Design, Synthesis, and Bioactivity Evaluation of Novel Isoxazole-Amide Derivatives Containing an Acylhydrazone Moiety as New Active Antiviral Agents
Abstract
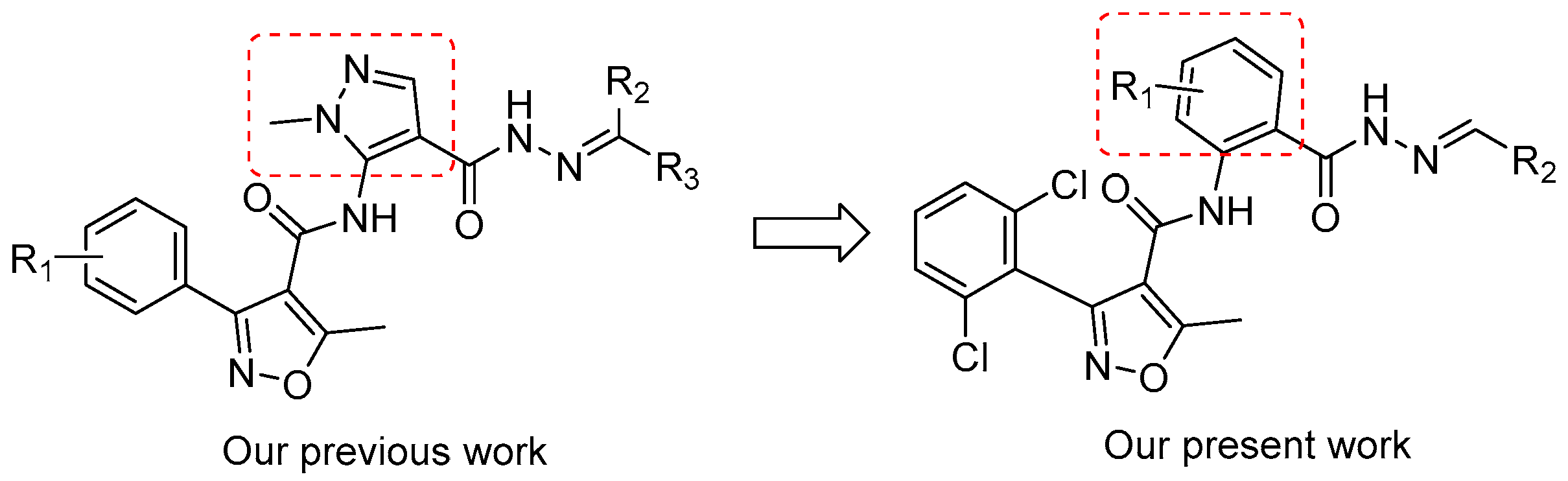
1. Introduction
2. Results
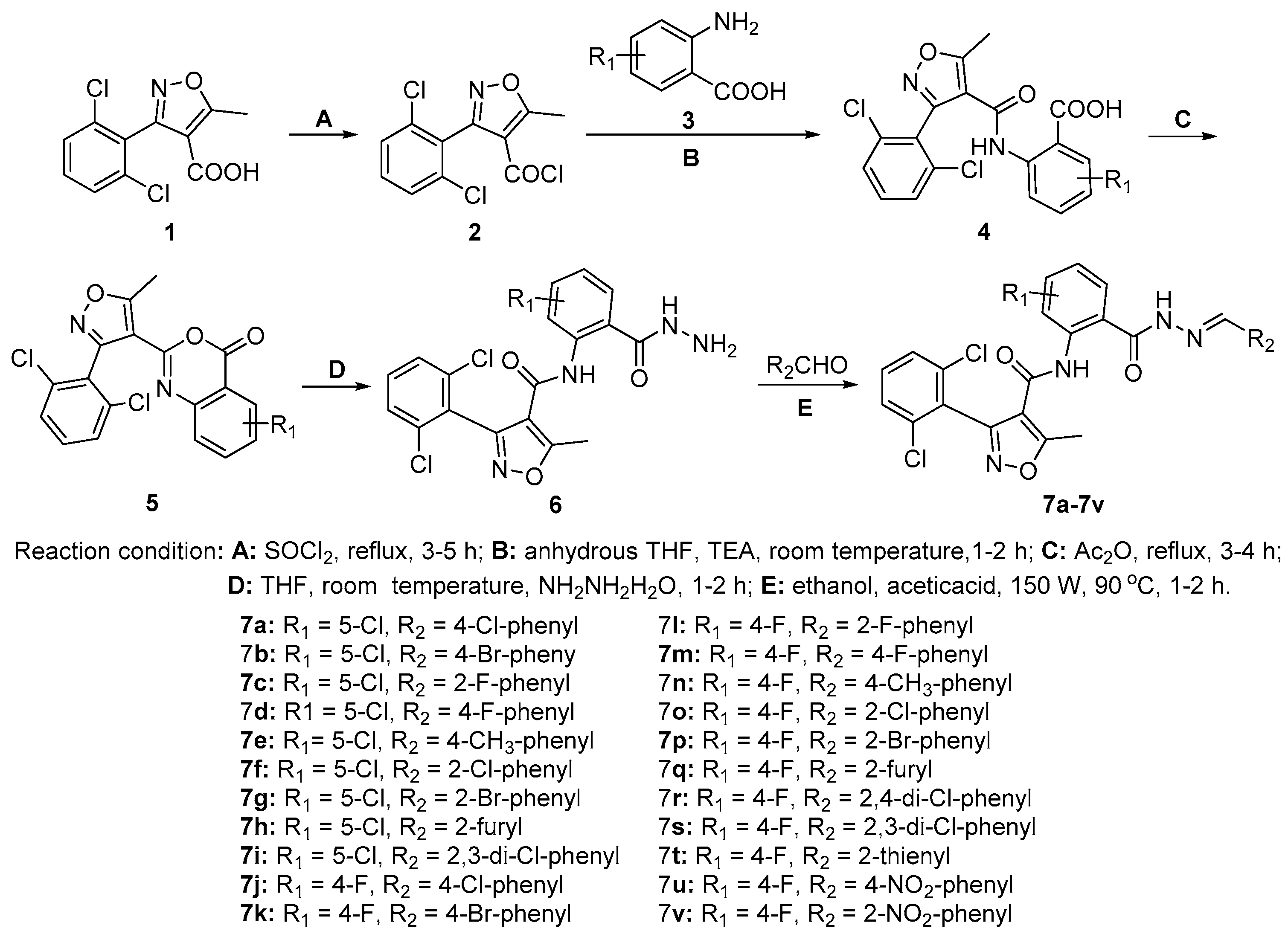
2.1. Chemistry
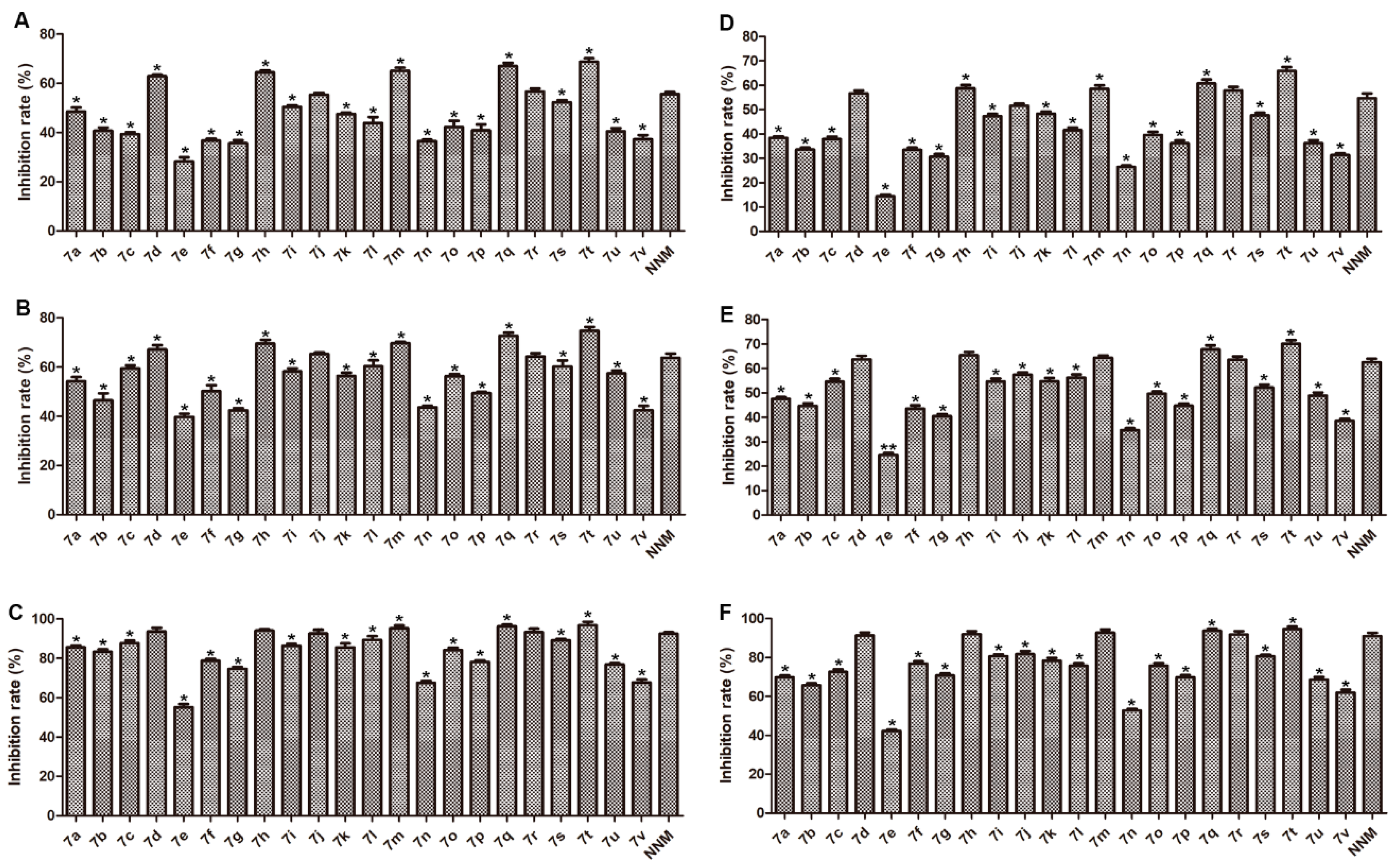
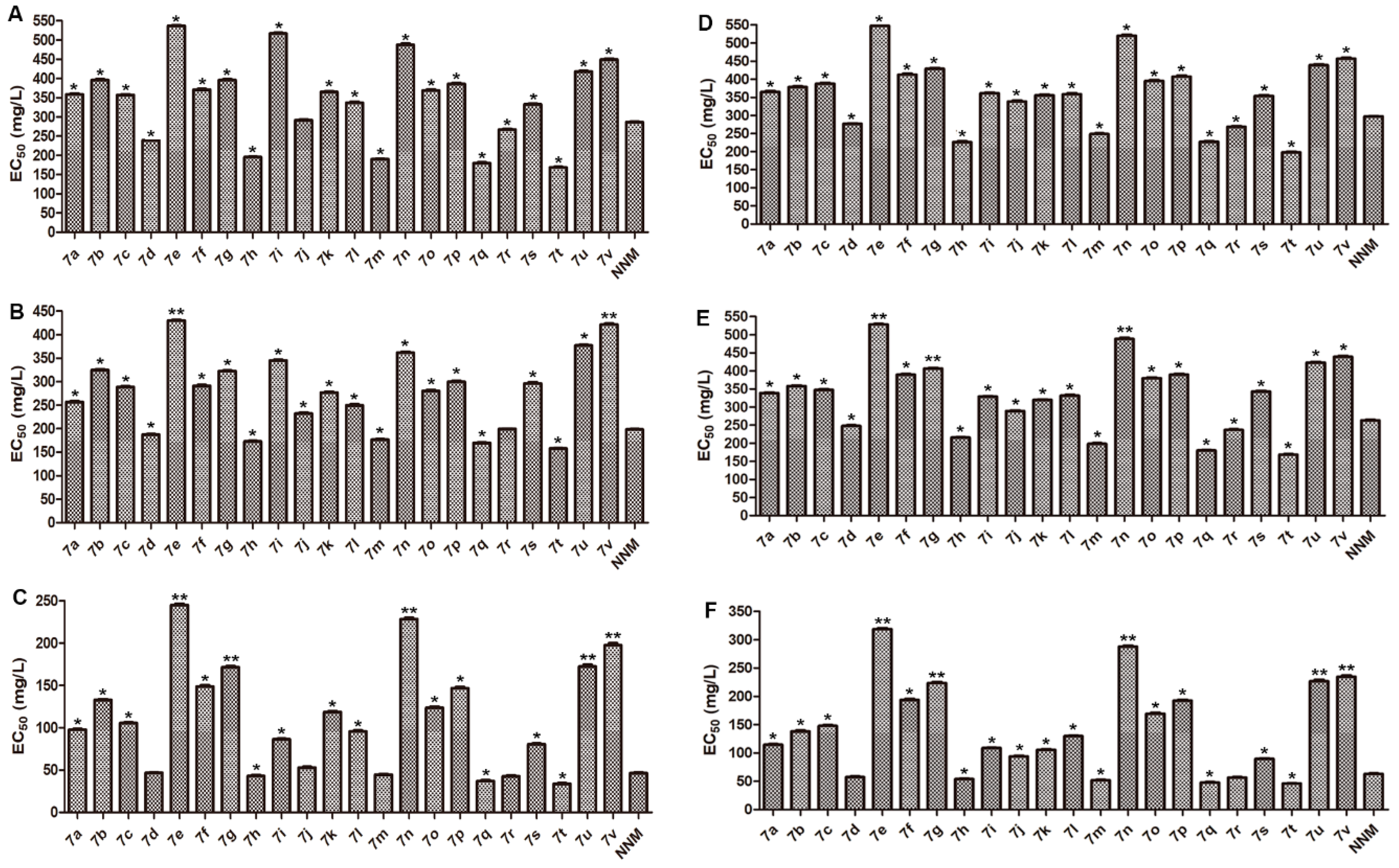
2.2. Biological Evaluations
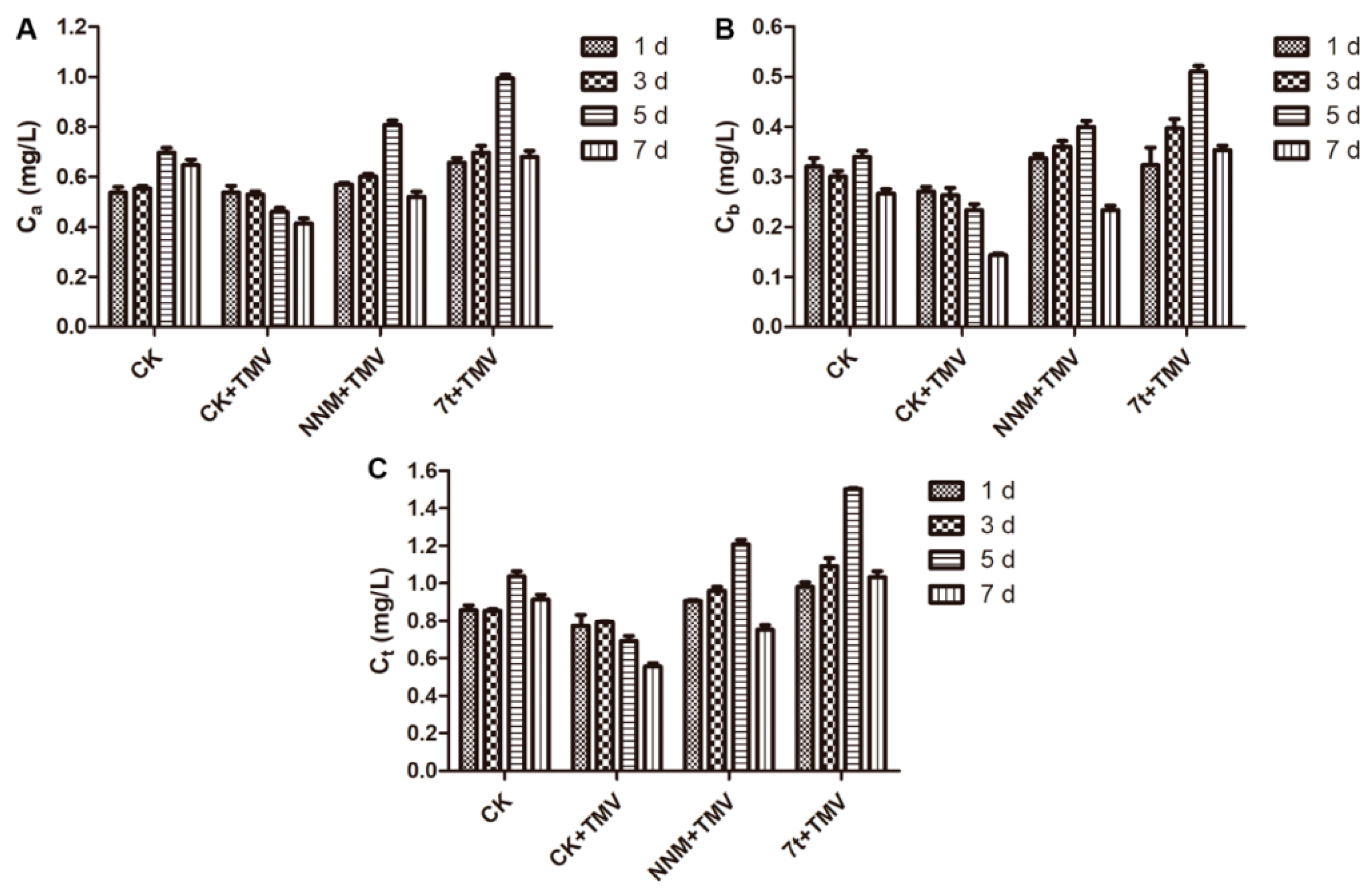
2.3. Effect on Chlorophyll Contents
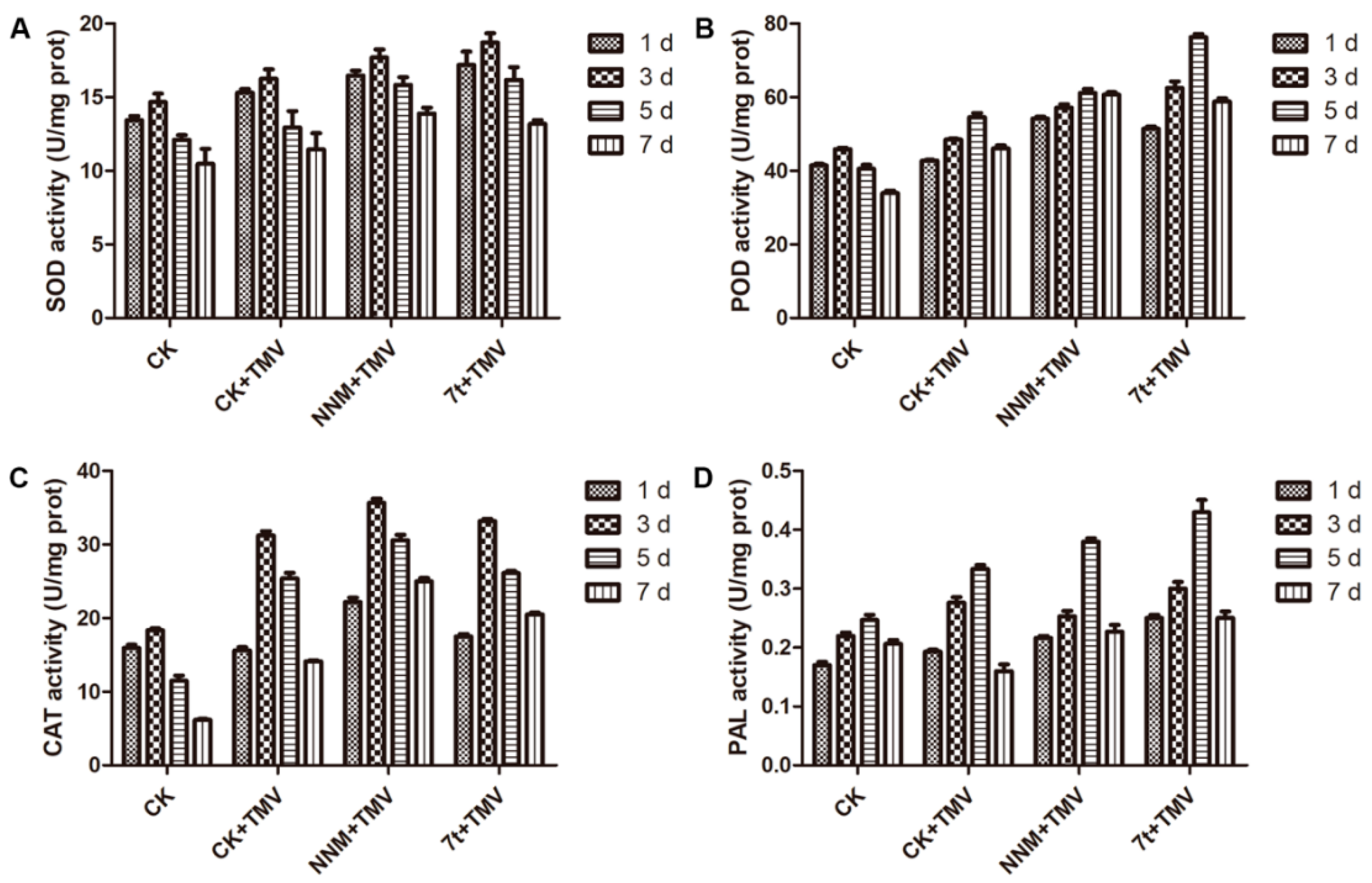
2.4. Effect on Defensive Enzyme Activities
3. Discussion
3.1. Structure–Activity Relationship (SAR) Analysis
3.2. Physiological and Biochemical Responses of Tobacco
4. Materials and Methods
4.1. General Methods
4.2. General Procedure for the Preparation of the Key Intermediate 6
4.3. Preparation of the Target Compounds 7a−7v
4.4. In Vivo Antiviral Activity Test
4.4.1. Purification of TMV and CMV
4.4.2. Curative Activity of the Target Compounds against TMV In Vivo
4.4.3. Protection Activity of the Target Compounds against TMV In Vivo
4.4.4. Inactivation Activity of the Target Compounds against TMV In Vivo
4.4.5. Curative Activity of the Target Compounds against CMV In Vivo
4.4.6. Protection Activity of the Target Compounds against CMV In Vivo
4.4.7. Inactivation Activity of the Target Compounds against CMV In Vivo
4.5. Physiological and Biochemical Analysis
4.5.1. Plant Growth and Sample Collection
4.5.2. Chlorophyll Content Test
4.5.3. Determination of Defensive Enzyme Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Creager, A.N.; Scholthof, K.B.; Citovsky, V.; Scholthof, H.B. Tobacco mosaic virus: Pioneering research for a century. Plant. Cell 1999, 11, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Cai, X.J.; Hu, D.Y.; Xue, W.; Zeng, S. Synthesis and antiviral activities of chiral thiourea derivatives containing an R-aminophosphonate moiety. J. Agric. Food Chem. 2009, 57, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Reichman, M.; Devash, Y.; Suhadolnik, R.J.; Sela, I. Human leukocyte interferon and the antiviral factor (AVF) from virus-infected plants stimulate plant tissues to produce nucleotides with antiviral activity. Virology 1983, 128, 240–244. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.P.; Mao, M.Z.; Zhang, X.G.; Zheng, X.R.; Huang, X.Y.; Xue, C.; Ning, B.K. Synthesis and biological activity of ethyl 2-(5-methyl-3-arylisoxazole-4-carboxamido)-4-alkylthiazole-5-carboxylate. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 164–167. [Google Scholar] [CrossRef]
- Yang, Z.B.; Li, P.; Gan, X.H. Novel pyrazole-hydrazone derivatives containing an isoxazole moiety: Design, synthesis, and antiviral activity. Molecules 2018, 23, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Sushama, K.; Sunil, T.; Manoj, D.; Jaiprakash, S.; Rajendra, P. Synthesis, biological evaluation, molecular docking, and ADMET studies of some isoxazole-based amides. Med. Chem. Res. 2018, 27, 429–441. [Google Scholar]
- Liu, P.F.; Zhang, J.F.; Yan, T.; Xiong, L.X.; Li, Z.M. Design, synthesis and insecticidal activities of novel phenyl substituted isoxazolecarboxamides. Chem. Res. Chin. Univ. 2012, 28, 430–433. [Google Scholar]
- Wu, J.; Kang, S.H.; Song, B.A.; Hu, D.Y.; He, M.; Jin, L.H.; Yang, S. Synthesis and antibacterial activity against ralstonia solanacearum for novel hydrazone derivatives containing a pyridine moiety. Chem. Cent. J. 2013, 7, 64. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Wang, K.L.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Synthesis and antiviral, insecticidal, and fungicidal activities of gossypol derivatives containing alkylimine, oxime or hydrazine moiety. Bioorg. Med. Chem. 2016, 24, 474–483. [Google Scholar] [CrossRef]
- Yang, X.D. Synthesis and biological activity of hydrazone derivatives containing pyrazole. J. Chem. Res. 2008, 9, 489–491. [Google Scholar] [CrossRef]
- Yang, Z.B.; Hu, D.Y.; Zeng, S.; Song, B.A. Novel hydrazone derivatives containing pyridine amide moiety: Design, synthesis, and insecticidal activity. Bioorg. Med. Chem. Lett. 2016, 26, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, D.D.; Shan, W.L.; Zhao, Y.H.; Zhang, W.; Song, B.A.; Yang, S.; Ma, J. Synthesis and insecticidal activity of anthranilic diamides with hydrazone substructure. Chem. Pap. 2015, 69, 993–1003. [Google Scholar] [CrossRef]
- Gan, X.H.; Hu, D.Y.; Li, P.; Wu, J.; Chen, X.W.; Xue, W.; Song, B.A. Design, synthesis, antiviral activity and three-dimensional quantitative structure–activity relationship study of novel 1,4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety. Pest. Manag. Sci. 2016, 72, 534–543. [Google Scholar] [CrossRef]
- Xu, W.M.; He, J.; He, M.; Han, F.F.; Chen, X.H.; Pan, Z.X.; Wang, J.; Tong, M.G. Synthesis and antifungal activity of novel sulfone derivatives containing 1,3,4-oxadiazole moieties. Molecules 2011, 16, 9129–9141. [Google Scholar] [CrossRef]
- Li, P.; Hu, D.Y.; Xie, D.D.; Chen, J.X.; Jin, L.H.; Song, B.A. Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agric. Food Chem. 2018, 66, 3093–3100. [Google Scholar] [CrossRef]
- Li, P.; Tian, P.Y.; Chen, Y.Z.; Song, X.P.; Xue, W.; Jin, L.H.; Hu, D.Y.; Yang, S.; Song, B.A. Novel bisthioether derivatives containing a 1,3,4-oxadiazole moiety: Design, synthesis, antibacterial and nematocidal activities. Pest. Manag. Sci. 2018, 74, 844–852. [Google Scholar] [CrossRef]
- Yang, Z.B.; Zhao, Y.; Li, P.; He, Y.J. Design, synthesis, and insecticidal activity of novel isoxazole derivatives containing bisamide moiety. J. Heterocycl. Chem. 2019. [Google Scholar] [CrossRef]
- Chen, J.X.; Chen, Y.Z.; Gan, X.H.; Song, B.J.; Hu, D.Y.; Song, B.A. Synthesis, nematicidal evaluation, and 3D-QSAR analysis of novel 1,3,4-oxadiazole–cinnamic acid hybrids. J. Agric. Food Chem. 2018, 66, 9616–9623. [Google Scholar] [CrossRef]
- Song, B.A.; Zhang, H.P.; Wang, H.; Yang, S.; Jin, L.H.; Hu, D.Y.; Pang, L.L.; Xue, W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891. [Google Scholar] [CrossRef]
- Long, C.W.; Li, P.; Chen, M.H.; Dong, L.R.; Hu, D.Y.; Song, B.A. Synthesis, anti-tobacco mosaic virus and cucumber mosaic virus activity, and 3D-QSAR study of novel 1,4-pentadien-3-one derivatives containing 4-thioquinazoline moiety. Eur. J. Med. Chem. 2015, 102, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.T.; Song, B.A.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Yang, S. Antiviral activity and mechanism of action of novel thiourea containing chiral phosphonate on tobacco mosaic virus. Int. J. Mol. Sci. 2011, 12, 4522–4535. [Google Scholar] [CrossRef]
- Rahoutei, J.; García-Luque, I.; Barón, M. Inhibition of photosynthesis by viral infection: Effect on PSII structure and function. Physiol. Plant. 2000, 110, 286–292. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Blackwell Sci.: Oxford, UK, 2002; pp. 149–151. [Google Scholar]
- Ghasemie, E.; Kazempour, M.; Padasht, F. Isolation and identification of Xathomonas oryzae pv. oryzae the causal agent of bacterial blight of rice in Iran. Eur. J. Plant. Pathol. 2008, 48, 53–62. [Google Scholar]
- Ahmad, I.; Cheng, Z.H.; Meng, H.W.; Liu, T.J.; Nan, W.C.; Khan, M.A.; Wasila, H.; Khan, A.R. Effect of intercropped garlic (Allium sativum) on chlorophyll contents, photosynthesis and antioxidant enzymes in pepper. Pak. J. Bot. 2013, 45, 1889–1896. [Google Scholar]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant. Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Helal, I.M. Use of biocides for controlling viral diseases that attack common bean and cucumber plants. Folia Hort. 2019, 31, 159–170. [Google Scholar] [CrossRef]
- Gan, X.H.; Hu, D.Y.; Wang, Y.J.; Yu, L.; Song, B.A. Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction. J. Agric. Food Chem. 2017, 65, 4367–4377. [Google Scholar] [CrossRef]
- Yin, L.M.; Gan, X.H.; Shi, J.; Zan, N.N.; Zhang, A.W.; Ren, X.L.; Li, M.; Xie, D.D.; Hu, D.Y.; Song, B.A. Induced resistance mechanism of novel curcumin analogs bearing a quinazoline moiety to plant virus. Int. J. Mol. Sci. 2018, 19, 4065. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, S.C.; Niranjana, S.R.; Umesha, S. Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. J. Phytopathol. 2009, 157, 552–557. [Google Scholar] [CrossRef]
- Schurter, R.; Kunz, W.; Nyfelder, R. New and Known 1,2,3-benzothiadiazole Derivatives-Useful for Immunising Plants against Microbial Infections. EU Patent 0313512, 21 August 1987. [Google Scholar]
- Yoshida, H.; Konishi, K.; Koike, K.; Nakagawa, T.; Sekido, S.; Yamaguchi, I. Effect of N-cyanomethyl-2-chloroisonicotinamide for control of rice blast. J. Pestic. Sci. 1990, 15, 413–417. [Google Scholar] [CrossRef]
- Yoshioka, K.; Nakashita, H.; Klessig, D.F.; Yamaguchi, I. Probenazole induces systemic acquired resistance in Arabidopsis with a novel type of action. Plant. J. 2001, 25, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M. Oxygen in biology and biochemistry: Role of free radicals. In Functional Metabolism: Regulation and Adaptation; Storey, K.B., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004; pp. 319–368. [Google Scholar]
- Moura, J.C.M.S.; Bonine, C.A.V.; De Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant. Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Du, Y.Y.; Wang, P.C.; Chen, J.; Song, C.P. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J. Integr. Plant. Biol. 2008, 50, 1318–1326. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant. Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Gooding, G.V.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar]
- Zhou, X.P.; Xu, Z.X.; Xu, J. The research of cucumber mosaic virus which infected loofah. J. South. Chin. Agric. Univ. 1995, 16, 74–79. [Google Scholar]
- Zhao, J.; Zhou, J.J.; Wang, Y.Y.; Gu, J.W.; Xie, X.Z. Positive regulation of phytochrome B on chlorophyll biosynthesis and chloroplast development in rice. Rice Sci. 2013, 20, 243–248. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 7a–7v are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.-B.; Li, P.; He, Y.-J. Design, Synthesis, and Bioactivity Evaluation of Novel Isoxazole-Amide Derivatives Containing an Acylhydrazone Moiety as New Active Antiviral Agents. Molecules 2019, 24, 3766. https://doi.org/10.3390/molecules24203766
Yang Z-B, Li P, He Y-J. Design, Synthesis, and Bioactivity Evaluation of Novel Isoxazole-Amide Derivatives Containing an Acylhydrazone Moiety as New Active Antiviral Agents. Molecules. 2019; 24(20):3766. https://doi.org/10.3390/molecules24203766
Chicago/Turabian StyleYang, Zai-Bo, Pei Li, and Yin-Ju He. 2019. "Design, Synthesis, and Bioactivity Evaluation of Novel Isoxazole-Amide Derivatives Containing an Acylhydrazone Moiety as New Active Antiviral Agents" Molecules 24, no. 20: 3766. https://doi.org/10.3390/molecules24203766
APA StyleYang, Z.-B., Li, P., & He, Y.-J. (2019). Design, Synthesis, and Bioactivity Evaluation of Novel Isoxazole-Amide Derivatives Containing an Acylhydrazone Moiety as New Active Antiviral Agents. Molecules, 24(20), 3766. https://doi.org/10.3390/molecules24203766






