | R | R | IR |
| Ciprofloxacin | 0.12–4 | R | NA | NA | NA |
| Chloramphenicol | 2–32 | R | R | S | S |
| Penicillin | 0.25–16 | R | R | NA | ORC |
| Daptomycin | 0.25–16 | NA | NA | S | S |
| Vancomycin | 0.25–32 | R | NA | S | S |
| Streptomycin | 512–2048 | R | NA | NA | NA |
| Nitrofurantoin | 2–64 | ORC | NA | NA | NA |
| Tylosin tartrate | 0.25–32 | R | NA | NA | NA |
| Gentamicin | 128–1024 | R | NA | NA | NA |
| Quinupristin/dalfopristin | 0.5–32 | R | NA | S | S |
| Lincomycin | 1–8 | S | NA | NA | NA |
| Linezolid | 0.5–8 | R | NA | S | S |
| Kanamycin | 128–1024 | R | NA | NA | NA |
| Species | Strain Designation | Gram Stain | Strain Type |
|---|---|---|---|
| Escherichia coli | ATCC 25922 | G− | reference |
| Escherichia coli | F18 | G− | wild |
| Salmonella enterica Typhimurium | ATCC 14028 | G− | reference |
| Salmonella enterica Typhimurium | Sample ID #4286 | G− | wild |
| Campylobacter jejuni | ATCC 33560 (CIP 702) | G− | reference |
| Campylobacter jejuni | Campy 8DLIS D12-1 | G− | wild |
| Enterococcus faecalis | ATCC 29212 | G+ | reference |
| Clostridium perfringens | ATCC 12915 | G+ | reference |
| Streptococcus pneumoniae | ATCC 49619 | G+ | reference |
| Streptococcus suis | ATCC 43765 | G+ | wild |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. https://doi.org/10.3390/molecules24203770
Kovanda L, Zhang W, Wei X, Luo J, Wu X, Atwill ER, Vaessen S, Li X, Liu Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules. 2019; 24(20):3770. https://doi.org/10.3390/molecules24203770
Chicago/Turabian StyleKovanda, Lauren, Wen Zhang, Xiaohong Wei, Jia Luo, Xixi Wu, Edward Robert Atwill, Stefan Vaessen, Xunde Li, and Yanhong Liu. 2019. "In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria" Molecules 24, no. 20: 3770. https://doi.org/10.3390/molecules24203770
APA StyleKovanda, L., Zhang, W., Wei, X., Luo, J., Wu, X., Atwill, E. R., Vaessen, S., Li, X., & Liu, Y. (2019). In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules, 24(20), 3770. https://doi.org/10.3390/molecules24203770





