Modulating Immune Response with Nucleic Acid Nanoparticles
Abstract
1. Introduction
2. Design of RNA Nanoparticles from Naturally Occurring Structural Motifs.
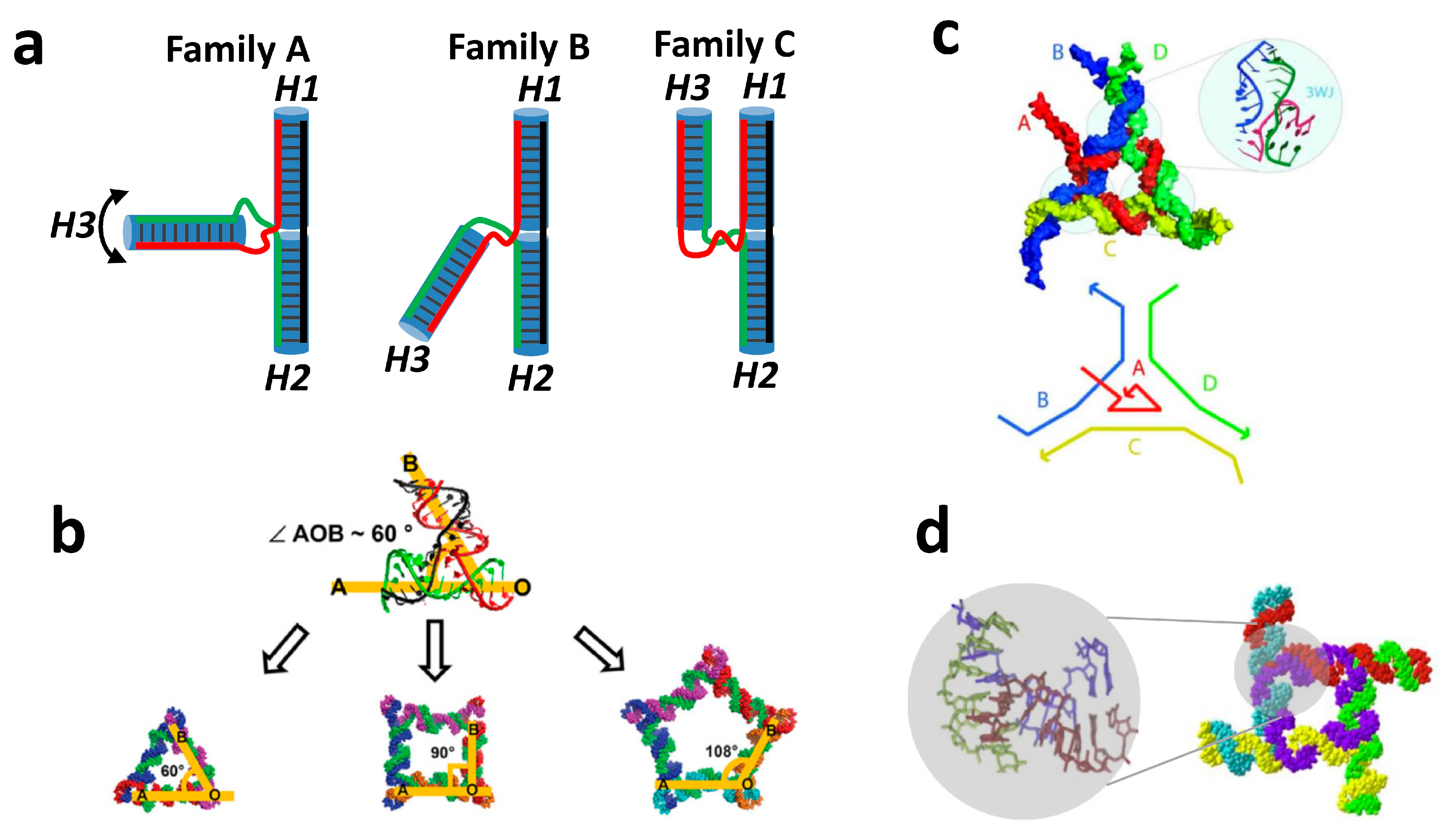
2.1. RNA Three Way and Other Multiway Junctions.
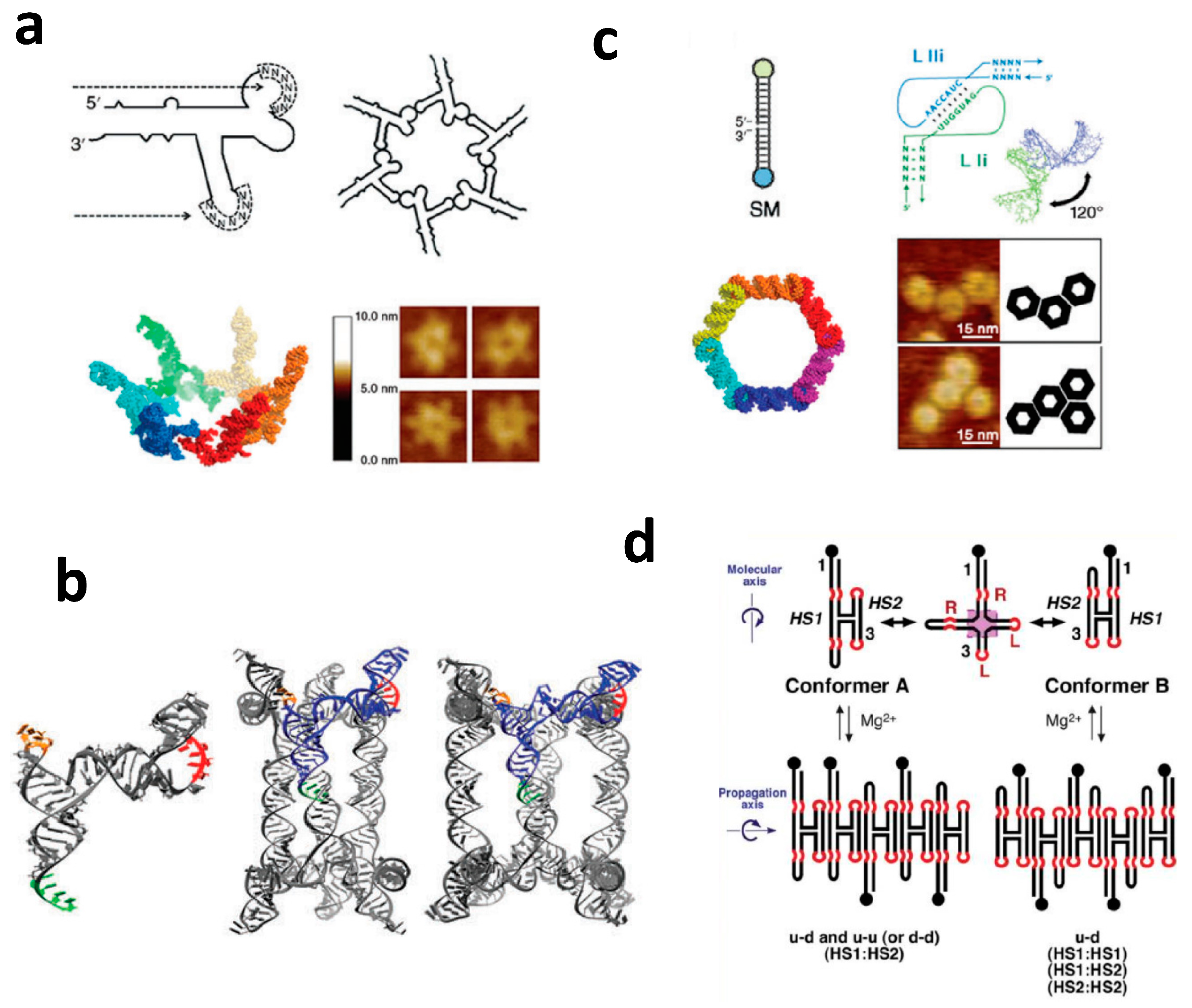
2.2. RNA Kink-Turn Motif and Other Helical Bends.
2.3. RNA Kissing Loop Motifs and Loop–Receptor Interaction.
3. Design of RNA Nanoparticles from Computationally Designed Structural Motifs.
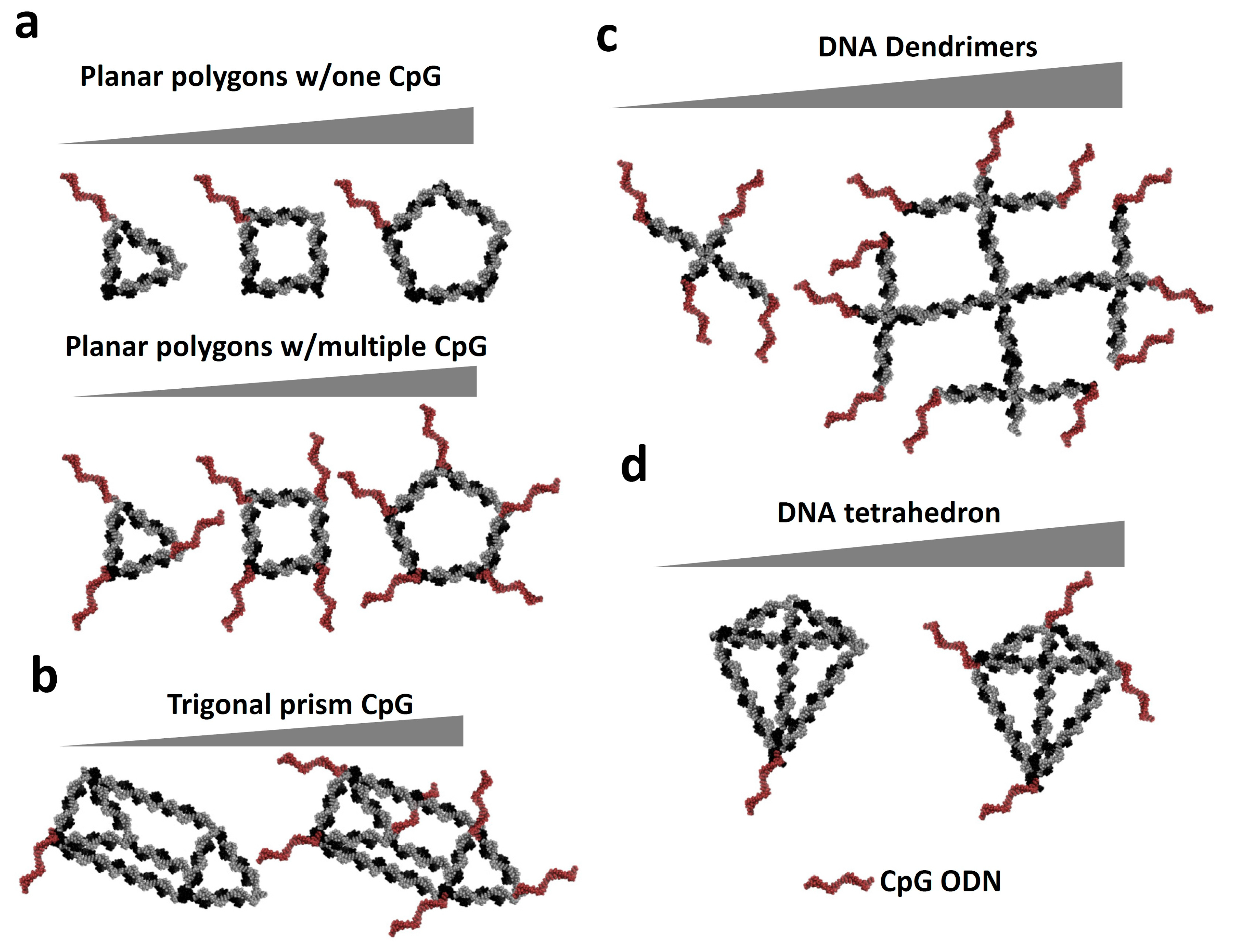
3.1. NANPs as Carriers of Immunostimulatory CpG Oligonucleotides.
3.2. NANPs as Stimulators of Innate Immunity.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Gordon, S. The role of scavenger receptors in pathogen recognition and innate immunity. Immunobiology 2004, 209, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kolar, S.S.; Baidouri, H.; McDermott, A.M. Role of Pattern Recognition Receptors in the Modulation of Antimicrobial Peptide Expression in the Corneal Epithelial Innate Response to F. solani. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Hunt, S.; Austyn, J. The expanding repertoire. Nature 1988, 332, 179–180. [Google Scholar] [CrossRef]
- Radovic-Moreno, A.F.; Chernyak, N.; Mader, C.C.; Nallagatla, S.; Kang, R.S.; Hao, L.; Walker, D.A.; Halo, T.L.; Merkel, T.J.; Rische, C.H.; et al. Immunomodulatory spherical nucleic acids. Proc. Natl. Acad. Sci. USA 2015, 112, 3892–3897. [Google Scholar] [CrossRef]
- Ke, W.; Hong, E.; Saito, R.F.; Rangel, M.C.; Wang, J.; Viard, M.; Richardson, M.; Khisamutdinov, E.F.; Panigaj, M.; Dokholyan, N.V.; et al. RNA-DNA fibers and polygons with controlled immunorecognition activate RNAi, FRET and transcriptional regulation of NF-kappaB in human cells. Nucleic Acids Res. 2018. [Google Scholar] [CrossRef]
- Hong, E.; Halman, J.R.; Shah, A.B.; Khisamutdinov, E.F.; Dobrovolskaia, M.A.; Afonin, K.A. Structure and Composition Define Immunorecognition of Nucleic Acid Nanoparticles. Nano Lett. 2018, 18, 4309–4321. [Google Scholar] [CrossRef]
- Hong, E.; Halman, J.R.; Shah, A.; Cedrone, E.; Truong, N.; Afonin, K.A.; Dobrovolskaia, M.A. Toll-Like Receptor-Mediated Recognition of Nucleic Acid Nanoparticles (NANPs) in Human Primary Blood Cells. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Chandler, M.; Afonin, K.A. Smart-Responsive Nucleic Acid Nanoparticles (NANPs) with the Potential to Modulate Immune Behavior. Nanomaterials (Basel) 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Khisamutdinov, E.F.; Bui, M.N.; Jasinski, D.; Zhao, Z.; Cui, Z.; Guo, P. Simple Method for Constructing RNA Triangle, Square, Pentagon by Tuning Interior RNA 3WJ Angle from 60 degrees to 90 degrees or 108 degrees. Methods Mol. Biol 2015, 1316, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Khisamutdinov, E.F.; Li, H.; Jasinski, D.L.; Chen, J.; Fu, J.; Guo, P. Enhancing immunomodulation on innate immunity by shape transition among RNA triangle, square and pentagon nanovehicles. Nucleic Acids Res. 2014, 42, 9996–10004. [Google Scholar] [CrossRef] [PubMed]
- Afonin, K.A.; Bindewald, E.; Yaghoubian, A.J.; Voss, N.; Jacovetty, E.; Shapiro, B.A.; Jaeger, L. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat. Nanotechnol. 2010, 5, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Afonin, K.A.; Kasprzak, W.; Bindewald, E.; Puppala, P.S.; Diehl, A.R.; Hall, K.T.; Kim, T.J.; Zimmermann, M.T.; Jernigan, R.L.; Jaeger, L.; et al. Computational and experimental characterization of RNA cubic nanoscaffolds. Methods 2014, 67, 256–265. [Google Scholar] [CrossRef]
- Khisamutdinov, E.F.; Jasinski, D.L.; Li, H.; Zhang, K.; Chiu, W.; Guo, P. Fabrication of RNA 3D Nanoprisms for Loading and Protection of Small RNAs and Model Drugs. Adv. Mater. 2016, 28, 10079–10087. [Google Scholar] [CrossRef]
- Bindewald, E.; Afonin, K.A.; Viard, M.; Zakrevsky, P.; Kim, T.; Shapiro, B.A. Multistrand Structure Prediction of Nucleic Acid Assemblies and Design of RNA Switches. Nano Lett. 2016, 16, 1726–1735. [Google Scholar] [CrossRef]
- Grabow, W.W.; Zakrevsky, P.; Afonin, K.A.; Chworos, A.; Shapiro, B.A.; Jaeger, L. Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett. 2011, 11, 878–887. [Google Scholar] [CrossRef]
- Guo, S.; Piao, X.; Li, H.; Guo, P. Methods for construction and characterization of simple or special multifunctional RNA nanoparticles based on the 3WJ of phi29 DNA packaging motor. Methods 2018, 143, 121–133. [Google Scholar] [CrossRef]
- Shu, D.; Khisamutdinov, E.F.; Zhang, L.; Guo, P. Programmable folding of fusion RNA in vivo and in vitro driven by pRNA 3WJ motif of phi29 DNA packaging motor. Nucleic Acids Res. 2014, 42, e10. [Google Scholar] [CrossRef]
- Shu, D.; Shu, Y.; Haque, F.; Abdelmawla, S.; Guo, P. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 2011, 6, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Shu, D.; Shu, Y.; Shlyakhtenko, L.S.; Rychahou, P.G.; Evers, B.M.; Guo, P. Ultrastable synergistic tetravalent RNA nanoparticles for targeting to cancers. Nano Today 2012, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.N.; Brittany Johnson, M.; Viard, M.; Satterwhite, E.; Martins, A.N.; Li, Z.; Marriott, I.; Afonin, K.A.; Khisamutdinov, E.F. Versatile RNA tetra-U helix linking motif as a toolkit for nucleic acid nanotechnology. Nanomedicine 2017, 13, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Afonin, K.A.; Bindewald, E.; Kireeva, M.; Shapiro, B.A. Computational and experimental studies of reassociating RNA/DNA hybrids containing split functionalities. Methods Enzymol. 2015, 553, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Halman, J.R.; Satterwhite, E.; Roark, B.; Chandler, M.; Viard, M.; Ivanina, A.; Bindewald, E.; Kasprzak, W.K.; Panigaj, M.; Bui, M.N.; et al. Functionally-interdependent shape-switching nanoparticles with controllable properties. Nucleic Acids Res. 2017, 45, 2210–2220. [Google Scholar] [CrossRef]
- Benkato, K.; O’Brien, B.; Bui, M.N.; Jasinski, D.L.; Guo, P.; Khisamutdinov, E.F. Evaluation of Thermal Stability of RNA Nanoparticles by Temperature Gradient Gel Electrophoresis (TGGE) in Native Condition. Methods Mol. Biol. 2017, 1632, 123–133. [Google Scholar] [CrossRef]
- Sajja, S.; Chandler, M.; Fedorov, D.; Kasprzak, W.K.; Lushnikov, A.; Viard, M.; Shah, A.; Dang, D.; Dahl, J.; Worku, B.; et al. Dynamic Behavior of RNA Nanoparticles Analyzed by AFM on a Mica/Air Interface. Langmuir 2018. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, C.; Chen, C.; Garver, K.; Trottier, M. Inter-RNA interaction of phage phi29 pRNA to form a hexameric complex for viral DNA transportation. Mol. Cell 1998, 2, 149–155. [Google Scholar] [CrossRef]
- Novikova, I.V.; Hassan, B.H.; Mirzoyan, M.G.; Leontis, N.B. Engineering cooperative tecto-RNA complexes having programmable stoichiometries. Nucleic Acids Res. 2011, 39, 2903–2917. [Google Scholar] [CrossRef]
- Afonin, K.A.; Cieply, D.J.; Leontis, N.B. Specific RNA self-assembly with minimal paranemic motifs. J. Am. Chem Soc. 2008, 130, 93–102. [Google Scholar] [CrossRef]
- Grabow, W.W.; Zhuang, Z.; Swank, Z.N.; Shea, J.E.; Jaeger, L. The right angle (RA) motif: A prevalent ribosomal RNA structural pattern found in group I introns. J. Mol. Biol 2012, 424, 54–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, L.; Lilley, D.M. The Kink Turn, a Key Architectural Element in RNA Structure. J. Mol. Biol 2016, 428, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.J.; Schmeing, T.M.; Moore, P.B.; Steitz, T.A. The kink-turn: A new RNA secondary structure motif. EMBO J. 2001, 20, 4214–4221. [Google Scholar] [CrossRef]
- Afonin, K.A.; Leontis, N.B. Generating new specific RNA interaction interfaces using C-loops. J. Am. Chem Soc. 2006, 128, 16131–16137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lescoute, A.; Westhof, E. Topology of three-way junctions in folded RNAs. RNA 2006, 12, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Kobayashi, T.; Kabata, R.; Endo, K.; Iwasa, T.; Yoshimura, S.H.; Takeyasu, K.; Inoue, T.; Saito, H. Synthetic RNA-protein complex shaped like an equilateral triangle. Nat. Nanotechnol. 2011, 6, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Inoue, T. RNA and RNP as new molecular parts in synthetic biology. J. Biotechnol 2007, 132, 1–7. [Google Scholar] [CrossRef]
- Khisamutdinov, E.F.; Jasinski, D.L.; Guo, P. RNA as a boiling-resistant anionic polymer material to build robust structures with defined shape and stoichiometry. ACS Nano 2014, 8, 4771–4781. [Google Scholar] [CrossRef]
- Shu, Y.; Pi, F.; Sharma, A.; Rajabi, M.; Haque, F.; Shu, D.; Leggas, M.; Evers, B.M.; Guo, P. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 74–89. [Google Scholar] [CrossRef]
- Bindewald, E.; Hayes, R.; Yingling, Y.G.; Kasprzak, W.; Shapiro, B.A. RNAJunction: A database of RNA junctions and kissing loops for three-dimensional structural analysis and nanodesign. Nucleic Acids Res. 2008, 36, D392–397. [Google Scholar] [CrossRef]
- Bindewald, E.; Afonin, K.; Jaeger, L.; Shapiro, B.A. Multistrand RNA secondary structure prediction and nanostructure design including pseudoknots. ACS Nano 2011, 5, 9542–9551. [Google Scholar] [CrossRef] [PubMed]
- Parlea, L.; Bindewald, E.; Sharan, R.; Bartlett, N.; Moriarty, D.; Oliver, J.; Afonin, K.A.; Shapiro, B.A. Ring Catalog: A resource for designing self-assembling RNA nanostructures. Methods 2016, 103, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Severcan, I.; Geary, C.; Verzemnieks, E.; Chworos, A.; Jaeger, L. Square-shaped RNA particles from different RNA folds. Nano Lett. 2009, 9, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Geary, C.; Chworos, A.; Verzemnieks, E.; Voss, N.R.; Jaeger, L. Composing RNA Nanostructures from a Syntax of RNA Structural Modules. Nano Lett. 2017, 17, 7095–7101. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Ikawa, Y.; Inoue, T. Biochemical characterization of the kink-turn RNA motif. Nucleic Acids Res. 2003, 31, 5544–5551. [Google Scholar] [CrossRef][Green Version]
- Goody, T.A.; Melcher, S.E.; Norman, D.G.; Lilley, D.M. The kink-turn motif in RNA is dimorphic, and metal ion-dependent. RNA 2004, 10, 254–264. [Google Scholar] [CrossRef]
- Huang, L.; Lilley, D.M. A quasi-cyclic RNA nano-scale molecular object constructed using kink turns. Nanoscale 2016, 8, 15189–15195. [Google Scholar] [CrossRef]
- Tiedge, H. K-turn motifs in spatial RNA coding. RNA Biol. 2006, 3, 133–139. [Google Scholar] [CrossRef]
- Huang, L.; Lilley, D.M.J. The kink-turn in the structural biology of RNA. Q Rev. Biophys. 2018, 51, e5. [Google Scholar] [CrossRef]
- Ohno, H.; Osada, E.; Saito, H. Design, assembly, and evaluation of RNA-protein nanostructures. Methods Mol. Biol. 2015, 1297, 197–211. [Google Scholar] [CrossRef]
- Shibata, T.; Suzuki, Y.; Sugiyama, H.; Endo, M.; Saito, H. Folding RNA-Protein Complex into Designed Nanostructures. Methods Mol. Biol. 2015, 1316, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Saito, H. RNA and RNP as Building Blocks for Nanotechnology and Synthetic Biology. Prog Mol. Biol. Transl. Sci. 2016, 139, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, S.M.; McLean, J.; Parsons, J.; Hermann, T. Self-assembling RNA square. Proc. Natl. Acad. Sci. USA 2011, 108, 6405–6408. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Haque, F.; Shu, D.; Li, W.; Zhu, Z.; Kotb, M.; Lyubchenko, Y.; Guo, P. Fabrication of 14 different RNA nanoparticles for specific tumor targeting without accumulation in normal organs. RNA 2013, 19, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Li, X.; Tian, C.; Jiang, W.; Wang, G.; Mao, C. Construction of RNA nanocages by re-engineering the packaging RNA of Phi29 bacteriophage. Nat. Commun. 2014, 5, 3890. [Google Scholar] [CrossRef]
- Yingling, Y.G.; Shapiro, B.A. Computational design of an RNA hexagonal nanoring and an RNA nanotube. Nano Lett. 2007, 7, 2328–2334. [Google Scholar] [CrossRef]
- Nasalean, L.; Baudrey, S.; Leontis, N.B.; Jaeger, L. Controlling RNA self-assembly to form filaments. Nucleic Acids Res. 2006, 34, 1381–1392. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Afonin, K.A.; Viard, M.; Koyfman, A.Y.; Martins, A.N.; Kasprzak, W.K.; Panigaj, M.; Desai, R.; Santhanam, A.; Grabow, W.W.; Jaeger, L.; et al. Multifunctional RNA nanoparticles. Nano Lett. 2014, 14, 5662–5671. [Google Scholar] [CrossRef]
- Hegele, A.; Dalpke, A.; Heeg, K.; Barth, P.; Varga, Z.; Hofmann, R.; Olbert, P. Immunostimulatory CpG oligonucleotides reduce tumor burden after intravesical administration in an orthotopic murine bladder cancer model. Tumour. Biol. 2005, 26, 274–280. [Google Scholar] [CrossRef]
- Bodera, P. Immunostimulatory oligonucleotides. Recent Pat. Inflamm Allergy Drug Discov. 2011, 5, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kerkmann, M.; Lochmann, D.; Weyermann, J.; Marschner, A.; Poeck, H.; Wagner, M.; Battiany, J.; Zimmer, A.; Endres, S.; Hartmann, G. Immunostimulatory properties of CpG-oligonucleotides are enhanced by the use of protamine nanoparticles. Oligonucleotides 2006, 16, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, C.; Schmidt, L.; Lanz, A.L.; Storch, B.; Wurzenberger, C.; Anz, D.; Sandholzer, N.; Mocikat, R.; Berger, M.; Poeck, H.; et al. Immunostimulatory RNA oligonucleotides induce an effective antitumoral NK cell response through the TLR7. J. Immunol. 2009, 183, 6078–6086. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Shibata, T.; Tanji, H.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Miyake, K.; Shimizu, T. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 2015, 520, 702–705. [Google Scholar] [CrossRef]
- Carpentier, A.F.; Auf, G.; Delattre, J.Y. CpG-oligonucleotides for cancer immunotherapy: Review of the literature and potential applications in malignant glioma. Front. Biosci. 2003, 8, e115–127. [Google Scholar] [CrossRef]
- Gupta, K.; Cooper, C. A review of the role of CpG oligodeoxynucleotides as toll-like receptor 9 agonists in prophylactic and therapeutic vaccine development in infectious diseases. Drugs R D 2008, 9, 137–145. [Google Scholar] [CrossRef]
- Mason, K.A.; Hunter, N.R. CpG plus radiotherapy: A review of preclinical works leading to clinical trial. Front. Oncol. 2012, 2, 101. [Google Scholar] [CrossRef]
- Razin, A.; Friedman, J. DNA methylation and its possible biological roles. Prog Nucleic Acid Res. Mol. Biol. 1981, 25, 33–52. [Google Scholar]
- Kim, I.Y.; Yan, X.; Tohme, S.; Ahmed, A.; Cordon-Cardo, C.; Shantha Kumara, H.M.; Kim, S.K.; Whelan, R.L. CpG ODN, Toll like receptor (TLR)-9 agonist, inhibits metastatic colon adenocarcinoma in a murine hepatic tumor model. J. Surg. Res. 2012, 174, 284–290. [Google Scholar] [CrossRef]
- Schuller, S.; Wisgrill, L.; Sadeghi, K.; Gindl, E.; Helmer, H.; Husslein, P.; Berger, A.; Spittler, A.; Forster-Waldl, E. The TLR-specific adjuvants R-848 and CpG-B endorse the immunological reaction of neonatal antigen-presenting cells. Pediatr. Res. 2016, 80, 311–318. [Google Scholar] [CrossRef]
- Sharma, S.; Dominguez, A.L.; Hoelzinger, D.B.; Lustgarten, J. CpG-ODN but not other TLR-ligands restore the antitumor responses in old mice: The implications for vaccinations in the aged. Cancer Immunol. Immunother 2008, 57, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Yeh, D.W.; Lai, C.Y.; Liu, Y.L.; Lu, C.H.; Tseng, P.H.; Yuh, C.H.; Yu, G.Y.; Liu, S.J.; Leng, C.H.; Chuang, T.H. CpG-oligodeoxynucleotides developed for grouper toll-like receptor (TLR) 21s effectively activate mouse and human TLR9s mediated immune responses. Sci. Rep. 2017, 7, 17297. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv. Immunol. 1999, 73, 329–368. [Google Scholar] [PubMed]
- Peer, D. Immunotoxicity derived from manipulating leukocytes with lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 1738–1748. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, J.; Zhan, P.; Liu, S.; Zhang, K.; Jiang, Q.; Li, C.; Ding, B. Self-Assembled DNA Dendrimer Nanoparticle for Efficient Delivery of Immunostimulatory CpG Motifs. ACS Appl. Mater. Interfaces 2017, 9, 20324–20329. [Google Scholar] [CrossRef]
- Mohri, K.; Kusuki, E.; Ohtsuki, S.; Takahashi, N.; Endo, M.; Hidaka, K.; Sugiyama, H.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Self-assembling DNA dendrimer for effective delivery of immunostimulatory CpG DNA to immune cells. Biomacromolecules 2015, 16, 1095–1101. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durbin, J.K.; Miller, D.K.; Niekamp, J.; Khisamutdinov, E.F. Modulating Immune Response with Nucleic Acid Nanoparticles. Molecules 2019, 24, 3740. https://doi.org/10.3390/molecules24203740
Durbin JK, Miller DK, Niekamp J, Khisamutdinov EF. Modulating Immune Response with Nucleic Acid Nanoparticles. Molecules. 2019; 24(20):3740. https://doi.org/10.3390/molecules24203740
Chicago/Turabian StyleDurbin, Jake K., Daniel K. Miller, Julia Niekamp, and Emil F. Khisamutdinov. 2019. "Modulating Immune Response with Nucleic Acid Nanoparticles" Molecules 24, no. 20: 3740. https://doi.org/10.3390/molecules24203740
APA StyleDurbin, J. K., Miller, D. K., Niekamp, J., & Khisamutdinov, E. F. (2019). Modulating Immune Response with Nucleic Acid Nanoparticles. Molecules, 24(20), 3740. https://doi.org/10.3390/molecules24203740





