Stapled Peptides—A Useful Improvement for Peptide-Based Drugs
Abstract
1. Proteins
Peptides as Drugs
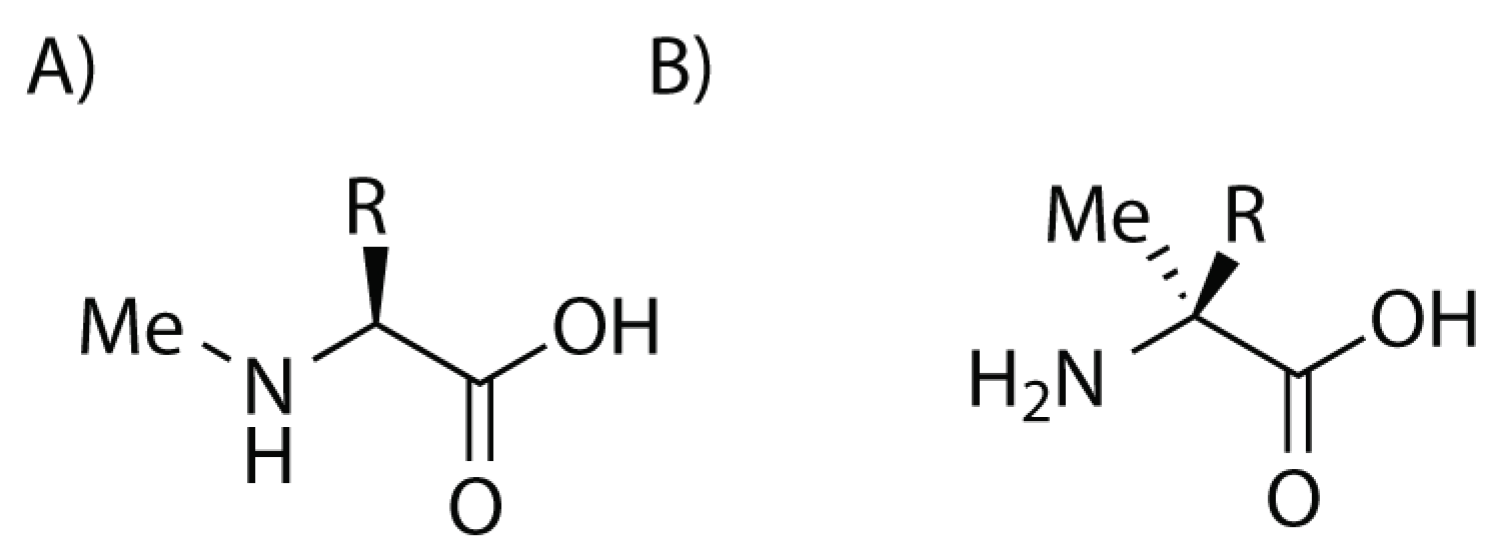
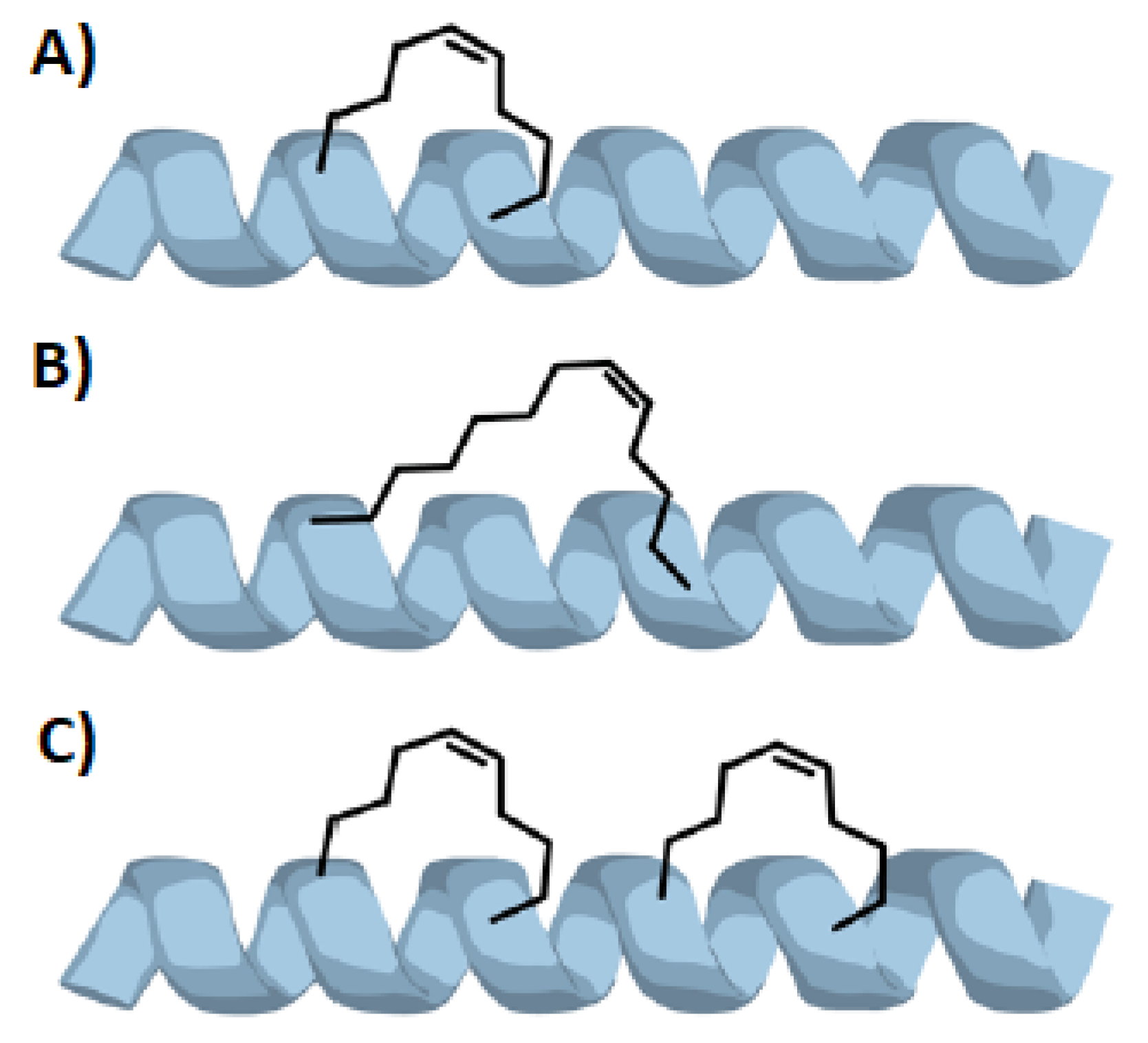
2. Stapled Peptides
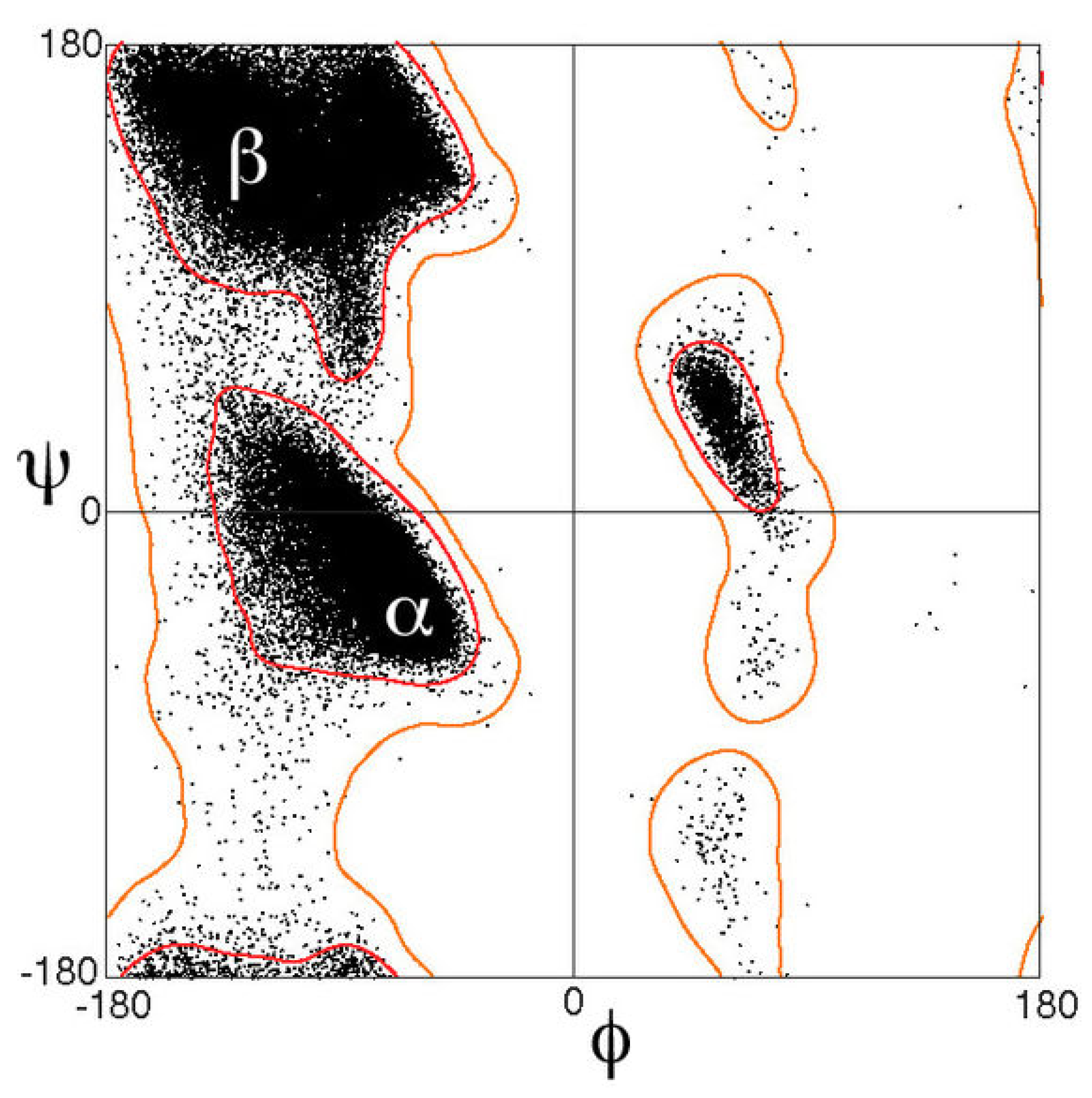
2.1. The -Helix
2.2. Peptide Properties
2.2.1. Protease Resistance
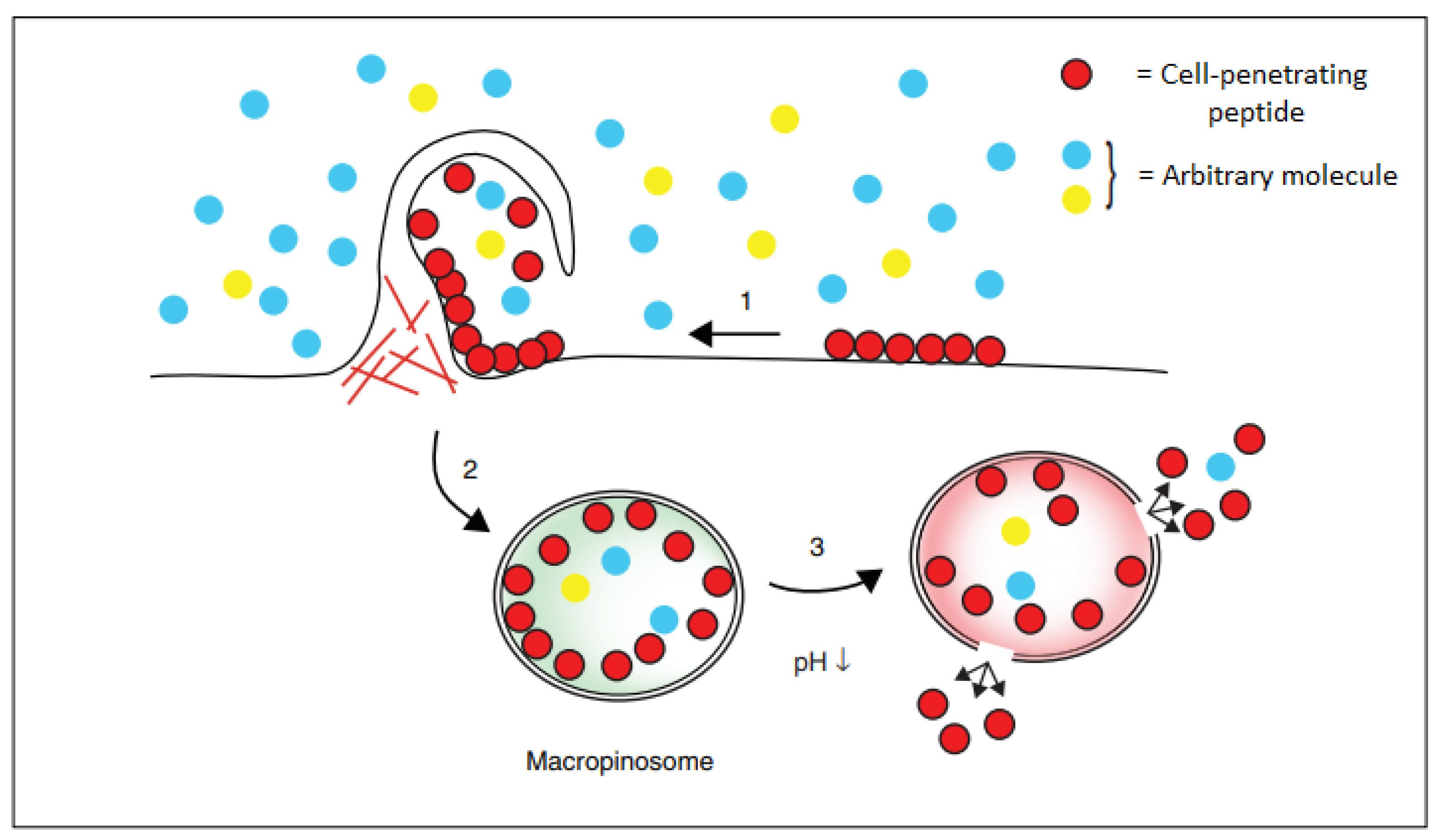
2.2.2. Cellular Uptake
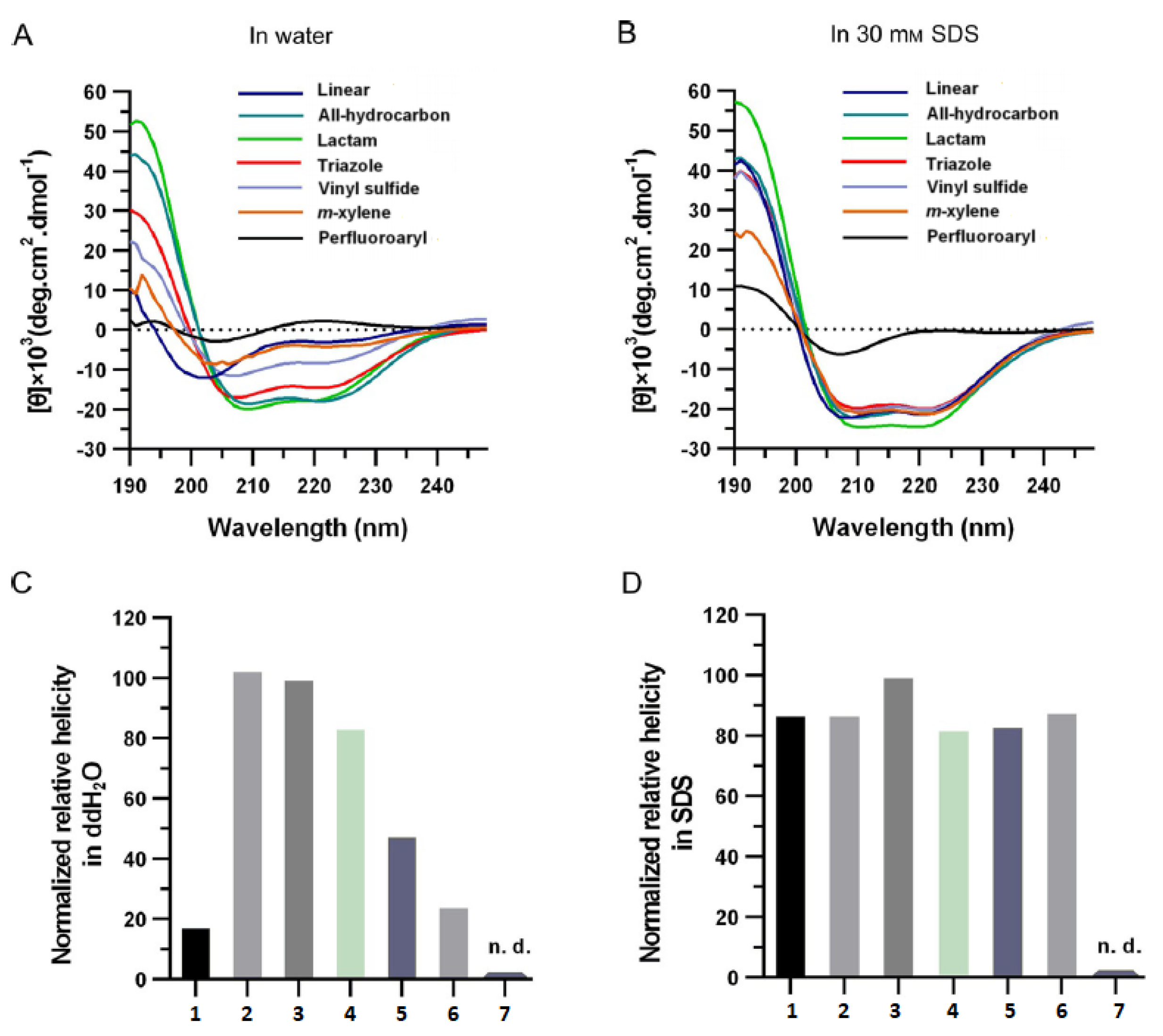
2.3. Experimental Determination
2.3.1. Helicity
2.3.2. Solubility
2.3.3. Proteolytic Resistance
2.3.4. Cellular Uptake
2.3.5. Biochemical Activity
2.4. Peptide Stapling
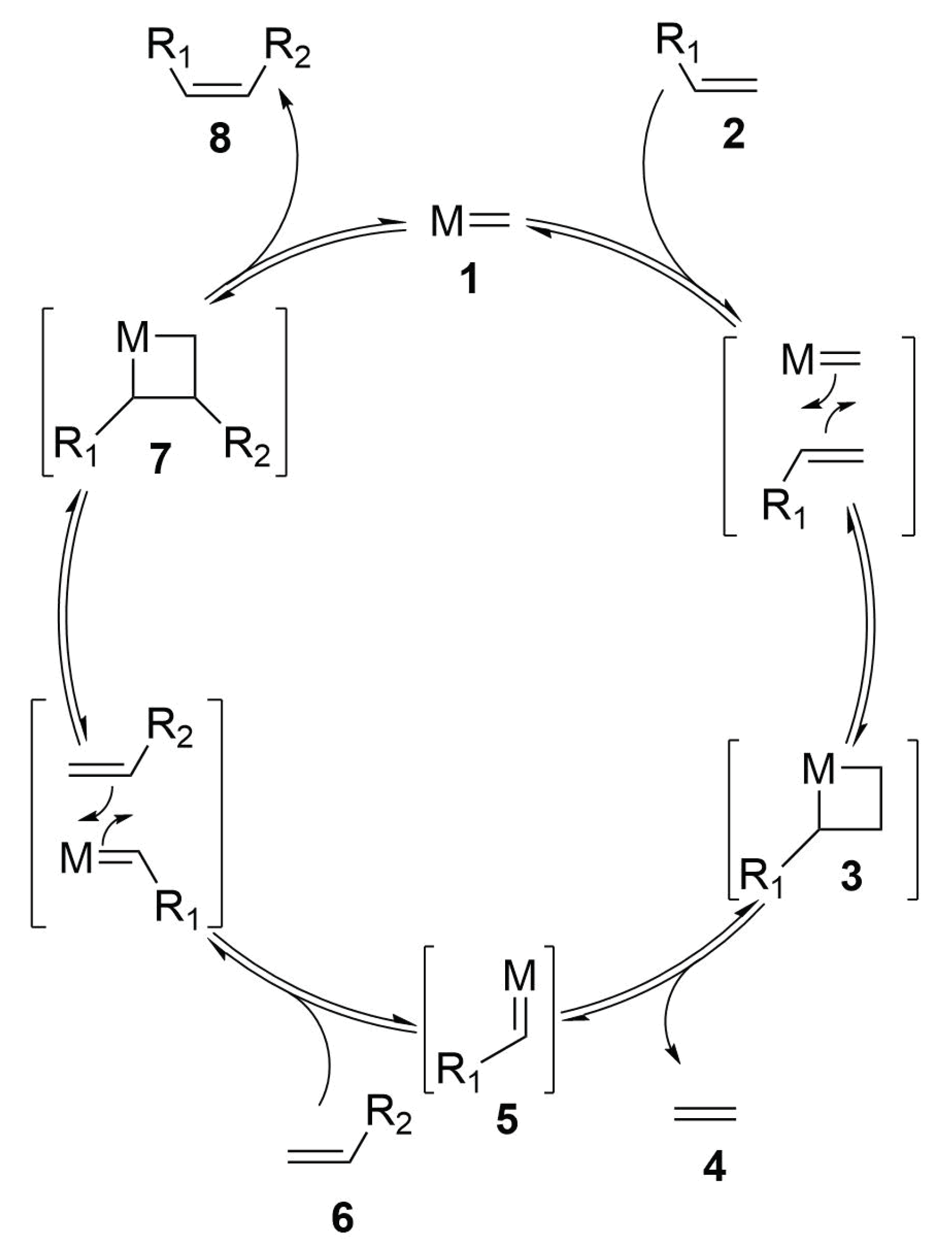
2.4.1. Ring Closing Metathesis
- First Generation Grubbs Catalysts—this kind of catalyst consists of a ruthenium core substituted with two phosphine groups [usually P(Cy)], two chlorine atoms and a carbene compound (usually a benzylidene carbene). These metal complexes are quite air-stable and so they are easy to handle [37].
- Second Generation Grubbs Catalysts—these catalysts are very similar to the first generation ones but they bring an N-Heterocyclic Carbene (NHC) instead of a phosphine substituent. The insertion of an NHC substituent enhance the activity of the catalyst, maintaining a quite good stability towards air and water [38].
- Schrock Catalysts—these are molybdenum-based catalysts. The electron deficient metal atom is coordinated in a pseudo-tetrahedral sphere by an aryl-imido carbene, a bulky alkylidene carbene and two electron withdrawing alkoxide substituents. These compounds display a great activity but are sensible towards air, water and some compounds with protons on the heteroatoms (-COOH, -SH, ...) [39].
Reaction Mechanism
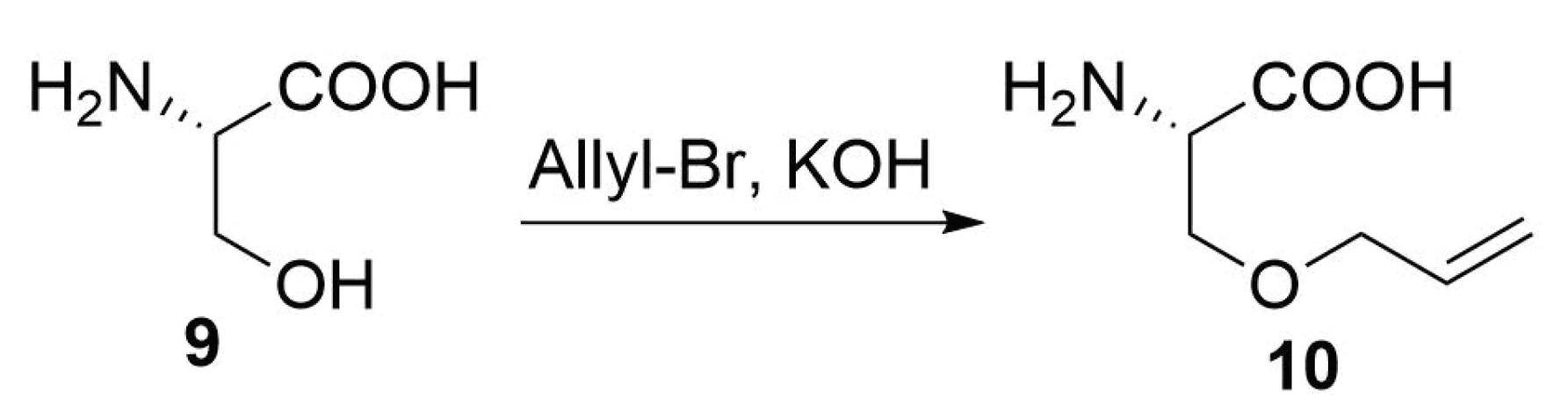
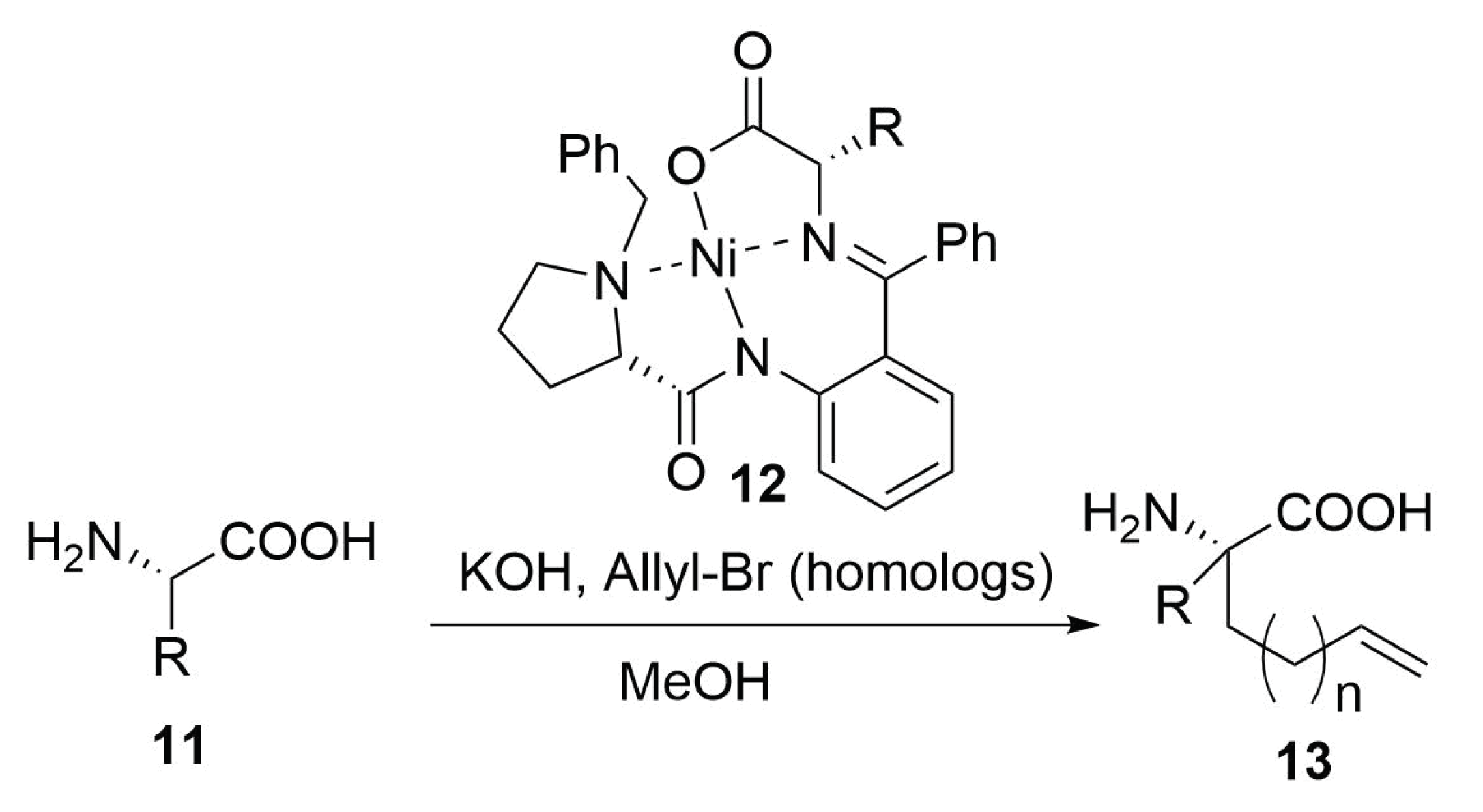
Modified Peptide Synthesis
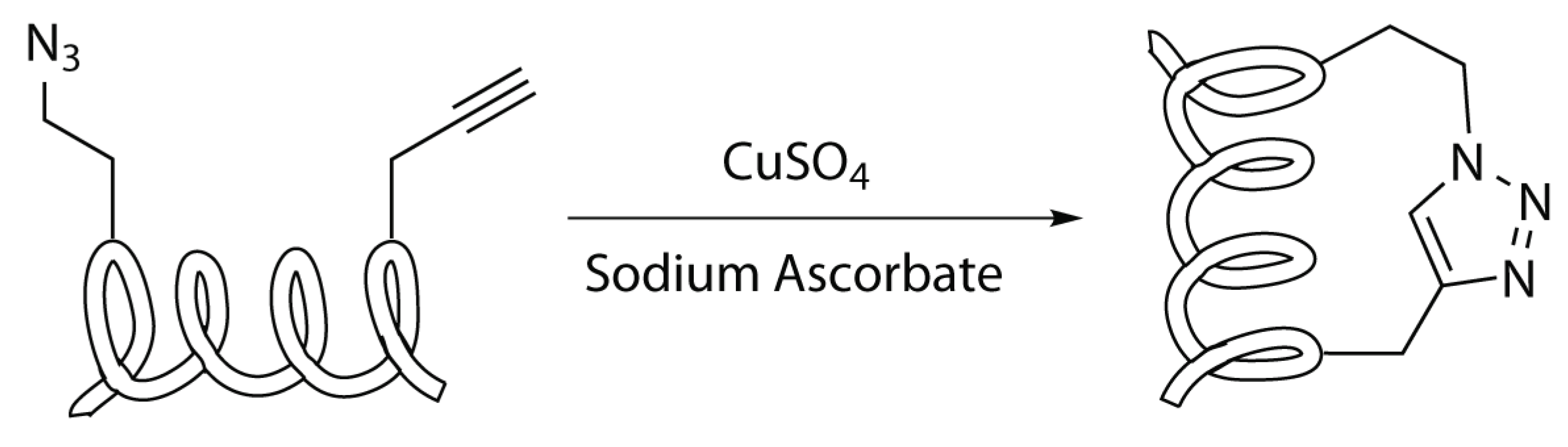
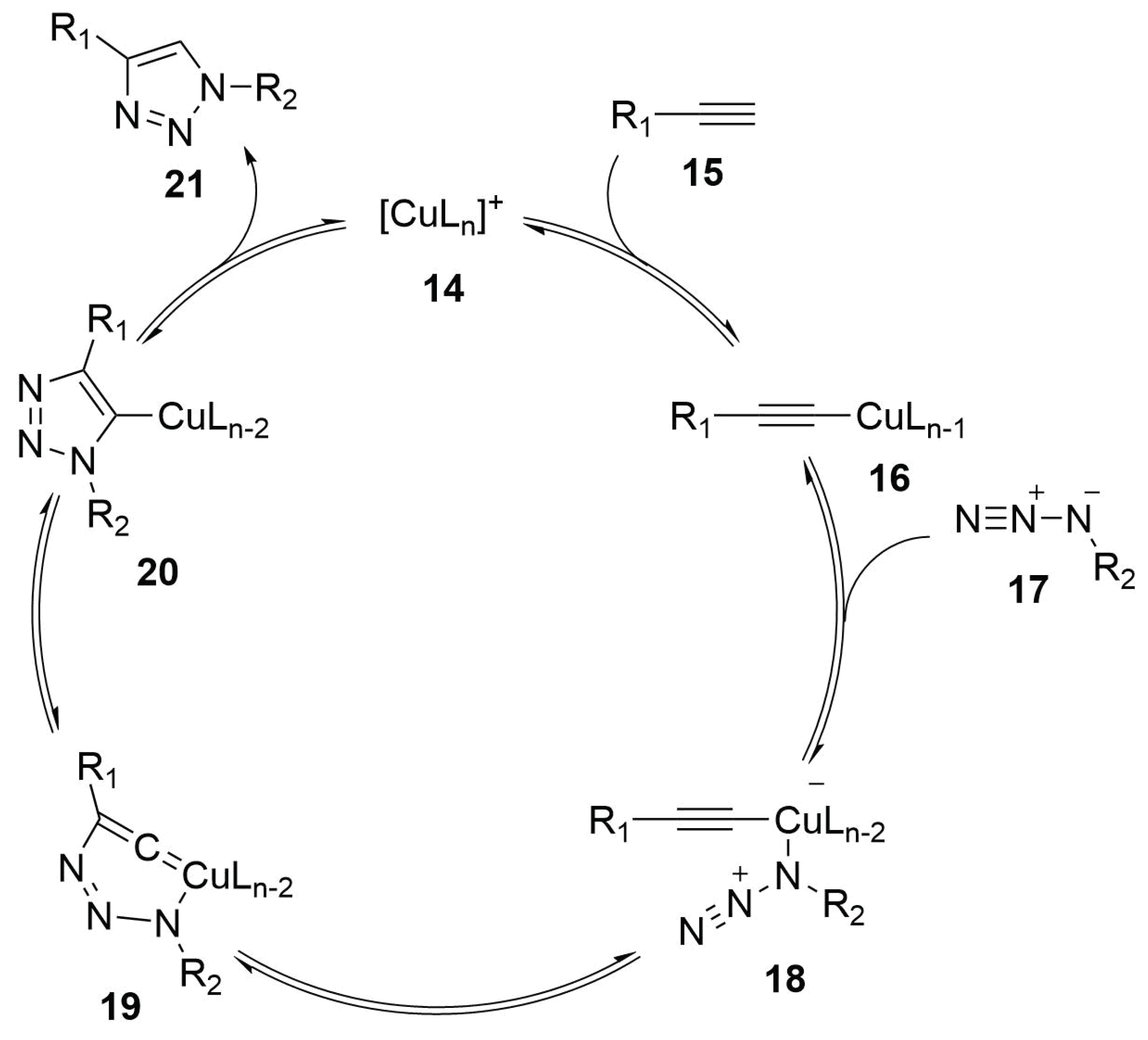
2.4.2. Copper Catalyzed Azide Alkyne Cycloaddition
Reaction Mechanism
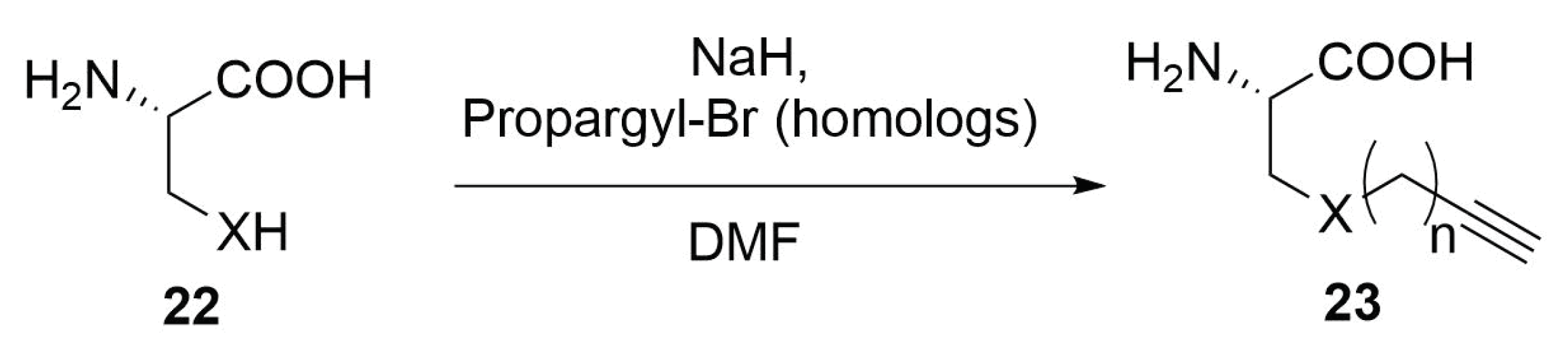
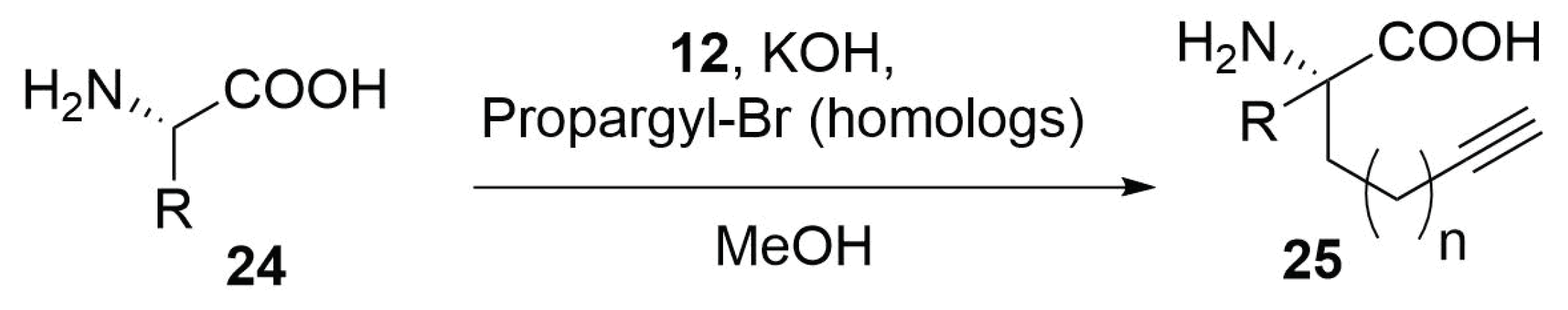
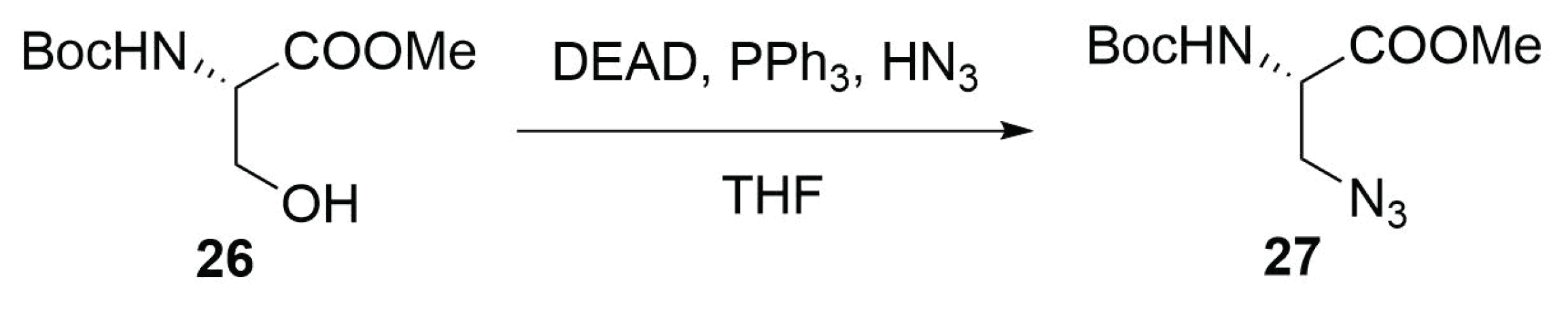
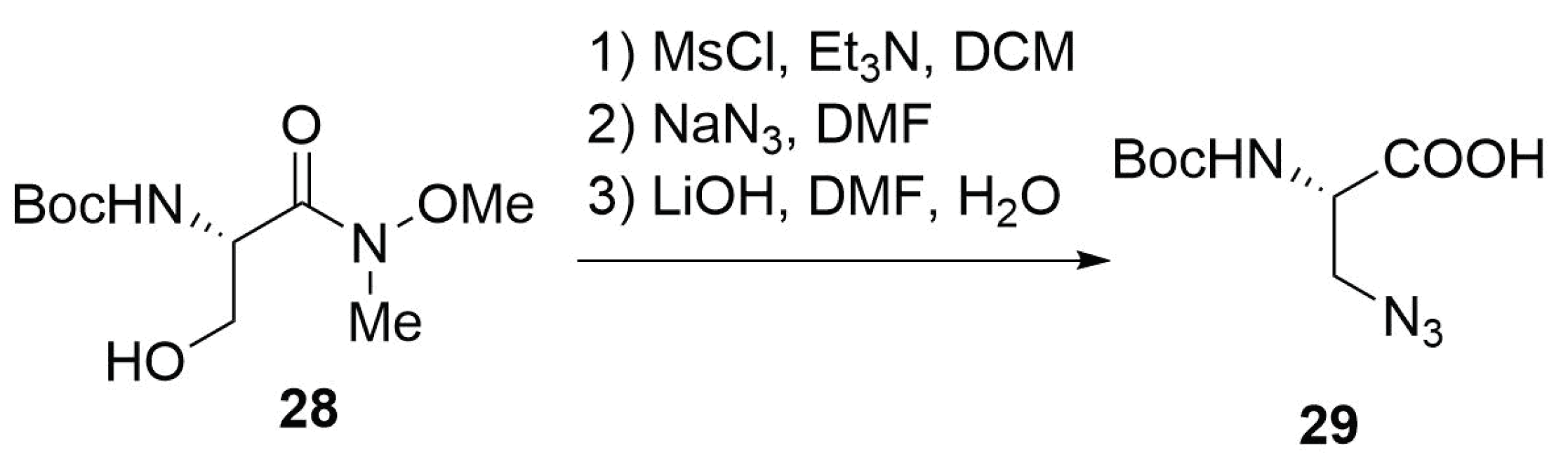
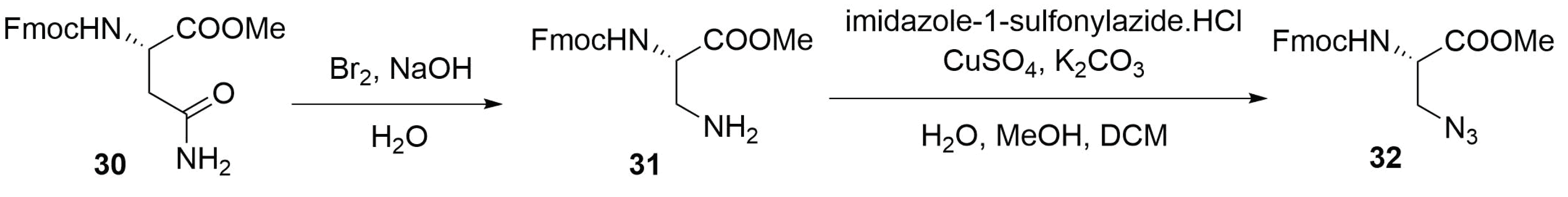
Modified Peptide Synthesis
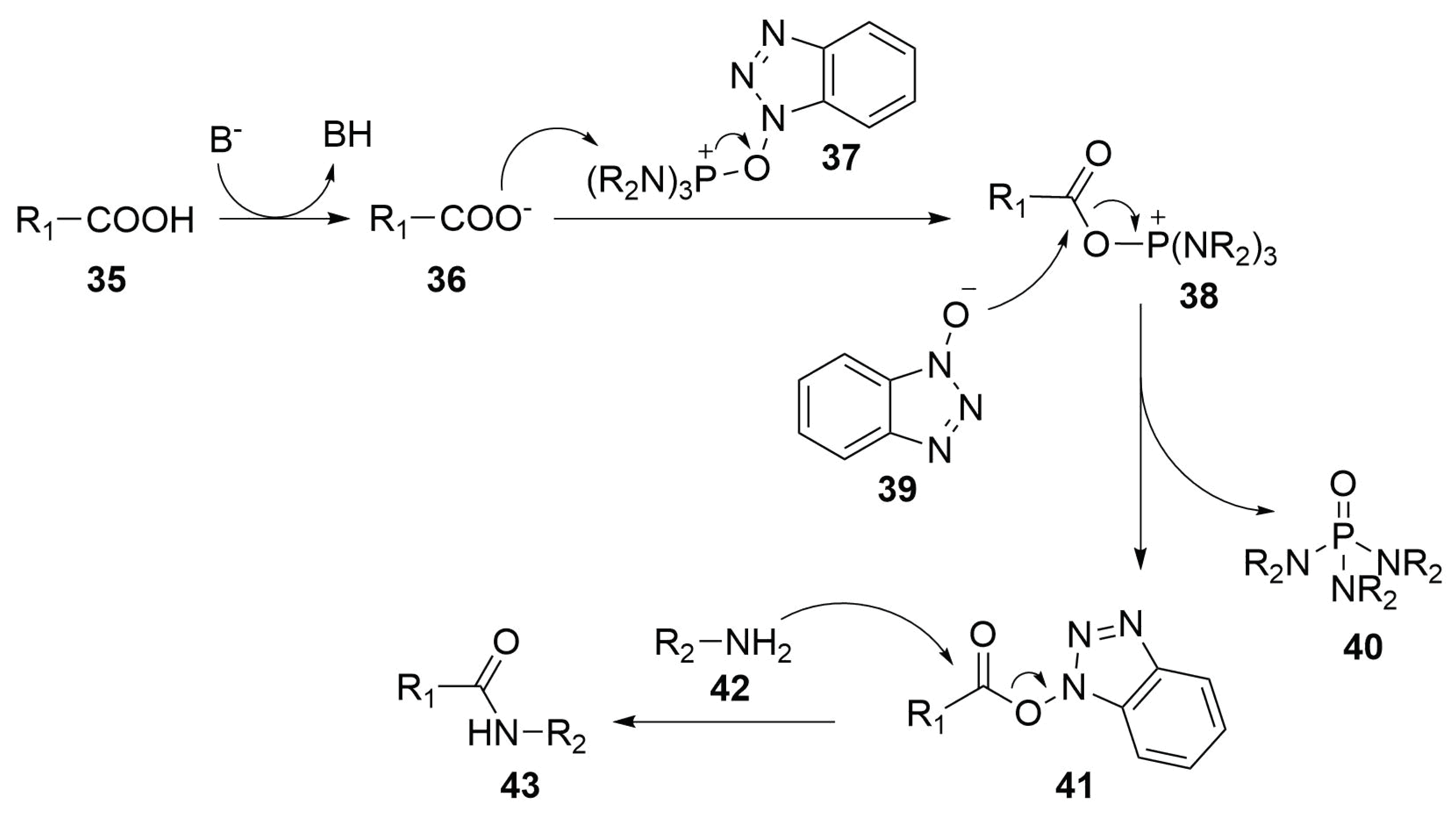
2.4.3. Lactamization Reaction
Reaction Mechanism
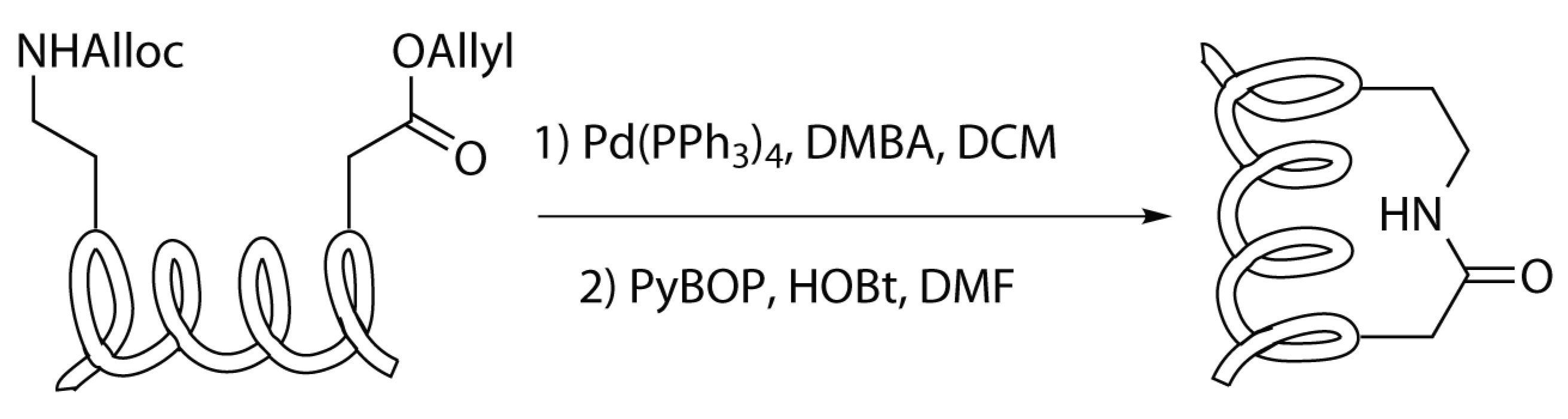
Modified Peptide Synthesis
2.4.4. Cysteine-Xylene Stapling
2.4.5. Cysteine-Perfluorobenzene Stapling
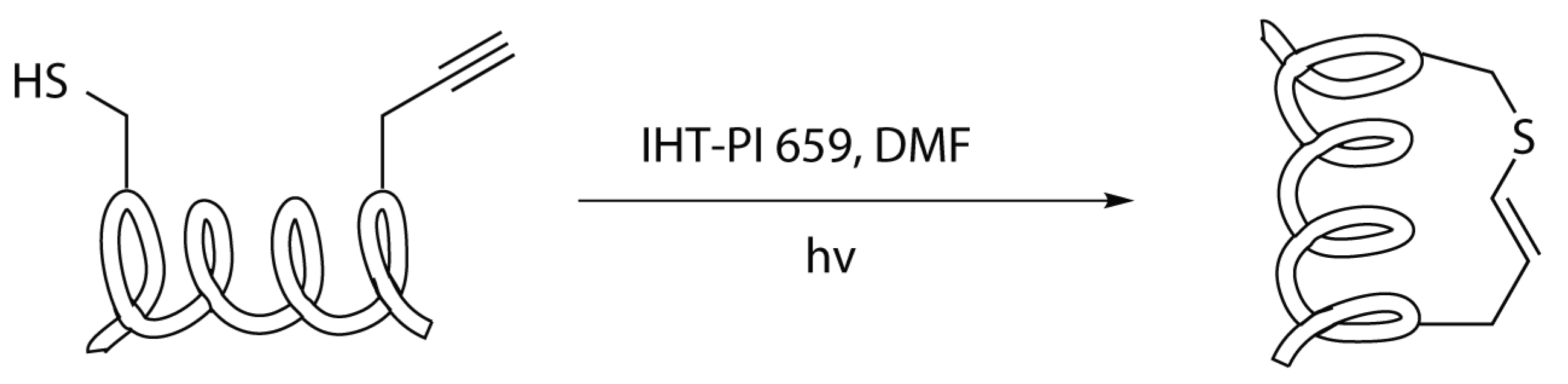
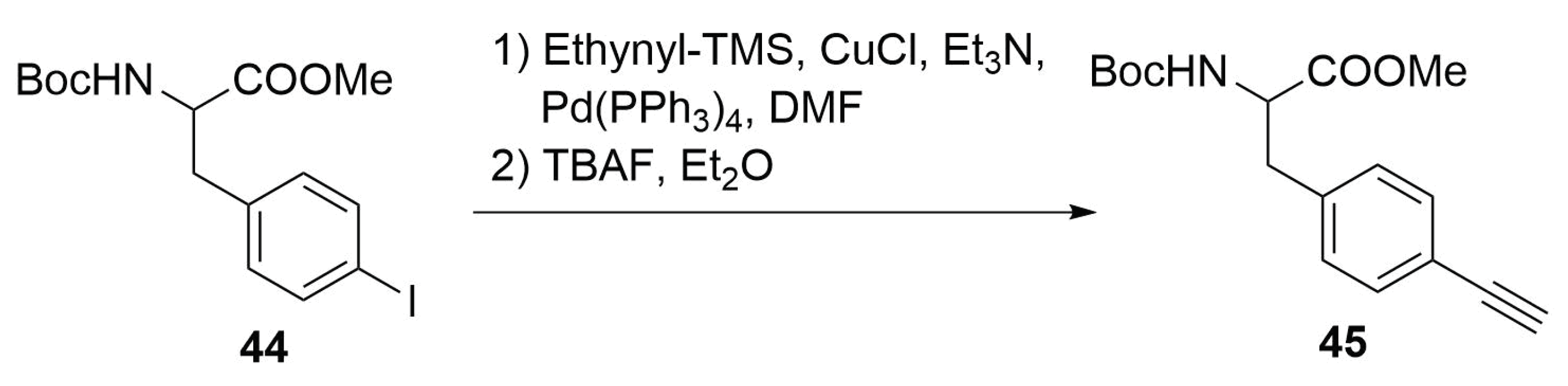
2.4.6. Thiol-yne/-ene Click Chemistry
Modified Peptide Synthesis
2.4.7. Selenocysteine Stapling
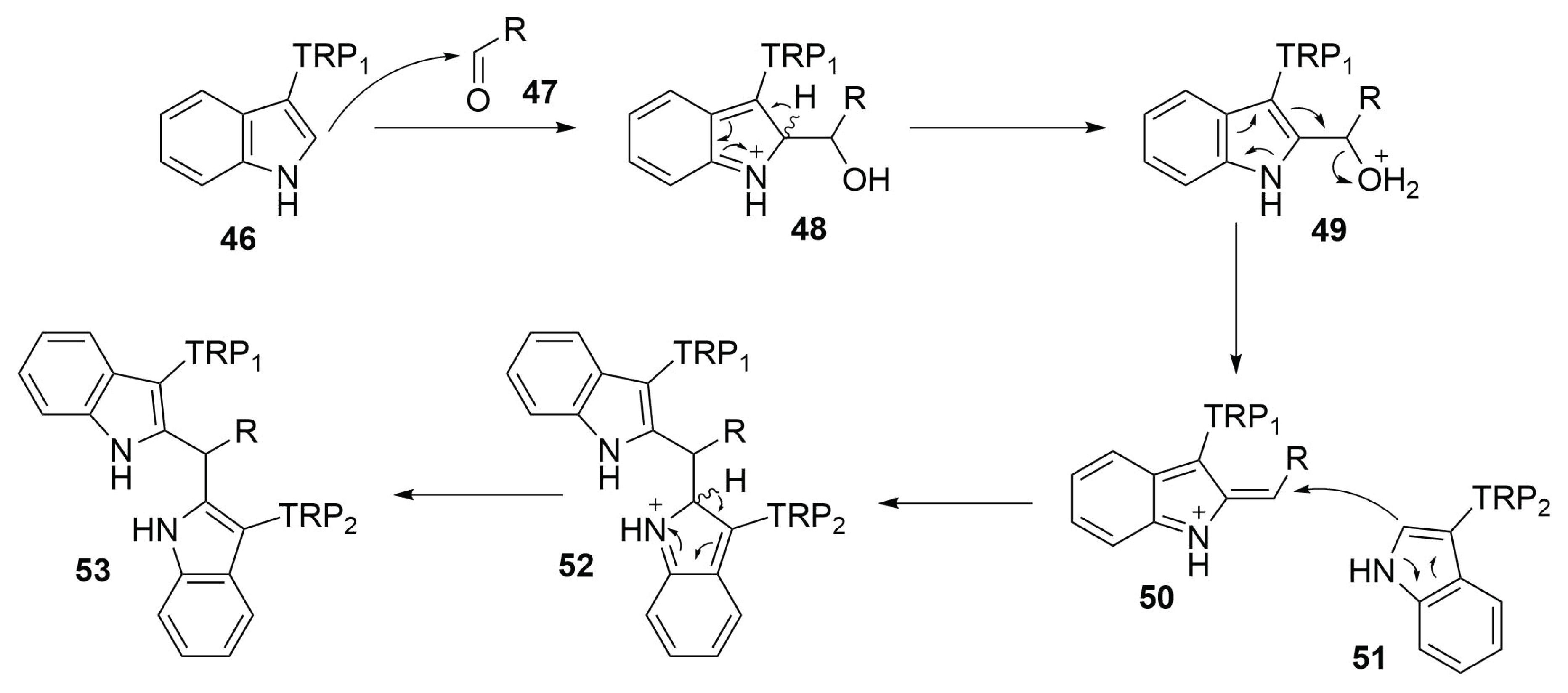
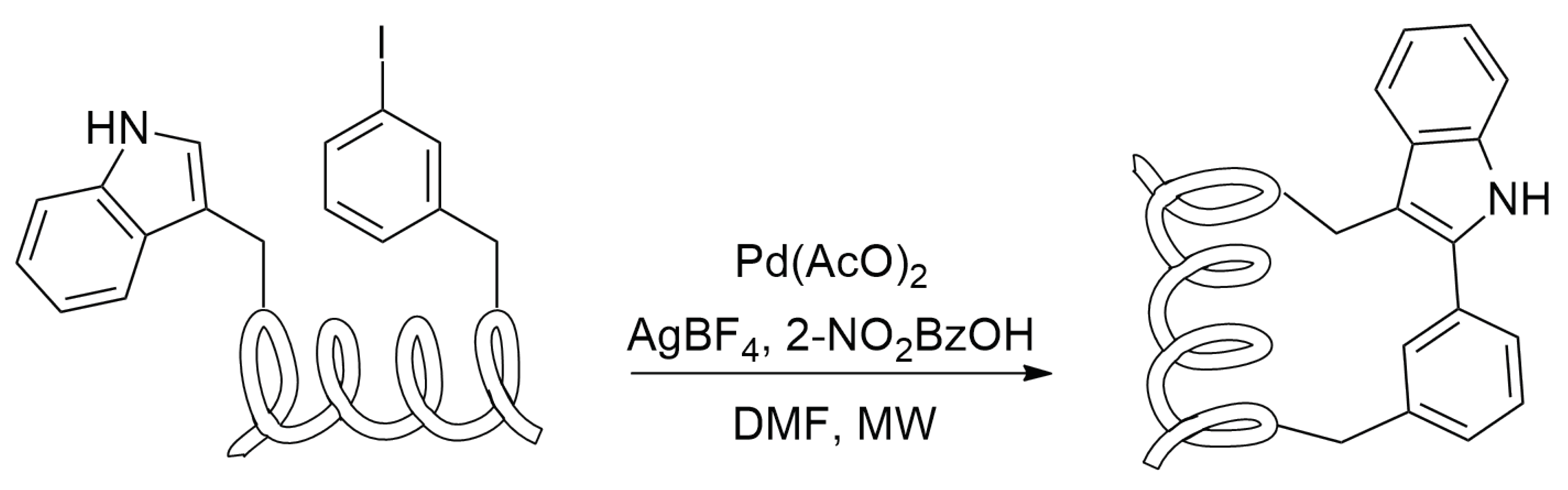
2.4.8. Tryptophan Condensation
Reaction Mechanism
2.4.9. C-H Activation
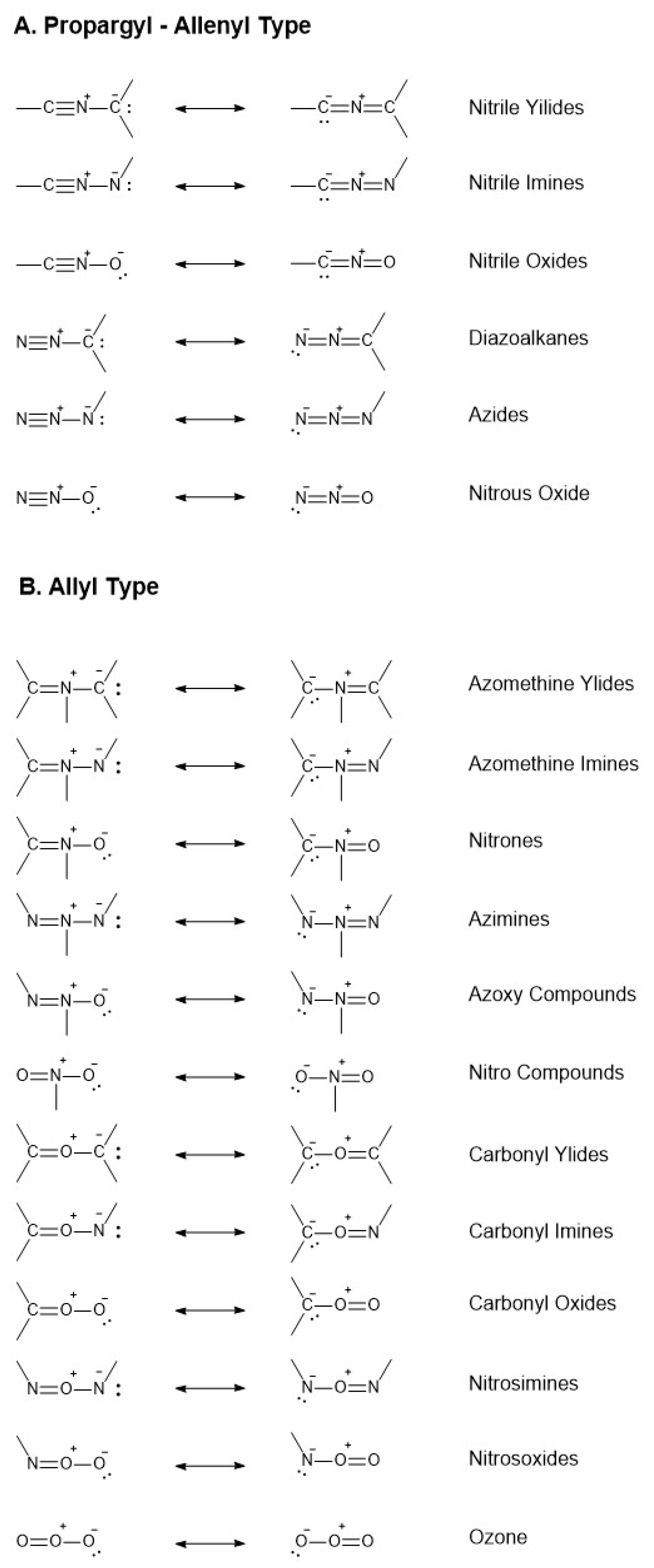
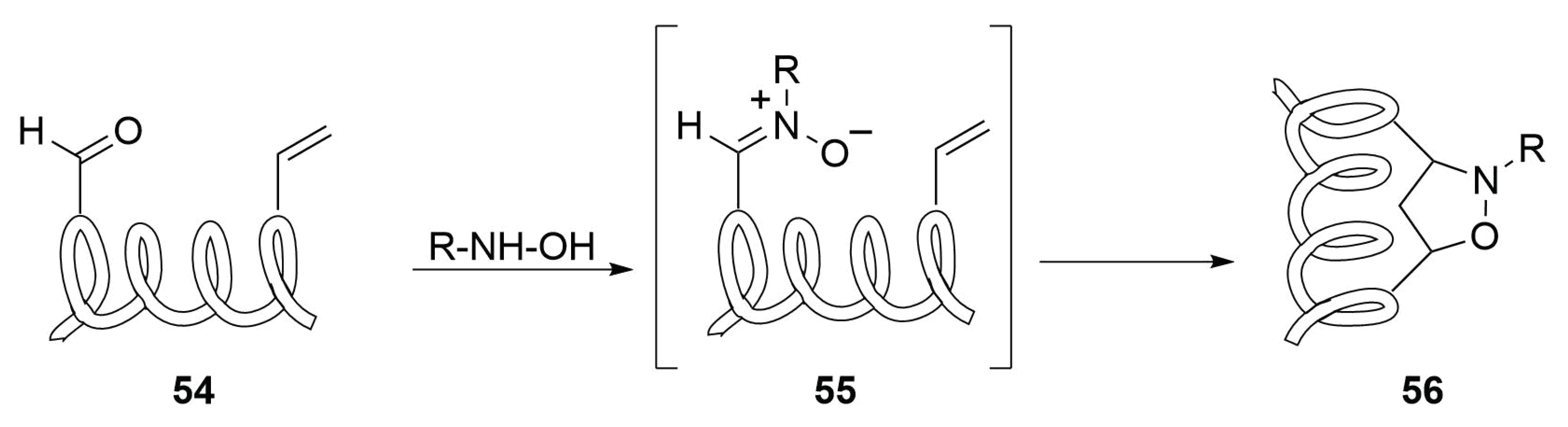
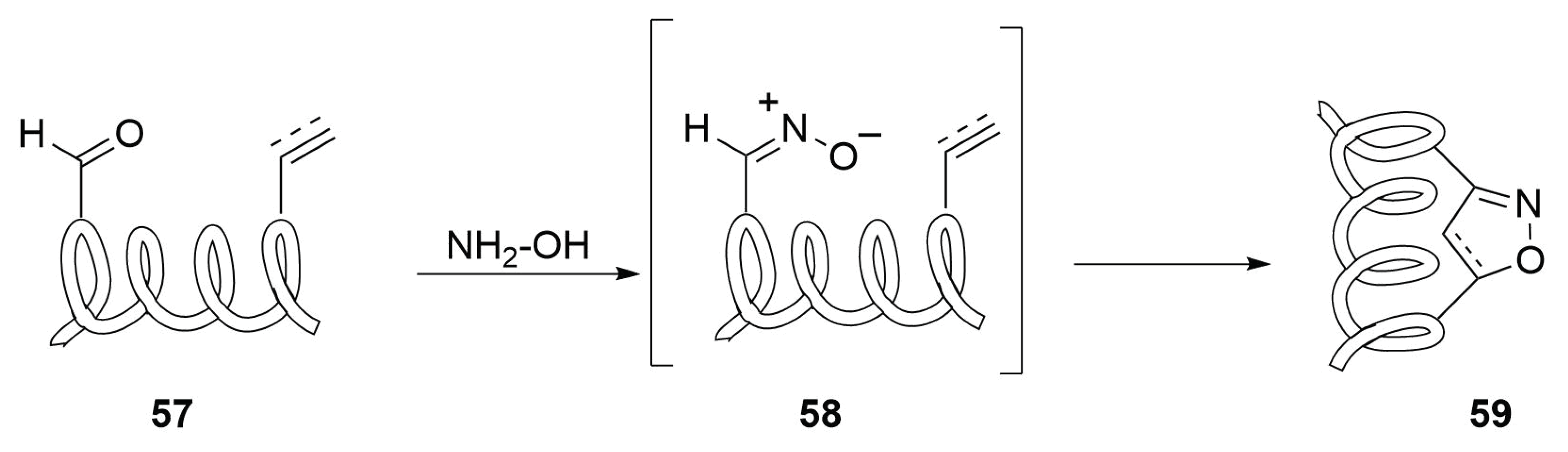
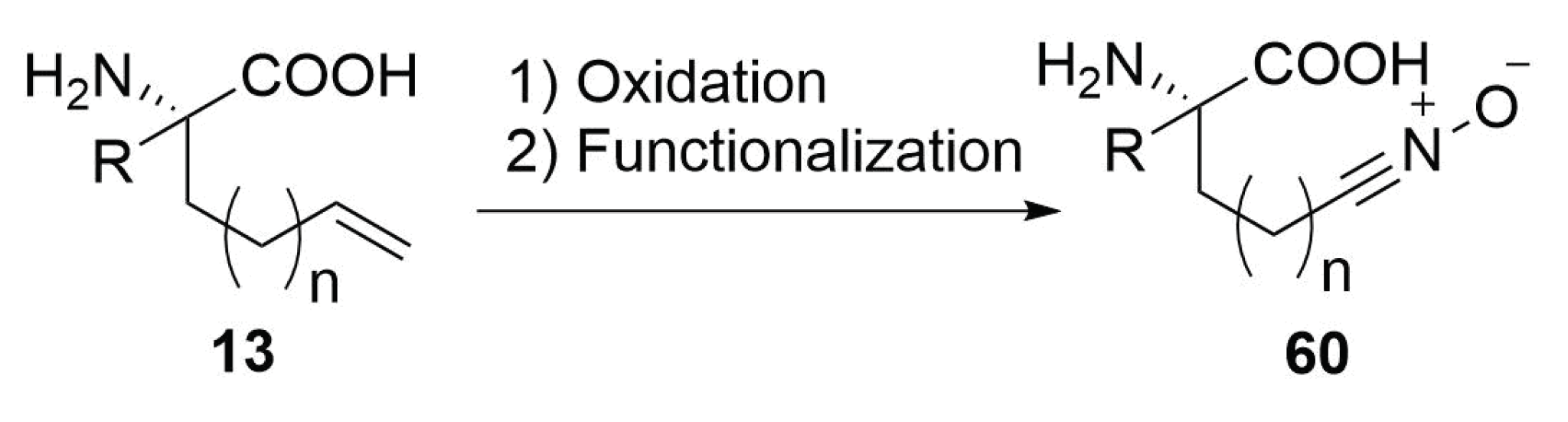
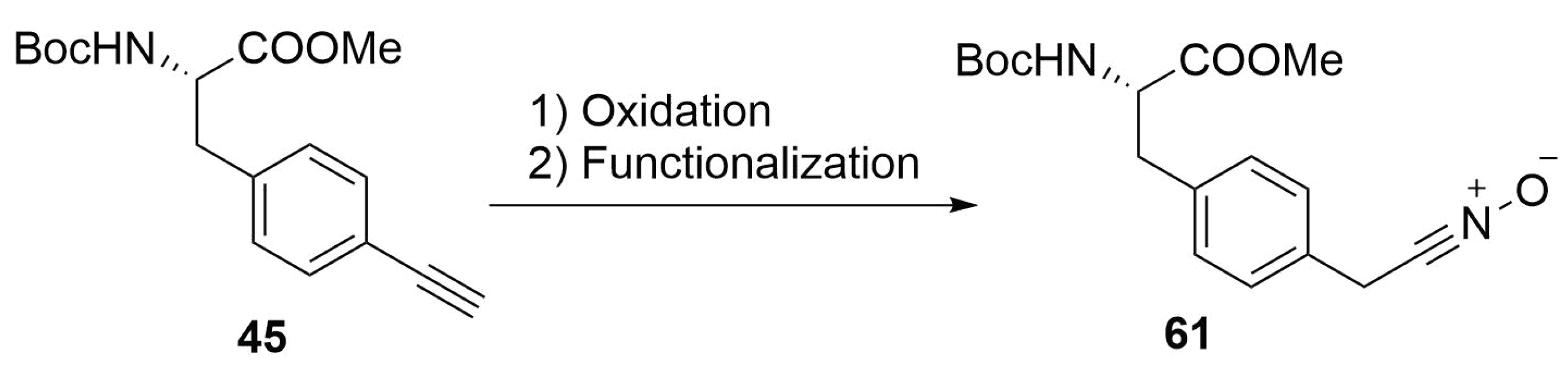
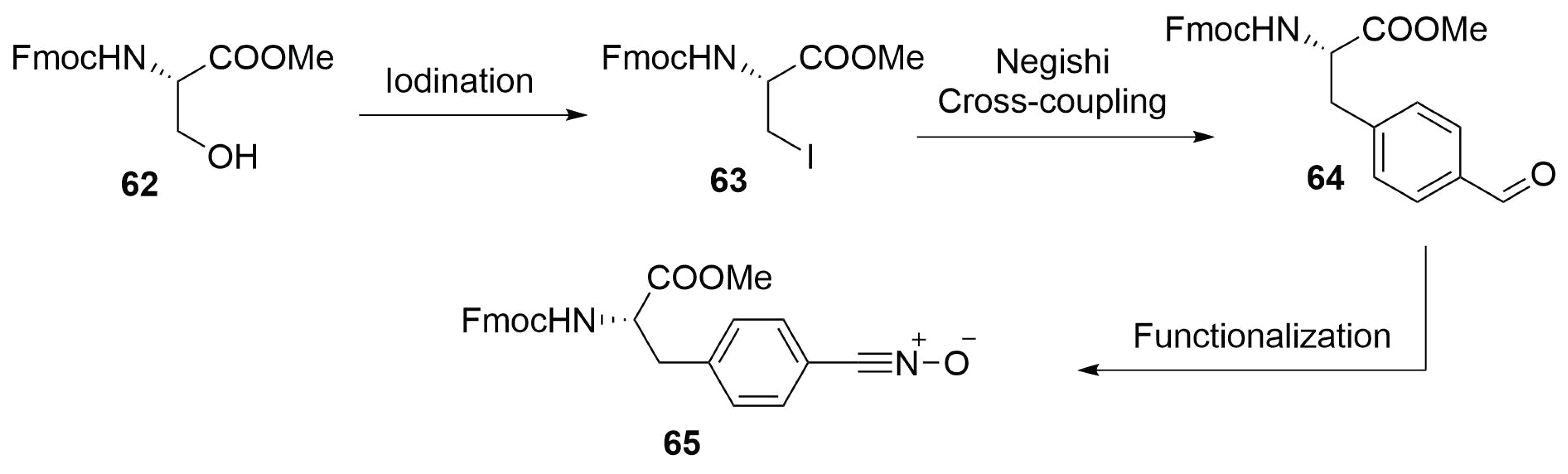
2.4.10. Future Perspectives on 1,3-Dipolar Cycloaddition for Stapling
Modified Peptide Synthesis
3. Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M. Lehninger’s Principles of Biochemistry, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2005. [Google Scholar]
- Osborne, T.B. The Vegetable Protein; Longman Harlow: London, UK, 1909. [Google Scholar]
- Mulder, G.J. Sur la composition de quelques substances animales. Bull. Sci. Phys. Nat. Neerl. 1838, 1, 104–119. [Google Scholar]
- Harold, H. Origin of the Word ‘Protein’. Nature 1951, 168, 244. [Google Scholar]
- Kendrew, J.C.; Bodo, G.; Dintzis, H.M.; Parrish, R.G.; Wyckoff, H.; Phillips, D.C. A three-dimensional model of the myoglobin molecule obtained by X-ray analysis. Nature 1958, 181, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Siddle, K.; Hutton, J.C. Peptide Hormone Secretion/Peptide Hormone Action: A Practical Approach; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Das, S.; Bahumik, A. Protein and Peptide Drug Delivery: A Fundamental Novel Approach and Future Perspective. World J. Pharm. Pharm. Sci. 2016, 5, 763–776. [Google Scholar]
- Bliss, M. Discovery of Insulin; University of Chicago Press: Chicago, IL, USA, 1982. [Google Scholar]
- Lilly, E. Discontinue Four Insulin Products; Eli Lilly: Indianapolis, IN, USA, 2005. [Google Scholar]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Chin, J.W. Expanding and reprogramming the genetic code of cells and animals. Annu. Rev. Biochem. 2014, 83, 379–408. [Google Scholar] [CrossRef]
- Maini, R.; Umemoto, S.; Suga, H. Ribosome-mediated synthesis of natural product-like peptides via cell-free translation. Curr. Opin. Chem. Biol. 2016, 34, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; de Andrade, P.; Quah, S.; Rossmann, M.; Laraia, L.; Skold, N.; Sum, T.J.; Rowling, P.J.E.; Joseph, T.L.; Verma, C.; et al. Functionalised staple linkages for modulating the cellular activity of stapled peptides. Chem. Sci. 2014, 5, 1804–1809. [Google Scholar] [CrossRef]
- Walensky, L.D.; Bird, G.H. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J. Med. Chem. 2014, 57, 6275–6288. [Google Scholar] [CrossRef] [PubMed]
- Schafmeister, C.E.; Po, J.; Verdine, G.L. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J. Am. Chem. Soc. 2000, 122, 5891–5892. [Google Scholar] [CrossRef]
- Klein, M.J.; Schmidt, S.; Wadhwani, P.; Bürck, J.; Reichert, J.; Afonin, S.; Berditsch, M.; Schober, T.; Brock, R.; Kansy, M.; et al. Lactam-Stapled Cell-Penetrating Peptides: Cell Uptake and Membrane Binding Properties. J. Med. Chem. 2017, 60, 8071–8082. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.H.; Madani, N.; Perry, A.F.; Princiotto, A.M.; Supko, J.G.; He, X.; Gavathiotis, E.; Sodroski, J.G.; Walensky, L.D. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Natl. Acad. Sci. USA 2010, 107, 14093–14098. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.S. The anatomy and taxonomy of protein structure. Adv. Protein Chem. 1981, 34, 167–339. [Google Scholar] [PubMed]
- Terwilliger, J.C. Rapid model building of alpha-helices in electron-density maps. Acta Crystallogr. Sect. D 2010, 66, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Lovell, S.C.; Davis, I.; Arendall, W.B.; de Bakker, P.I. Structure validation by Cα geometry: ϕ,ψ and Cβ deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Pace, C.N.; Scholtz, J.M. A helix propensity scale based on experimental studies of peptides and proteins. Biophys. J. 1998, 75, 422–427. [Google Scholar] [CrossRef]
- Verdine, G.L.; Hilinski, G.J. Stapled Peptides for Intracellular Drug Targets. Methods Enzymol. 2012, 503, 3–33. [Google Scholar]
- Xie, X.; Gao, L.; Shull, A.Y.; Teng, Y. Stapled peptides: Providing the best of both worlds in drug development. Future Med. Chem. 2016, 8, 1969–1980. [Google Scholar] [CrossRef]
- van den Berg, A.; Dowdy, S.F. Protein transduction domain delivery of therapeutic macromolecules. Curr. Opin. Biotechnol. 2011, 22, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jiang, Y.; Li, J.; Wang, D.; Zhao, H.; Li, Z. Effect of Stapling Architecture on Physiochemical Properties and Cell Permeability of Stapled α-Helical Peptides: A Comparative Study. ChemBioChem 2017, 18, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, A.D.; Hoang, H.N.; Kok, W.M.; Diness, F.; Gupta, P.; Hill, T.A.; Driver, R.W.; Price, D.A.; Liras, S.; Fairlie, D.P. Comparative α-Helicity of Cyclic Pentapeptides in Water. Angew. Chem. Int. Ed. 2014, 53, 6965–6969. [Google Scholar] [CrossRef] [PubMed]
- Rezgui, R.; Blumer, K.; Yeoh-Tan, G.; Trexler, A.J.; Magzoub, M. Precise Quantification of Cellular Uptake of Cell-penetrating Peptides Using Fluorescence-activated Cell Sorting and Fluorescence Correlation Spectroscopy. Biochim. Biophys. Acta 2016, 1858, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.S.T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, P.L.; Bonvin, A.M.J.J. On the Binding Affinity of Macromolecular Interactions: Daring to Ask Why Proteins Interact. J. R. Soc. Interface 2013, 10, 20120835. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.Y.; Rout, B.; Tan, Y.S.; Verma, C.S.; Chan, K.; Johannes, C.W. An intramolecular tryptophan-condensation approach for peptide stapling. Org. Biomol. Chem. 2018, 16, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, J.; Zhao, H.; Zeng, X.; Wang, D.; Liu, Q.; Niu, X.; Huang, X.; Xu, N.; Li, Z. Stapling of unprotected helical peptides via photo-induced intramolecular thiol-yne hydrothiolation. Chem. Sci. 2016, 7, 3325–3330. [Google Scholar] [CrossRef]
- Sakagami, K.; Masuda, T.; Kawano, K.; Futaki, S. Importance of Net Hydrophobicity in the Cellular Uptake of All-Hydrocarbon Stapled Peptides. Mol. Pharm. 2018, 15, 1332–1340. [Google Scholar] [CrossRef]
- Monfette, S.; Fogg, D.E. Equilibrium Ring-Closing Metathesis. Chem. Rev. 2009, 109, 3783–3816. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Johnson, L.K.; Grubbs, R.H.; Ziller, J.W. Ring-opening metathesis polymerization (ROMP) of norbornene by a Group VIII carbene complex in protic media. J. Am. Chem. Soc. 1992, 114, 3974–3975. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R.; Murdzek, J.S.; Bazan, G.C.; Robbins, J.; Dimare, M.; O’Regan, M. Synthesis of molybdenum imido alkylidene complexes and some reactions involving acyclic olefins. J. Am. Chem. Soc. 1990, 112, 3875–3886. [Google Scholar] [CrossRef]
- Yuen, T.Y.; Brown, C.J.; Xue, Y.; Sing Tan, Y.; Ferrer Gago, F.J.; Lee, X.E.; Neo, J.Y.; Thean, D.; Kristal Kaan, H.Y.; Partridge, A.W.; et al. Stereoisomerism of Stapled Peptide Inhibitors of the p53-Mdm2 Interaction: An Assessment of Synthetic Strategies and Activity Profiles. Chem. Sci. 2019, 10, 6457–6466. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals, 6th ed.; Wiley J. & Sons: New York, NY, USA, 2014. [Google Scholar]
- Grossman, R.B. Transition-Metal-Catalyzed and Mediated Reactions. The Art of Writing Reasonable Organic Reaction Mechanisms, 2nd ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Tatum, N.J.; Yufit, D.S.; Cobb, S.L.; Coxon, C.R. Synthesis, Ni(II) Schiff base complexation and structural analysis of fluorinated analogs of the ligand (S)-2-[N-(N’-benzylprolyl)amino]benzophenone (BPB). J. Fluor. Chem. 2015, 173, 77–83. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef]
- Johansson, H.; Pedersen, D.S. Azide- and Alkyne-Derivatised α-Amino Acids. Eur. J. Org. Chem. 2012, 2012, 4267–4281. [Google Scholar] [CrossRef]
- Coste, J.; Le-Nguyen, D.; Castro, B. PyBOP: A new peptide coupling reagent devoid of toxic by-product. Tetrahedron Lett. 1990, 31, 4267–4281. [Google Scholar] [CrossRef]
- Wall, L.A.; Pummer, W.J.; Fearn, J.E.; Antonucci, J.M. Reactions of Polyfluorobenzenes with Nucleophilic Reagents. J. Res. Natl. Bur. Stand. A Phys. Chem. 1963, 67A, 481–504. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Zou, Y.; Ling, J.J.; Yu, H.; Lin, Y.; Pentelute, B.L. A Perfluoroaryl-Cysteine SNAr Chemistry Approach to Unprotected Peptide Stapling. J. Am. Chem. Soc. 2013, 135, 5946–5949. [Google Scholar] [CrossRef] [PubMed]
- Perell, G.T.; Staebell, R.L.; Hairani, M.; Cembran, A.; Pomerantz, W.C.K. Tuning Sulfur Oxidation States on Thioether-Bridged Peptide Macrocycles for Modulation of Protein Interactions. ChemBioChem 2017, 18, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Santandrea, J.; Minozzi, C.; Cruché, C.; Collins, S.K. Photochemical Dual-Catalytic Synthesis of Alkynyl Sulfides. Angew. Chem. 2017, 56, 12255–12259. [Google Scholar] [CrossRef]
- de Araujo, A.D.; Perry, S.R.; Fairlie, D.P. Chemically Diverse Helix-Constrained Peptides Using Selenocysteine Crosslinking. Org. Lett. 2018, 20, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Akgun, E.; Tunali, M. Acid catalyzed aldehyde-indole reaction, synthesis of diindolylantipyrylmethane dyes. Archiv Pharm. 1988, 321, 921–924. [Google Scholar] [CrossRef]
- Mendive-Tapia, L.; Preciado, S.; Garcia, J.; Ramon, R.; Kielland, N.; Albericio, F.; Lavilla, R. New Peptide Architectures Through C–H Activation Stapling Between Tryptophan–phenylalanine/tyrosine Residues. Nat. Commun. 2015, 6, 7160–7168. [Google Scholar] [CrossRef]
- Wang, X.; Lane, B.S.; Sames, D. Direct C-Arylation of Free (NH)-Indoles and Pyrroles Catalyzed by Ar-Rh(III) Complexes Assembled In Situ. J. Am. Chem. Soc. 2005, 127, 4996–4997. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lorion, M.M.; Ackermann, L. Air-StableManganese(I)-Catalyzed C-H Activation for Decarboxylative C-H/C-O Cleavages in Water. Angew. Chem. Int. Ed. 2017, 56, 6339–6342. [Google Scholar] [CrossRef]
- Phipps, R.J.; Grimster, N.P.; Gaunt, M.J. Cu(II)-catalyzed Direct and Site-selective Arylation of Indoles Under Mild Conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Larrosa, I. On Water, Phosphine-free Palladium-catalyzed Room Temperature C-H Arylation of Indoles. Chem. A Eur. J. 2013, 19, 15093–15096. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhang, G.; Liu, T.; Giri, R.; Yu, J. Site-selective C(sp3)-H Functionalization of Di-, Tri-, and Tetrapeptides at the N-Terminus. J. Am. Chem. Soc. 2014, 136, 16940–16946. [Google Scholar] [CrossRef] [PubMed]
- Noisier, A.F.M.; Garcia, J.; Ionut, I.A.; Albericio, F. Stapled Peptides by Late-Stage C(sp3)-H Activation. Angew. Chem. Int. Ed. 2017, 56, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A. (Ed.) 1,3-Dipolar Cycloaddition Chemistry; Wiley J. & Sons: New York, NY, USA, 1984; Volumes 1 and 2. [Google Scholar]
- Padwa, A.; Pearson, W.H. (Eds.) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products; Wiley J. & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon Press: Oxford, UK, 1990; pp. 269–331. [Google Scholar]
- Tufariello, J.J. Nitrones; Wiley J. & Sons: New York, NY, USA, 1984; Volume 2, pp. 83–115. [Google Scholar]
- Caramella, P.; Grunanger, P. Nitrile Oxides and Imines; Wiley J. & Sons: New York, NY, USA, 1984; Volume 1, pp. 291–392. [Google Scholar]
- Heaney, F. Nitrile Oxide/Alkyne Cycloadditions—A Credible Platform for Synthesis of Bioinspired Molecules by Metal-Free Molecular Clicking. Eur. J. Org. Chem. 2012, 2012, 3043–3058. [Google Scholar] [CrossRef]
- Minuti, L.F.; Memeo, M.G.; Crespi, S.; Quadrelli, P. Fluorescent Probes from Stable Aromatic Nitrile Oxides. Eur. J. Org. Chem. 2016, 4, 821–829. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Qu, J.; Madden, M.M.; Lin, Q. A Photoinducible 1,3-Dipolar Cycloaddition Reaction for Rapid, Selective Modification of Tetrazole-Containing Proteins. Angew. Chem. Int. Ed. 2008, 47, 2832–2835. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Bratz, M. Dialkyl Mesoxalates by Ozonolysis of Dialkyl Benzalmalonates. Org. Synth. Collect. Vol. 2003, 9, 31. [Google Scholar]
- Tomio, S.; Yoshiyuki, H.; Hiroshi, S.; Kazuhiro, T. A Convenient Preparative Method of Nitrile Oxides by the Dehydration of Primary Nitro Compounds with Ethyl Chloroformate or Benzenesulfonyl Chloride in the Presence of Triethylamine. Bull. Chem. Soc. Jpn. 1986, 59, 2827–2831. [Google Scholar]
- Mukaiyama, T.; Hoshino, T. The Reactions of Primary Nitroparaffins with Isocyanates. J. Am. Chem. Soc. 1960, 82, 5339–5342. [Google Scholar] [CrossRef]
- Loudon, M.G. Organic Chemistry—Addition Reactions of Alkenes, 4th ed.; Oxford University: Oxford, UK, 2002. [Google Scholar]
- Oswald, C.L.; Carrillo-Marquez, T.; Caggiano, L.; Jackson, R.F.W. Negishi Cross-Coupling Reactions of α-Amino Acid-Derived Organozinc Reagents and Aromatic Bromides. ChemInform 2008, 39, 681–687. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, F.; Zhao, B.; Lin, H.; Xie, M.; Bai, G.; Gilbert, A.M.; Goetz, G.H.; Liras, S.; Mathiowetz, A.A.; et al. Chiral Sulfoxide-Induced Single Turn Peptide α-Helicity. Sci. Rep. 2016, 6, 38573. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Moellering, R.E.; Hilinsku, G.J.; Kim, Y.; Grossmann, T.N.; Yeh, J.T.; Verdine, G.L. Towards Understanding Cell Penetration by Stapled Peptides. Med. Chem. Commun. 2015, 6, 111–119. [Google Scholar] [CrossRef]
- Serrano, J.C.; Sipthorp, J.; Xu, W.; Itzhaki, L.S.; Ley, S.V. A New Methodology for Incorporating Chiral Linkers into Stapled Peptides. ChemBioChem 2017, 18, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Geng, H.; Zhang, Q.; Liu, Q.; Xie, M.; Sun, C.; Li, W.; Lin, H.; Jiang, F.; Wang, T.; et al. An In-tether Chiral Center Modulates the Helicity, Cell Permeability, and Target Binding Affinity of a Peptide. Angew. Chem. Int. Ed. 2016, 55, 8013–8071. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.; Ruiz-Gomez, G.; Hill, T.A.; Hoang, H.N.; Fairlie, D.P.; Mason, J.M. Truncated and Helix-Constrained Peptides with High Affinity and Specificity for the cFos Coiled-Coil of AP-1. PLoS ONE 2013, 8, e59415. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Murcia, P.A.; Ruiz-Santaquiteria, M.; Toro, M.A.; de Lucio, H.; Jimenez, M.A.; Gago, F.; Jimenez-Ruiz, A.; Camarasa, M.; Velazquez, S. Comparison of Hydrocarbon- and Lactam- Bridged Cyclic Peptides as Dimerization Inhibitors of Leishmania Infantum Trypanothione Reductase. RSC Adv. 2015, 5, 55784–55794. [Google Scholar] [CrossRef]
- Wang, Y.; Bruno, B.J.; Cornillie, S.; Nogieira, J.M.; Chen, D.; Cheatham, T.E.; Lim, C.S.; Chou, D.H. Application of Thiol-yne/Thiol–ene Reactions for Peptide and Protein Macrocyclizations. Chem. A Eur. J. 2017, 23, 7087–7092. [Google Scholar] [CrossRef] [PubMed]
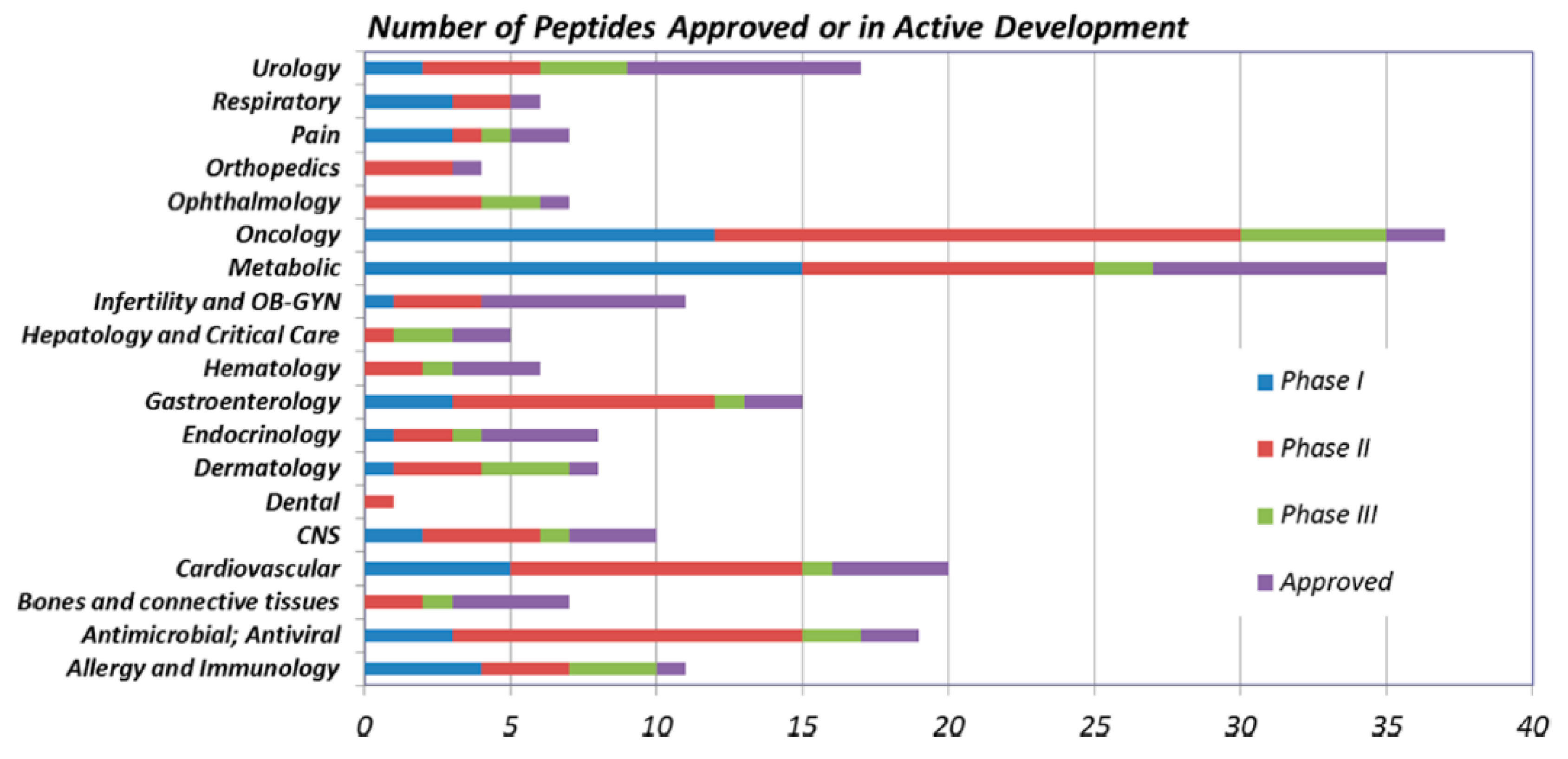







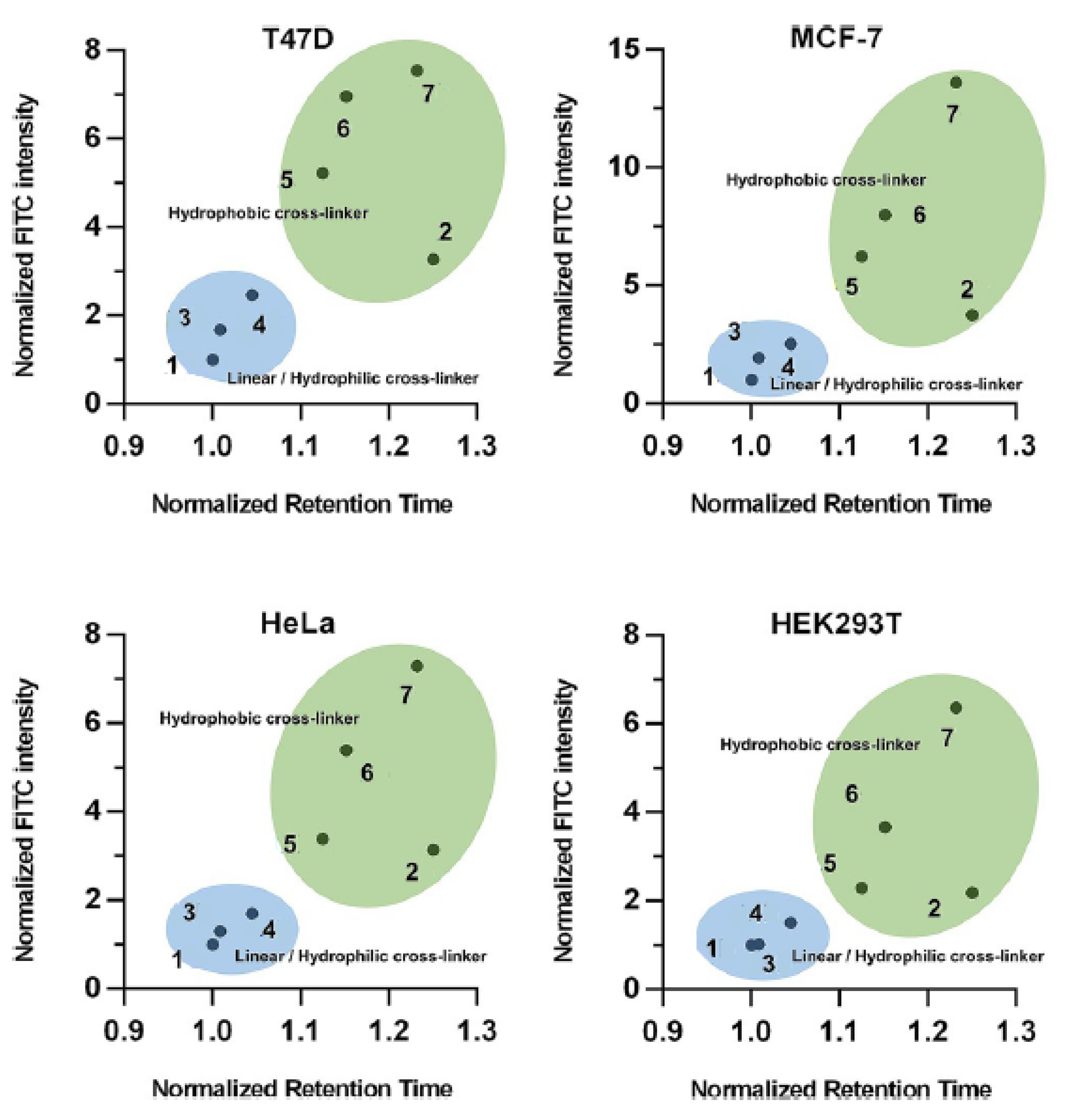
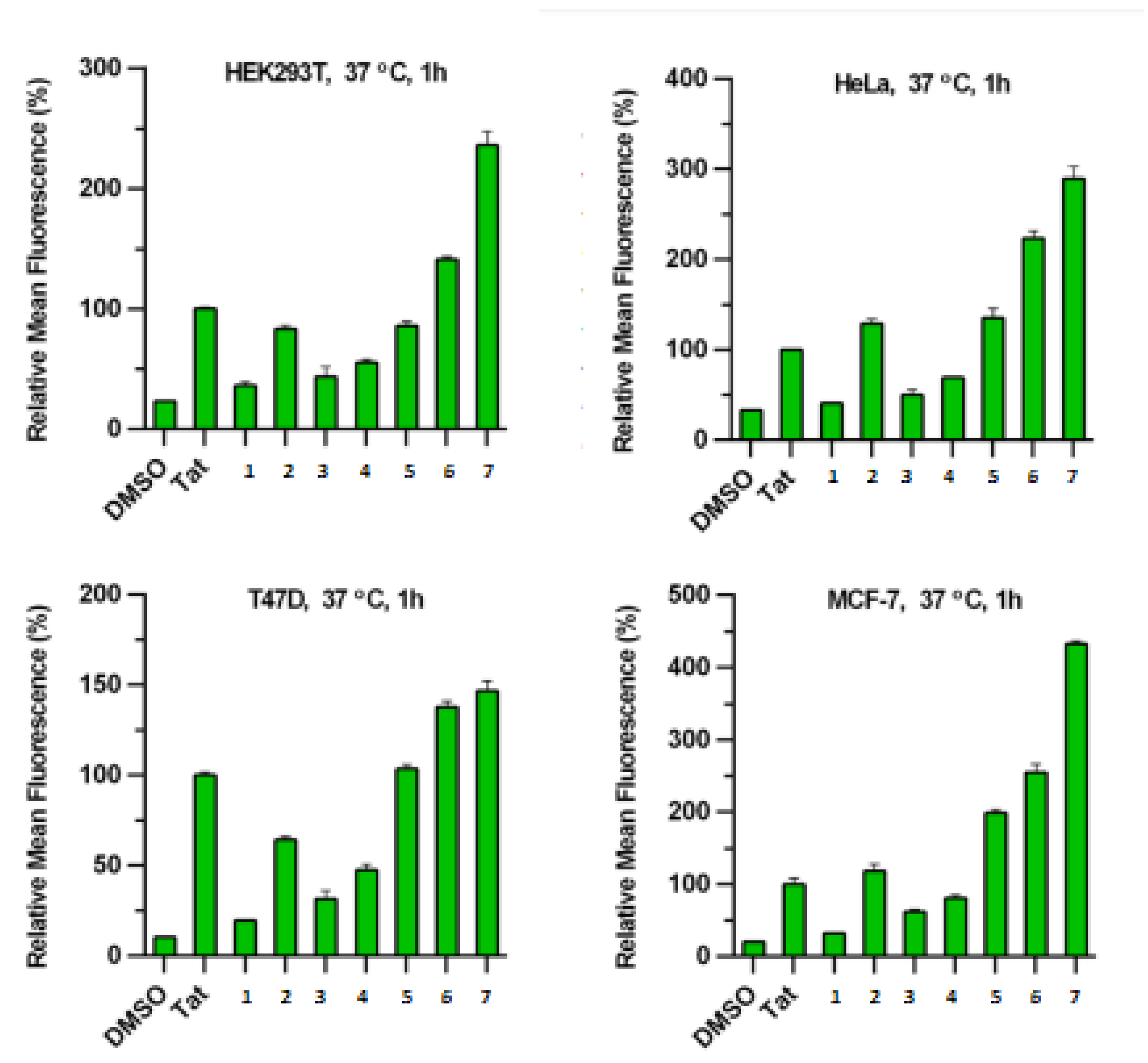
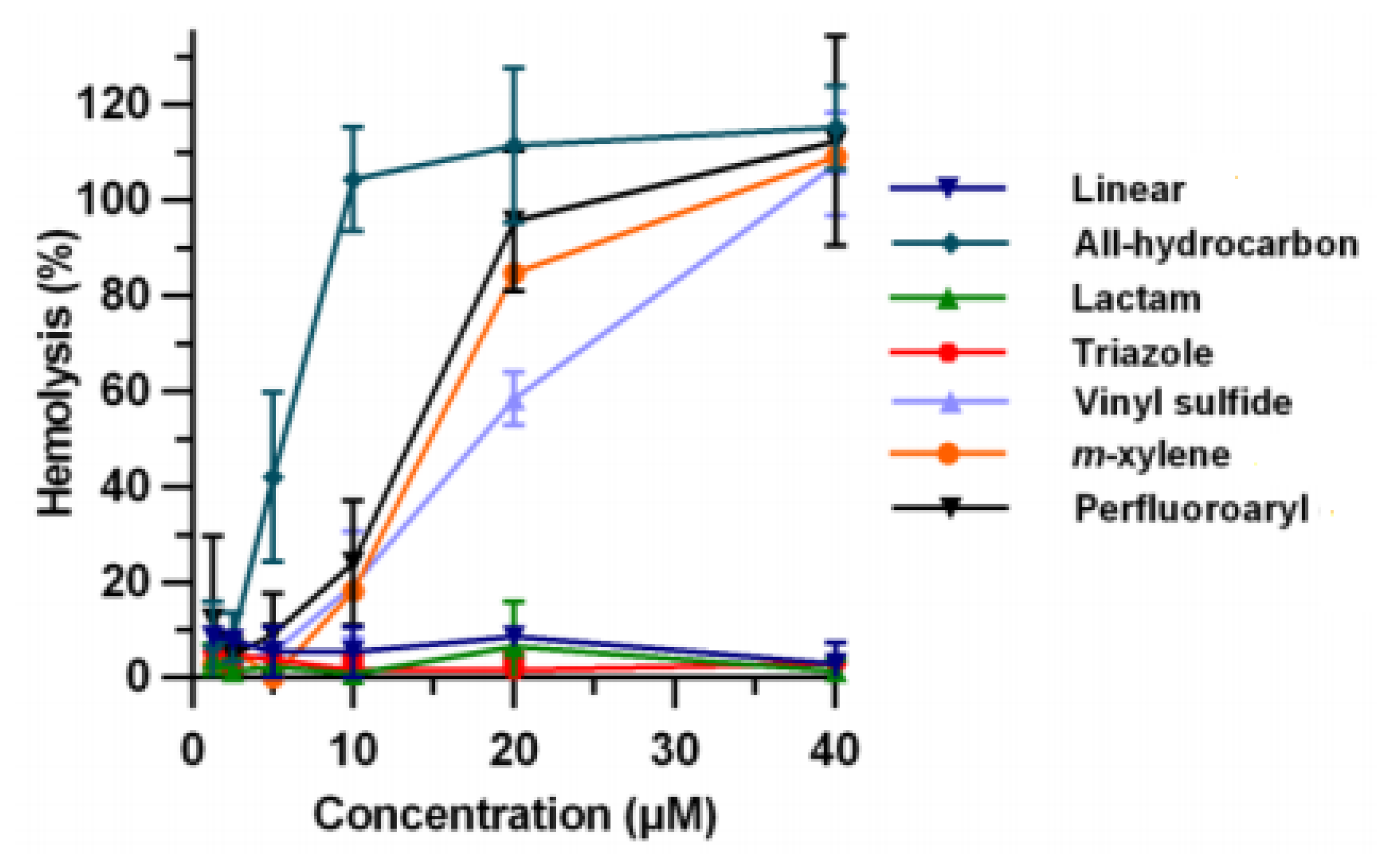
| Name | Year of Approval | Therapeutic Area | Name | Year of Approval | Therapeutic Area |
|---|---|---|---|---|---|
| atosiban | 2000 | obstetrics | mifamurtide | 2009 | oncology |
| taltirelin | 2000 | CNS | liraglutide | 2009 | metabolic disease |
| aviptadil | 2000 | urology | tesamorelin | 2010 | antiinfective |
| carbetocin | 2001 | obstetrics | lucinactant | 2012 | pulmnary |
| nesiritide | 2001 | cardiovascular | peginesatide | 2012 | hematology |
| teriparatide | 2002 | osteoporosis | pasireotide | 2012 | endocinology |
| enfuvirtide | 2003 | antiinfective | carfilzomib | 2012 | oncology |
| abarelix | 2003 | oncology | linaclotide | 2012 | gastroenterology |
| ziconotide | 2004 | pain | teduglutide | 2012 | gastroenterology |
| pramlintide | 2005 | metabolic disease | lixisenatide | 2013 | metabolic disease |
| exenatide | 2005 | metabolic disease | albiglutide | 2014 | metabolic disease |
| icatibant | 2008 | hematology | oritavancin | 2014 | antiinfective |
| romiplostim | 2008 | hematology | dulagutide | 2014 | metabolic disease |
| degarelix | 2008 | oncology | afamelanotide | 2014 | dermatology |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiola, M.; Memeo, M.G.; Quadrelli, P. Stapled Peptides—A Useful Improvement for Peptide-Based Drugs. Molecules 2019, 24, 3654. https://doi.org/10.3390/molecules24203654
Moiola M, Memeo MG, Quadrelli P. Stapled Peptides—A Useful Improvement for Peptide-Based Drugs. Molecules. 2019; 24(20):3654. https://doi.org/10.3390/molecules24203654
Chicago/Turabian StyleMoiola, Mattia, Misal G. Memeo, and Paolo Quadrelli. 2019. "Stapled Peptides—A Useful Improvement for Peptide-Based Drugs" Molecules 24, no. 20: 3654. https://doi.org/10.3390/molecules24203654
APA StyleMoiola, M., Memeo, M. G., & Quadrelli, P. (2019). Stapled Peptides—A Useful Improvement for Peptide-Based Drugs. Molecules, 24(20), 3654. https://doi.org/10.3390/molecules24203654






