Abstract
[TiCp2S5] (phase A), [TiCp2Se5] (phase F), and five solid solutions of mixed titanocene selenide sulfides [TiCp2SexS5−x] (Cp = C5H5−) with the initial Se:S ranging from 1:4 to 4:1 (phases B–E) were prepared by reduction of elemental sulfur or selenium or their mixtures by lithium triethylhydridoborate in thf followed by the treatment with titanocene dichloride [TiCp2Cl2]. Their 77Se and 13C NMR spectra were recorded from the CS2 solution. The definite assignment of the 77Se NMR spectra was based on the PBE0/def2-TZVPP calculations of the 77Se chemical shifts and is supported by 13C NMR spectra of the samples. The following complexes in varying ratios were identified in the CS2 solutions of the phases B–E: [TiCp2Se5] (51), [TiCp2Se4S] (41), [TiCp2Se3S2] (31), [TiCp2SSe3S] (36), [TiCp2SSe2S2] (25), [TiCp2SSeS3] (12), and [TiCp2S5] (01). The disorder scheme in the chalcogen atom positions of the phases B–E observed upon crystal structure determinations is consistent with the spectral assignment. The enthalpies of formation calculated for all twenty [TiCp2SexS5−x] (x = 0–5) at DLPNO-CCSD(T)/CBS level including corrections for core-valence correlation and scalar relativistic, as well as spin-orbit coupling contributions indicated that within a given chemical composition, the isomers of most favourable enthalpy of formation were those, which were observed by 77Se and 13C NMR spectroscopy.
1. Introduction
Titanocene pentasulfide [bis(cyclopentadienyl)pentasulfidotitanium] and titanocene penta-selenide [bis(cyclopentadienyl)pentaselenidotitanium] {the general formula [TiCp2E5] (Cp = C5H5− or its alkyl-substituted derivative; E = S, Se)}, as well as the related dinuclear complexes [TiCp2(μ-En)2TiCp2] (n = 2 or 3, the latter in case of sulfur) have long been known as convenient chalcogen atom transfer reagents (for some selected reviews see [1,2,3,4,5,6,7,8]). These reagents are particularly useful in the preparation of homocyclic sulfur and selenium ring molecules as well as individual heterocyclic selenium sulfides, which are generally formed only in complicated molecular mixtures.
Crystal structures have been reported for mononuclear titanocene pentasulfide [9,10,11,12], pentaselenide [13,14], and mixed titanocene selenide sulfides [15,16,17], as well as the related dinuclear complexes [18,19,20,21]. Their NMR spectroscopic properties are also well known [15,16,18,19,20,21,22,23,24,25,26,27]. This information was utilized in the preliminary identification of individual molecular components in the mixtures of titanocene selenide sulfides [TiCp2SexS5−x] [15]. The assignment of the 77Se NMR spectra of mixtures containing different initial molar ratios of sulfur and selenium was based on the consideration of the spectroscopic data of [TiCp2Se5] [25], on the constant intensities between some resonances, as the Se:S ratio is varied, and on the trends in the 77Se chemical shifts [28,29,30]. This semi-quantitative analysis has then been utilized in resolving the disorder in the chalcogen atom positions in the crystalline [TiCp2SexS5−x] phase, which was prepared using the initial Se:S molar ratio of 3:2 [15]. The composition was further verified by treating the [TiCp2SexS5−x] mixture with Se2Cl2 or S2Cl2 and determining the composition of the two product mixtures using 77Se NMR spectroscopy [31].
In this contribution, we extend these earlier studies by considering the stabilities of different [TiCp2SexS5−x] complexes using high-level DFT and domain based local pair natural orbital coupled cluster [DLPNO-CCSD(T)] computations. We also verify the earlier spectroscopic assignments by computing the 77Se shielding tensors and chemical shifts utilizing the methodology, which has proven suitable for molecular selenium species [32]. For understanding the trends in the formation of individual complexes, we have considered six different syntheses by varying the initial Se:S molar ratio in the preparations. The designation of the product phases are as follows: Phase A (only sulfur), phase B (Se:S = 1:4), phase C (Se:S = 2:3), phase D (Se:S = 3:2), phase E (Se:S = 4:1), and phase F (only selenium). Since the crystal structures of phase A {[TiCp2S5]} [9,10,11,12], phase F {[TiCp2Se5]} [13,14], and phase D [15] have been determined previously, in this contribution we have determined only the crystal structures of Phases B, C, and E. The 77Se NMR spectra of phases C, D, and F are also known [15,24]. In this work, we augment this information by those of B and E. The 13C NMR spectra of all five mixed phases B–E as well as those of [TiCp2S5] (A) and [TiCp2Se5] (F) are reported. The main objective of the present work is to get further information of the composition of the crystalline solid solutions of the [TiCp2SexS5−x] phases.
2. Results and Discussion
2.1. Crystal Structures of Phases B, C, and E
It has been well-established that the reduction of sulfur-selenium mixtures by Li[AlEt3H] followed by the reaction with [TiCp2Cl2] forms a mixture of [TiCp2SexS5−x] complexes [15] in an analogous manner to the formation of [TiCp2S5] and [TiCp2Se5] from sulfur and selenium [1,2,3,4,5,6,7,8]. There are 20 possible complexes in the [TiCp2SexS5−x] (x = 0–5) series, which are shown in Figure 1 together with their abbreviated designations. Upon crystallization, they form solid solutions.
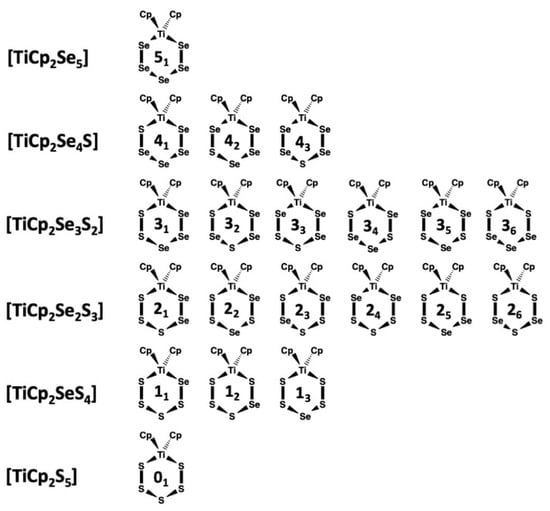
Figure 1.
Possible isomers of [TiCp2SexS5−x] (x = 0–5).
The crystal structures of [TiCp2S5] (Phase A) [9,10,11], [TiCp2Se5] (Phase F) [13,14], and Phase D [15] are well-known. The crystal structures of Phases B, C, and E have been determined in this work. All phases A–F show similar structures of the bidentate anionic chelating E52− ligand (E = S, Se) coordinating to a titanium atom of the bis(cyclopentadienyl)titanium fragment, as shown in Figure 2.
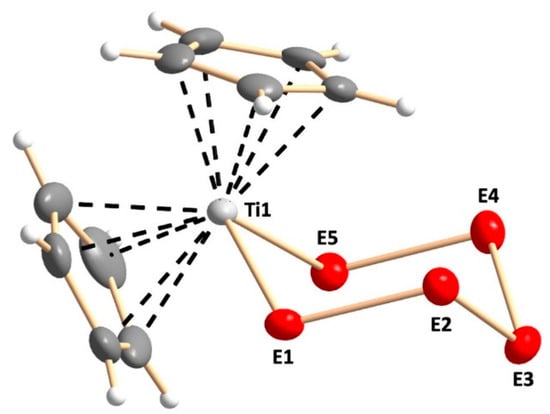
Figure 2.
Molecular structure of [TiCp2SexS5−x] as exemplified by Phase C. In phases B–E the chalcogen atom positions marked with “Ei” (i = 1–5) are disordered with sulfur and selenium in random positions. The site occupancy factors are shown in Table S1 in the Supporting Information. The anisotropic displacement parameters are shown at 50% probability level.
Four different isomorphic series have been observed for crystalline phases A–F. [TiCp2S5] (Phase A) shows two different monoclinic polymorphs [9,10,11]. The mixed sulfur-selenium phases B–D are mutually isomorphic and are also isomorphic with [ZrCp2Se5] [14]. [TiCp2Se5] (phase F) is triclinic and is isomorphic with phase E [13,14].
With the exception of atoms E2, E3, and E4 in phase E, all chalcogen atom positions in phases B–E are disordered with sulfur and selenium distributed randomly in the atomic sites. The site occupancy factors of the chalcogen atoms in Phases B–E are shown in the Supporting Information (Table S1). Because of the disorder, the bond distances between the chalcogen atoms only reflect the average disordered composition of the atomic sites and therefore carry no accurate physical significance. These interatomic distances are also listed in the Supporting Information (Table S2).
2.2. 77Se and 13C-NMR Spectra of the [TiCp2SexS5−x] (x = 0–5) Phases A–F
A typical 77Se NMR spectrum of the [TiCp2SexS5−x] mixture has been recorded previously for the Phase D [15]. The resonances were tentatively assigned to [TiCp2Se5] (51), [TiCp2Se4S] (41), [TiCp2Se3S2] (31), [TiCp2SSe3S] (36), [TiCp2SSe2S2] (25), and [TiCp2SSeS3] (12) [see Figure 3a]. The spectrum of phase E is somewhat simpler but shows only resonances, which are also observed for other phases [see Figure 3b]. The current study indicates that no new resonances were observed in the complete range of the Se:S ratio from 1:4 to 4:1. Only the relative intensities of the resonances varied.
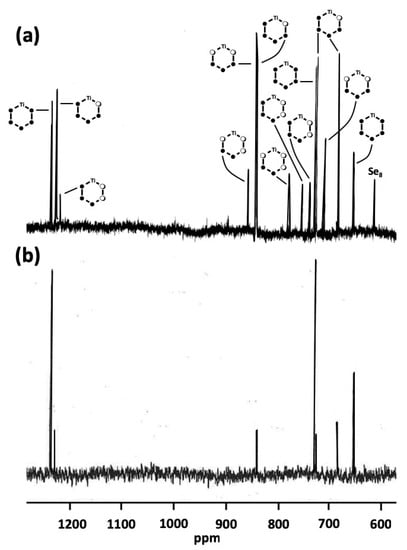
Figure 3.
(a) The 77Se-NMR spectrum of phase D recorded previously in CS2 together with the tentative assignment [15] (adapted with permission from Pekonen, P.; Hiltunen, Y.; Laitinen, R.S.; Valkonen, J. Selenium-77 NMR Spectroscopic and X-ray Crystallographic Characterization of Bis(cyclopentadienyl)titanium Selenide Sulfide Mixtures [Ti(C5H5)2SexS5-x]. Inorg. Chem. 1991, 30, 1874–1878. Copyright 1991 American Chemical Society). (b) The 77Se-NMR spectrum of phase E recorded in CS2.
It was observed that by varying the Se:S ratio of the chalcogen reagent the relative intensities of some groups of resonances remained constant, while the relative intensities between the groups varied. The groups exhibiting constant intensity ratios are shown in Table 1 and formed the basis for the initial assignment reported earlier [15].

Table 1.
Computed and observed chemical shifts of [TiCp2SexS5−x] a.
The assignment was verified by PBE0/def2-TZVPP calculations of nuclear magnetic shielding tensors using methodology described previously [32]. The computed 77Se chemical shifts of all nineteen selenium-containing [TiCp2SexS5−x] complexes are presented in Table 1 and compared with those of the experimental resonances. It can be seen from Table 1 that the current calculations agree very well with the previous tentative assignment [15] of the 77Se resonances. The agreement between the computed and observed 77Se chemical shifts is excellent and provides the best fit between the experiment and theory. It was reported earlier [15] that it is not possible to assign unambiguously the 77Se NMR resonance at 936 ppm either to [TiCp2SSeS3] (12) or [TiCp2S2SeS2] (13). The current computations, however, indicate that 12 is a more likely candidate.
Since it has been deduced earlier from the crystallographic disorder scheme that the Phase D must also contain [TiCp2S5] (01) [15], which cannot be detected by 77Se NMR spectroscopy, the 13C NMR spectra of all phases A–F were recorded in this contribution. All complexes present in the mixtures show 13C NMR resonances due to the carbon atoms in the cyclic η5-C5H5 ligand. 1H NMR spectroscopy has shown that in solution at room temperature, the ligand is rotationally fluxional [23,24]. Since it is also known that conformational chair-chair inversion of the hexaatomic TiE5 chelate ring does not take place under ambient conditions [23,24], two resonances are observed in 1H and 13C NMR spectra for each [TiCp2SexS5−x] complex (for a typical 13C NMR spectrum, see Figure 4).
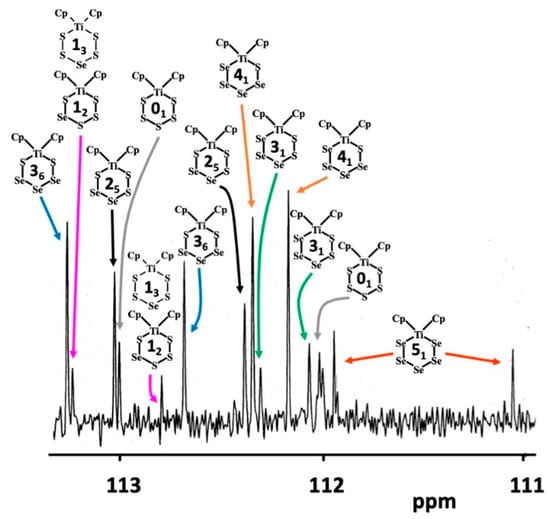
Figure 4.
13C-NMR spectrum of phase D in CS2.
The assignment in the 13C NMR spectrum shown in Figure 4 was based on the comparison of relative intensities of the pairs 13C resonances of equal intensity with those obtained from the corresponding 77Se spectrum. The possibility to prepare and record the 13C NMR spectra of pure [TiCp2S5] (01) and [TiCp2Se5] (51) served to verify the assignments. Further support to the assignments in Figure 4 is obtained by considering the trends in the chemical shifts. In case of [TiCp2Se5] (51), which contains two Ti–Se bonds, the average of the two carbon shifts is 111.5 ppm. [TiCp2Se4S] (41) and [TiCp2Se3S2] (31) have one Ti–S bond and one Ti–Se bond. Their average 13C chemical shifts span a narrow range of 112.1–112.3 ppm. Other complexes, which contain two Ti–S bonds, i.e., [TiCp2SSe3S] (36), [TiCp2SSe2S2] (25), [TiCp2SSeS3] (12), and [TiCp2S5] (01) show their average chemical shifts in the range 112.5–113.0 ppm. The trend of decreasing shielding in the carbon atoms is to be expected, since the electronegativity of sulfur is slightly higher than that of selenium.
Abel et al. [24] have inferred that the 13C resonance of fluxional cyclopentadienyl ring in the axial position is more shielded than that in the equatorial position. The trend in the difference between the two chemical shifts is very informative in the [TiCp2SexS5−x] series, as shown in Figure 5. It can be seen that the numerical values of the differences can be classified in three distinct groups. The interpretation of the trend can be explained as follows:
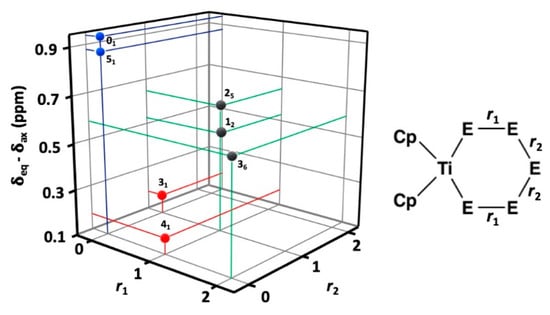
Figure 5.
The difference between the 13C chemical shifts as a function of heteronuclear Se–S bonds in positions r1 and/or r2 (ri = 0, 1, 2). The data points in blue mark complexes with no heteronuclear chalcogen-chalcogen bonds (01 and 51), those marked in red indicate complexes with one Se–S bond either in position r1 or r2, and those in grey indicate two Se–S bonds either in positions r1 or r2.
The twenty complexes can have their five chalcogen atom positions occupied either by sulfur or selenium resulting in a varying number of homo- and heteronuclear chalcogen-chalcogen bonds. [TiCp2S5] (01) and [TiCp2Se5] (51) contain no heteronuclear S–Se bonds and both show a large difference of 0.94 and 0.88 ppm between the chemical shifts of the fluxional equatorial and axial C5H5− ligands. [TiCp2Se4S] (41) and [TiCp2Se3S2] (31) both contain one heteronuclear S–Se bond either in the position marked by r1 or r2 (see Figure 5) and show a very small chemical difference of 0.17–0.18 ppm. Other [TiCp2SexS5−x] complexes show a total of two Se–S bonds either in the position r1 or r2. They show chemical differences in the range 0.47–0.65 ppm.
2.3. Composition of [TiCp2SexS5−x] Phases B–E
The approximate compositions of the phases B–E, which have been determined by considering the intensities of the 77Se and 13C resonances, are shown in Table 2.

Table 2.
Semiquantitative determination of the contents (mol%) of the [TiCp2SexS5−x] complexes in phases B–E based on the relative intensities observed in the 77Se and 13C NMR spectra of the reaction mixtures recorded in carbon disulfide.
The composition of the solid phases B–E crystallized from the reaction mixtures can be estimated considering the disorder scheme in the crystal structures and assuming that the solid solutions contain only complexes, which have been detected in solution (for details, see Supporting Information). These semiquantitative analytical results are presented in Table 3.

Table 3.
The composition of solid solutions B–E based on the observed disorder in the crystal structures (see Supporting Information).
Expectedly, all solid solutions are richer in selenium than the solutions or the initial reagents of the elemental selenium-sulfur mixtures. This is due to the decreasing solubility of the complexes as the selenium-content increases. It can be inferred, however, that disorder scheme observed in the crystal structures is consistent with the complexes, which are formed in the reactions. The justification for these conclusions is presented in Supporting Information.
2.4. Relative Stabilities of Individual [TiCp2SexS5−x] (x = 0–5) Complexes
Enthalpies of formation for individual [TiCp2SexS5−x] species have been predicted using DLNPO-CCSD(T)/CBS energies. Test computations on reference molecules Se2 and S2 showed that the present calculations are capable of predicting the enthalpies of formation of chalcogen species with very good accuracy {c.f. ΔfH(Se2) calc. +146.8 vs. exptl. +144.9 ± 1.1 kJ mol−1 [33] and ΔfH(S2) calc. +130.6 vs. exptl. +128.6 ± 0.3 kJ mol−1 [34]}. The calculated formation enthalpies of [TiCp2SexS5−x] species shown in Figure 6 and in Table S4 in Supporting Information indicate that the formation reactions of all complexes are exothermic in the gas phase. The calculated ΔfH values span a relatively small range (<30 kJ mol−1), as would be expected for the species forming an equilibrium in solution. [TiCp2S5] (01) is predicted to be the most stable of [TiCp2SexS5−x] species. The sulfur-rich [TiCp2SexS5−x] complexes are in general more stable than the selenium-rich species. It can be seen from Figure 6 that the formation enthalpies can be classified in three distinct, but slightly overlapping groups based on the number of Ti–S and Ti–Se bonds.
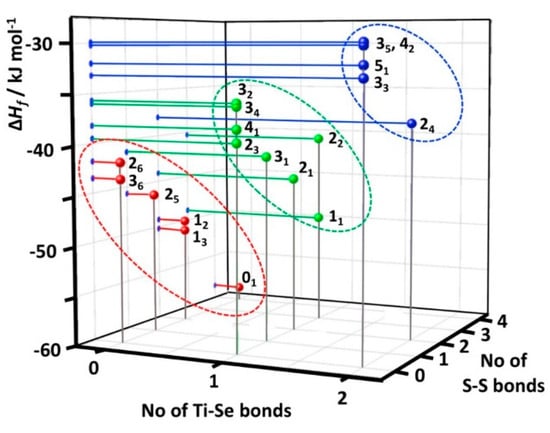
Figure 6.
Enthalpies of formation of the [TiCp2SexS5−x] complexes as a function of the number of Ti–Se and S–S bonds. Complexes with two Ti–Se bonds have been marked in blue, those with one Ti–S and one Ti–Se bond in green, and those with two Ti–Se bonds in red.
ΔfHnonrel. values, which have been calculated without scalar relativistic correction ΔE (C + R) and atomic spin-orbit ΔE (SO) correction, have been included in Table S6 (see Supporting Information) for comparison. They show that the inclusion of core-valence correlation and scalar relativistic correction to energies stabilizes the calculated structures significantly compared to free elements [35,36]. Addition of the spin-orbit corrections has an opposite effect on the calculated enthalpies especially in the case of selenium-rich species and changes the stability order in the [TiCp2SexS5−x] series to favor sulfur-rich species.
Each observed [TiCp2SexS5−x] species in the CS2 solutions correspond to the most stable structures in the series of isomers with the same composition (see Figure 7). The only exception is the complex 12 which lies 1.1 kJ mol−1 higher in energy than the lowest energy isomer 13. It should be noted that the solvent effects were not considered in calculated ΔfH values. They do not have a significant effect on the relative stabilities.
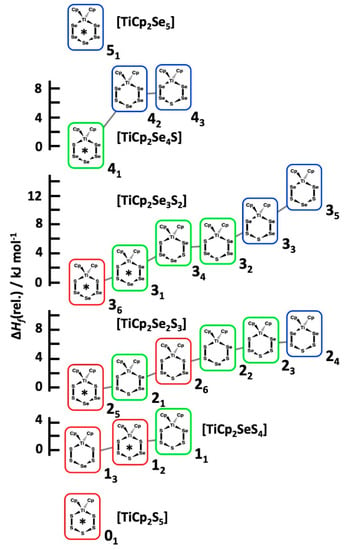
Figure 7.
The relative enthalpy of formation of the [TiCp2SexS5−x] isomers of the same composition. The most stable isomer is given the relative value of 0 for each composition. The red frames indicate two Ti–S bonds, green frames one Ti–S and one Ti–Se bond, and the blue frames two Ti–Se bonds. The complexes observed in the reaction mixtures by 77Se and 13C NMR spectroscopy have been indicated by an asterisk.
The current computations show that isomeric structures with maximal number of homopolar chalcogen bonds are stabilized over structures with chalcogen heteroatom bonds (S–Se). This has been observed to be the case also for heterocyclic SexS8−x molecules [28,30]. This is also consistent with the observation that the formation of SeS(g) from S2(g) and Se2(g) is slightly endothermic (5.2 kJ mol−1) [32]. The structures with S–Ti bonds are favoured over Se–Ti bonds.
3. Experimental
3.1. Preparation of [TiCp2SexS5−x]
All reactions and manipulations of air- and moisture-sensitive materials were carried out under an argon atmosphere by using a standard drybox or Schlenk techniques. Tetrahydrofuran (LabScan, Bangkok, Thailand) was dried before use by distillation over Na/benzophenone in a nitrogen atmosphere, and carbon disulfide (Thermo Fisher Scientific, Waltham, MA, USA) was distilled over P4O10 also under a nitrogen atmosphere. Sulfur (Merck, Darmstadt, Germany), selenium (Merck), and [TiCp2Cl2] (Cp = C5H5−, Sigma-Aldrich, Darmstadt, Germany) were used as provided. [TiCp2S5], [TiCp2Se5] and all [TiCp2SexS5−x] (x = 1–4) mixtures were prepared as described previously modifying the method by Gladysz et al. [15,37] (for synthetic details, see Table S8 in Supporting Information). After the reaction, the solutions were filtered, the solvent was evaporated, and the solid material was extracted by carbon disulfide. The 77Se- and 13C-NMR spectra were recorded from the thus formed CS2 solution. The crystals obtained upon recrystallization of these CS2 solutions were involved in the crystal structure determinations. The designation of the final crystalline products was based on the initial Se:S molar ratio of the elemental chalcogen mixture reagent, as follows: Phase B (Se:S = 1:4), phase C (Se:S = 2:3), phase D (Se:S = 3:2), and phase E (Se:S = 4:1).
3.2. NMR Spectroscopy
The 77Se and 13C NMR spectra were recorded in the CS2 solution on a DPX-400 spectrometer (Bruker, Karlsruhe, Germany) operating at 76.31 and 100.61 MHz, respectively. The spectra were recorded unlocked. Typical respective spectral widths for 77Se and 13C were 76,000, and 30,000 kHz, and the respective pulse widths were 6.7 and 4.00 µs. The pulse delay for selenium was 0.43 s and for carbon 0.81 s. The 77Se-NMR spectra were referenced externally to a saturated aqueous solution of selenium dioxide. The chemical shifts are reported relative to neat Me2Se [δ(Me2Se) = δ(SeO2) + 1302.6] [38]. In case of 13C spectra, the chemical shifts were referenced and reported relative to TMS.
3.3. X-ray Crystallography
Diffraction data for crystal phases B, C, and E were collected on a Nonius Kappa CCD diffractometer (Bruker, Karlsruhe, Germany) at 120 K using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å; 55 kV, 25 mA). Crystal data and the details of structure determinations are given in Table 4. All structures were solved by direct methods using SHELXS-2016 and refined using SHELXL-2016 [39,40]. After the full-matrix least-squares refinement of the non-hydrogen atoms with anisotropic thermal parameters, the hydrogen atoms were placed in calculated positions in the cyclopentadienyl groups (C–H = 0.95 Å). The isotropic thermal parameters of the hydrogen atoms were fixed at 1.2 times to that of the corresponding carbon or nitrogen. The scattering factors for the neutral atoms were those incorporated with the program.

Table 4.
Crystal data and details of structure determination of crystals from phases B (initial Se:S ratio 1:4) C (initial Se:S ratio 2:3), and E (initial Se:S ratio 4:1).
All chalcogen atom positions in phases B and C were disordered with sulfur and selenium statistically distributed over the atomic sites. In case of phase E the chalcogen atoms 1 and 5 were also disordered, while those of 2–4 were only occupied by selenium. The following constraints were applied due to the correlation between the thermal parameters and the occupation factor:
where sof(Sei), sof(Si), U(Sei), and U(Si) are the occupation factors and isotropic thermal parameters or the main diagonal parameters in the anisotropic thermal parameter tensor of selenium and sulfur atoms at the ith atomic position. Sulfur and selenium atoms of the disordered pairs were also constrained in the same atomic positions. Information on physically meaningful bond lengths and bond angles was thereby lost. The method, however, enables the reliable refinement of the occupation factors of selenium and sulfur at the disordered chalcogen atom sites and thus serves in the identification of the molecular species.
sof(Sei) + sof(Si) = 1
U(Sei) = U(Si)
The X-ray data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax (+44) 1223-336-033; e-mail: deposit@ccdc.cam.ac.uk (for CCDC registry numbers, see Table 4).
4. Computational Details
All structures were optimized using PBE0 hybrid DFT [41,42,43] with def2-TZVPP basis sets [44] on all atoms and employing empirical D3BJ correction [45] to treat dispersion forces. Nuclear magnetic shielding tensors were calculated on optimized stationary points with GIAO method [46,47,48,49] as implemented in Gaussian 09 [50] that was used for all DFT calculations. 77Se chemical shifts were determined from calculated nuclear shielding tensors using a previously described calibration [32].
Enthalpies of formation were determined using electronic energies calculated with DLPNO-CCSD(T) method [51,52,53,54] implemented in ORCA 4.0 program suite [55] that has been recently shown to provide accurate enthalpy estimations with moderate computational cost when used together with thermal energy corrections from DFT calculations [56,57]. TightPNO setting, that controls the cut-off parameters for the treatment of the domain based localized pair natural orbitals [58], and VeryTightSCF (energy change 1 × 10−9 au) option to achieve better converged reference wavefunctions were used in the energy calculations. DLPNO-CCSD(T) energies calculated with def2-XZVPP (X = T, Q) basis sets [41], and corresponding auxiliary basis sets def2-XZVPP/C [59], were extrapolated to complete basis set (CBS) limit using separate extrapolations for reference Hartree-Fock energy [60] and correlation energy [61].
where α (7.88) and β (2.97) are optimized coefficients for the basis set couple taken from Neese and Valeev [62] and A and B are parameters to be obtained by combining the results of the two basis set levels.
Neglect of core-valence correlation and scalar relativistic effects have been shown to be potential sources of significant error in relative energies [35]. However, calculation of core-valence correlation and scalar relativistic effects are likely to be the most time-consuming step in the calculations if treated at the same level as the valence correlation calculations [63,64]. To account for core-valence correlation and scalar relativistic contributions to energies while conserving computational resources we adopted a similar approach used by Chan and Radom in W1X-1 composite method [64] and calculated the contributions at DLPNO-MP2/cc-pwCVTZ level [36,65,66,67,68] as differences between frozen-core and all-electron-DKH energies and added the contribution as correction ΔE (C + R) term to total energies. Spin-orbit coupling corrections ΔE (SO) were applied to atomic reference energies using weighted J-averaged values derived from experimental data in Moore’s tables [69].
Enthalpies of formation were calculated from atomization enthalpies using reference values taken from tables published by the committee on Data of the International Council for Science (CODATA) [34] for other elements and from the work of Drowart and Smoes [33] for selenium.
5. Conclusions
It has long been known that the reduction of elemental sulfur or selenium by lithium triethylhydridoborate in THF followed by the treatment with titanocene dichloride [TiCp2Cl2] (Cp = C5H5− or its alkyl-substituted derivative) affords [TiCp2S5] or [TiCp2Se5], respectively [9,10,11,12,13,14]. When mixtures of elemental sulfur and selenium are used as the reagent in the reaction, mixtures of [TiCp2SexS5−x] were formed [15]. Tentative identification of the molecular species formed in the reactions with the initial Se:S molar ratio of 2:3 and 3:2 indicated the formation of [TiCp2Se5] (51), [TiCp2Se4S] (41), [TiCp2Se3S2] (31), [TiCp2SSe3S] (36), [TiCp2SSe2S2] (25), and [TiCp2SSeS3] (12). In addition, the presence of [TiCp2S5] (01) was postulated.
In the current contribution, the preliminary work was extended to the preparation of five solid solutions of mixed titanocene selenide sulfides [TiCp2SexS5−x] (Cp = C5H5−) with the initial Se:S ranging from 1:4 to 4:1 (phases B–E). Their 77Se- and 13C-NMR spectra were recorded from the CS2 solution. The definite assignment of the NMR spectra was based on the PBE0/def2-TZVPP calculation of the 77Se chemical shifts that verified the earlier, tentative inferences. Further confirmation was provided by 13C-NMR spectra of the samples. The presence of [TiCp2S5] (01) and [TiCp2Se5] (51) was unambiguously verified. By comparison of the relative intensities of the 13C NMR resonances with those of 77Se resonances, a complete, consistent assignment of the 13C spectra could be made.
All crystal structures of the mixed Se–S phases showed disorder in the chalcogen-atom positions. The site occupancy factors of selenium and sulfur in the solid phases B–E were consistent with classification of the phases as solid solutions of the species observed in the corresponding CS2 solutions even though the selenium contents of the solid phases B–E were higher than those in the corresponding solutions due to the rapidly decreasing solubility of the [TiCp2SexS5−x] complexes, as the selenium content increases.
The enthalpies of formation were calculated for all twenty [TiCp2SexS5−x] (x = 0–5) species at DLPNO-CCSD(T)/CBS level of theory and augmented to account for core-valence correlation and scalar relativistic, as well as spin-orbit coupling contributions to energies. The formation enthalpies could be divided into three distinct, but slightly overlapping groups. The most favourable group contained complexes with two Ti–S bonds, the intermediate group consisted of complexes with one Ti–S and one Ti–Se bond, and group with least exothermic enthalpy of formation contained complexes with two Ti–Se bonds. Within a given chemical composition, the isomers of the most favourable enthalpy of formation were those, which were observed by 77Se and 13C-NMR spectroscopy.
Supplementary Materials
The following are available online, Table S1. The site occupancy factors of selenium in the disordered chalcogen atom sites of the crystals of phases B–E; Table S2. The interatomic chalcogen-chalcogen and chalcogen-titanium distances; Table S3. The composition of the solid solution of phases B–E based on the site occupancy factors of selenium in different chalcogen atom positions; Table S4. Energy terms of atoms (in a.u.) used in the DLPNO-CCSD(T)/CBS enthalpy of formation calculations; Table S5. Energy terms of atoms (in Hartree) used in the DLPNO-CCSD(T)/CBS enthalpy of formation calculations; Table S6. The DLPNO-CCSD(T)/CBS formation enthalpies (298 K) of TiCp2SexS5−x molecules calculated at non-relativistic ΔfHnonrel.. And relativistic level ΔfH with core-correlation and scalar relativistic [ΔE (C + R)] and spin-orbit [[ΔE (SO)] energy corrections included; Table S7. Isotropic 77Se NMR shielding values calculated at PBE0/def2-TZVPP level of theory.
Author Contributions
Conceptualization, R.S.L.; methodology, R.S.L. and J.M.R.; validation, J.M.R. and R.S.L.; formal analysis, J.M.R. and R.S.L.; investigation, H.L., J.I., J.M.R., M.S.; resources, R.S.L.; writing—original draft preparation, R.S.L. and J.M.R.; writing—review and editing, H.L., J.I., M.S., J.M.R., R.S.L.; visualization, R.S.L.; supervision, R.S.L.; project administration, R.S.L.; funding acquisition, H.L., J.M.R., R.S.L.
Funding
This research was funded by Academy of Finland, University of Jyväskylä (J.M.R.), and Neste Oy Foundation (H.L.).
Acknowledgments
We are grateful to Jari Rajala, M. Sc. for his help in syntheses. We are also grateful for provision of computational capacity by the Finnish Grid and Cloud infrastructure (persistent identifier urn:nbn:fi:research-infras-2016072533).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roof, L.C.; Kolis, J.W. New developments in the coordination chemistry of inorganic selenide and telluride ligands. Chem. Rev. 1993, 93, 1037–1080. [Google Scholar] [CrossRef]
- Laitinen, R.S.; Pekonen, P.; Suontamo, R.J. Homo- and heteroatomic chalcogen rings. Coord. Chem. Rev. 1994, 130, 1–62. [Google Scholar] [CrossRef]
- Kanatzidis, M.G.; Huang, S.P. Coordination chemistry of heavy polychalcogenide ligands. Coord. Chem. Rev. 1994, 130, 509–621. [Google Scholar] [CrossRef]
- Steudel, R.; Kustos, M.; Pridöhl, M.; Westphal, U. The utilization of titanocene polysulfide complexes for the synthesis of organic polysulfanes. Phosphorus Sulfur Silicon Relat. Elem. 1994, 93–94, 61–72. [Google Scholar] [CrossRef]
- Steudel, R.; Eckert, B. Solid sulfur allotropes. Top. Curr. Chem. 2003, 230, 1–79. [Google Scholar] [CrossRef]
- Takeda, N.; Tokitoh, N.; Okazaki, R. Polysulfido complexes of main group and transition metals. Top. Curr. Chem. 2003, 231, 153–202. [Google Scholar]
- Laitinen, R.S.; Oilunkaniemi, R. Catenated Compounds: Group 16 (Se, Te). In Comprehensive Inorganic Chemistry II, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 197–231. [Google Scholar]
- Horácek, M. Titanocene sulfide chemistry. Coord. Chem. Rev. 2016, 314, 83–102. [Google Scholar] [CrossRef]
- Muller, E.G.; Petersen, J.L.; Dahl, L.F. Synthesis and characterization of Di-π-cyclopentadienyl-metal pentasulfides of Titanium(IV) and Vanadium(IV): An operational test of the influence of an unpaired electron on the molecular geometry. J. Organomet. Chem. 1976, 111, 91–112. [Google Scholar] [CrossRef]
- Epstein, E.F.; Bernal, I. Pentachalcogenide dianions in transition-metal complexes: Crystal structure of Bis(π-cyclopentedienyl)titanium pentasulfide. J. Chem. Soc. Chem. Commun. 1970, 0, 410–411. [Google Scholar] [CrossRef]
- Epstein, E.F.; Bernal, I.; Kopf, H. The crystal and molecular structures of di-π-cyclopentadienyltitanium pentachalcogenides (C5H5)2TiS5−xSex: I. The structural properties of π-(η5-C5H5)2TiS5. J. Organomet. Chem. 1971, 26, 229–245. [Google Scholar] [CrossRef]
- Klouras, N.; Demakopoulos, I.; Terzis, A.; Raptopoulou, C.P. Crystal and molecular structure of Bis(η5-methylcyclopentadienyl)titana(IV)cyclohexasulfane Ti(η5-C5H4CH3)2S5. Z. Anorg. Allg. Chem. 1995, 621, 113–116. [Google Scholar] [CrossRef]
- Fenske, D.; Adel, J.; Dehnicke, K. Die Kristallstruktur von Bis(η-cyclopentadienyl)titana(TiIV)cyclohexaselenan, Cp2TiSe5. Z. Naturforsch. 1987, 42, 931–933. [Google Scholar] [CrossRef]
- Albrecht, N.; Weiss, E. Bis(η-cyclopentadienyl)(pentaselenido)metall-Komplexe Cp2MSe5 des Ti, Zr, Hf und (μ2-O) (μ2-Se4) (Cp2Hf)2, eine zweikernige Verbindung mit Se4- und O-Brücken. J. Organomet. Chem. 1988, 355, 89–98. [Google Scholar] [CrossRef]
- Pekonen, P.; Hiltunen, Y.; Laitinen, R.S.; Valkonen, J. 77Se NMR spectroscopic and X-ray crystallographic characterization of Bis(cyclopentadienyl)titanium selenide sulfides mixtures [Ti(C5H5)2SexS5−x]. Inorg. Chem. 1991, 30, 1874–1878. [Google Scholar] [CrossRef]
- Papavassiliou, M.; Pickardt, J.; Steudel, R. Synthesis, structures, and reactions of Cp2′TiSe3S2 and Cp2′TiSe4S (Cp′ = η5-C5H4CH3). Phosphorus Sulfur Silicon Relat. Elem. 1992, 65, 161–164. [Google Scholar] [CrossRef]
- Raptopoulou, C.P.; Terzis, A.; Tzavellas, N.; Klouras, N. Sulfure-selenium chelates: Crystal structure of the first titanocene derivative [Ti(η5-C5H5)2SSe4]. Z. Anorg. Allg. Chem. 1995, 621, 1800–1802. [Google Scholar] [CrossRef]
- Bolinger, C.M.; Rauchfuss, T.B.; Wilson, S.R. Synthesis and characterization of a cyclic bimetallic complex of the trisulfide ion. J. Am. Chem. Soc. 1981, 103, 5620–5621. [Google Scholar] [CrossRef]
- Bolinger, C.M.; Hoots, J.E.; Rauchfuss, T.B. Intermetallic chalcogenide atom transfer and the synthesis of 1,4-[(CH3C5H4)2Ti]2S4. Organometallics 1982, 1, 223–225. [Google Scholar] [CrossRef]
- Giolando, D.M.; Rauchfuss, T.B.; Rheingold, A.L.; Wilson, S.R. Chemistry of (RC5H4)2TiS5. New information on its reactions with nucleophiles, syntheses and reactions of 1,4-[(RC5H4)2Ti]2S4, and a second isomer of (RC5H4)2TiS2C2(CO2Me)2. Organometallics 1987, 6, 667–675. [Google Scholar] [CrossRef]
- Giolando, D.M.; Papavassiliou, M.; Pickardt, J.; Rauchfuss, T.B.; Steudel, R. Synthesis and structure of 1,4-[(RCp)2Ti]2Se4 and its applications to the “Chalcogenospecific” synthesis of 1,2,5,6-Se4S4. Inorg. Chem. 1988, 27, 2596–2600. [Google Scholar] [CrossRef]
- Chandra, K.; Soni, P.; Garg, B.S.; Singh, R.P. Pentachalcogenide dianions in titanium complexes. J. Ind. Chem. Soc. 1981, 58, 10–12. [Google Scholar]
- Koepf, H.; Block, B.; Schmidt, M. Di-p-cyclopentadienyltitanium(IV)pentaselenide and pentasulfide, two heterocyclohexachalcogens in fixed conformation. Chem. Ber. 1968, 101, 272–276. [Google Scholar]
- Abel, E.W.; Booth, M.; Orrell, K.G. The inversion of the titanium pentasulfide ring in Di((η5-cyclopentadienyl)titanium pentasulfide. J. Organomet. Chem. 1978, 160, 75–79. [Google Scholar] [CrossRef]
- Pekonen, P.; Hiltunen, Y.; Laitinen, R.S. The Se-Se coupling in Bis(cyclopentadienyl)titanium pentaselenide [Ti(C5H5)2Se5]. Acta Chem. Scand. 1989, 43, 914–916. [Google Scholar] [CrossRef]
- Diaz, A.; Radzewich, C.; Wicholas, M. Synthesis and variable-temperature 1H NMR conformational analysis of Bis (η5-cyclopentadienyl)titanium pentasulfide. J. Chem. Ed. 1995, 72, 937–938. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Dohm, S.; Hansen, A.; Pisarek, J.; Pracht, P.; Seibert, J.; Neese, F. Fully automated quantum-chemistry-based computation of spin-spin-coupled nuclear magnetic resonance spectra. Angew. Chem. Int. Ed. 2017, 56, 14763–14769. [Google Scholar] [CrossRef]
- Laitinen, R.S.; Pakkanen, T.A. 77Se NMR spectroscopic characterization of selenium sulfide ring molecules SenS8−n. Inorg. Chem. 1987, 26, 2598–2603. [Google Scholar] [CrossRef]
- Pekonen, P.; Taavitsainen, J.; Laitinen, R.S. New routes to heterocyclic selenium sulfides. Acta Chem. Scand. 1998, 52, 1188–1193. [Google Scholar] [CrossRef]
- Komulainen, J.; Laitinen, R.S.; Suontamo, R.J. A theoretical study of the 77Se NMR and vibrational spectroscopic properties of SenS8−n ring molecules. Can. J. Chem. 2002, 80, 1435–1443. [Google Scholar] [CrossRef]
- Pekonen, P.; Laitinen, R.S.; Hiltunen, Y. Selenium-77 nuclear magnetic resonance identification of seven-membered selenium sulfide ring molecules. J. Chem. Soc. Dalton Trans. 1992, 0, 2885–2887. [Google Scholar] [CrossRef]
- Karhu, A.J.; Pakkanen, O.J.; Rautiainen, J.M.; Oilunkaniemi, R.; Chivers, T.; Laitinen, R.S. Experimental and computational 77Se NMR investigations of the cyclic eight-membered selenium imides 1,3,5,7-Se4(NR)4 (R = Me, tBu) and 1,5-Se6(NMe)2. Inorg. Chem. 2015, 54, 4990–4997. [Google Scholar] [CrossRef] [PubMed]
- Drowart, J.; Smoes, S. Determination by the mass spectrometric knudsen cell method and discussion of the dissociation energies of the molecules Se2(g), SSe(g) and SeTe(g). J. Chem. Soc. Faraday Trans. II 1977, 73, 1755–1767. [Google Scholar] [CrossRef]
- Garvin, D.; White, H.J. Comprehensive, Consistent Thermodynamic Tables, CODATA Bulletin No. 58: Thermodynamic Databases; Pergamon Press: Oxford, UK, 1985; pp. 1–5. [Google Scholar]
- Minenkov, Y.; Bistoni, G.; Riplinger, C.; Auer, A.A.; Neese, F.; Cavallo, L. Pair natural orbital and canonical coupled cluster reaction enthalpies involving light to heavy alkali and alkaline earth metals: The importance of sub-valence correlation. Phys. Chem. Chem. Phys. 2017, 19, 9374–9391. [Google Scholar] [CrossRef] [PubMed]
- DeYonker, N.J.; Peterson, K.A.; Wilson, A.K. Systematically convergent correlation consistent basis sets for molecular core-valence correlation effects: The third-row atoms gallium through krypton. J. Phys Chem. A 2007, 111, 11383–11393. [Google Scholar] [CrossRef]
- Gladysz, J.A.; Hornby, J.L.; Garbe, J.E. Convenient one-flask synthesis of dialkyl selenides and diselenides via lithium triethylborohydride reduction of Sex. J. Org. Chem. 1978, 43, 1204–1208. [Google Scholar] [CrossRef]
- Burns, R.C.; Collins, M.J.; Gillespie, R.J.; Schrobilgen, G.J. 77Se nuclear magnetic resonance study of Se82+ and Se102+: Examples of rigid and fluxional species. Disproportionation of Se102+ at low temperatures. Inorg. Chem. 1986, 25, 4465–4469. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- McWeeny, R. Perturbation theory for the fock-dirac density matrix. Phys. Rev. 1962, 126, 1028–1034. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Riplinger, C.; Neese, F. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys. 2013, 138, 034106. [Google Scholar] [CrossRef]
- Riplinger, C.; Sandhoefer, B.; Hansen, A.; Neese, F. Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys. 2013, 139, 134101. [Google Scholar] [CrossRef]
- Riplinger, C.; Pinski, P.; Becker, U.; Valeev, E.F.; Neese, F. Sparse maps—A systematic infrastructure for reduced-scaling electronic structure methods. II. Linear scaling domain based pair natural orbital coupled cluster theory. J. Chem. Phys. 2016, 144, 24109. [Google Scholar] [CrossRef]
- Saitow, M.; Becker, U.; Riplinger, C.; Valeev, E.F.; Neese, F. A new near-linear scaling, efficient and accurate, open-shell domain-based local pair natural orbital coupled cluster singles and doubles theory. J. Chem. Phys. 2017, 146, 164105. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Paulechka, E.; Kazakov, A. Efficient DLPNO–CCSD(T)-based estimation of formation enthalpies for C-, H-, O-, and N-containing closed-shell compounds validated against critically evaluated experimental data. J. Phys. Chem. A 2017, 121, 4379–4387. [Google Scholar] [CrossRef]
- Minenkov, Y.; Wang, H.; Wang, Z.; Sarathy, S.M.; Cavallo, L. Heats of formation of medium-sized organic compounds from contemporary electronic structure methods. J. Chem. Theory Comput. 2017, 13, 3537–3560. [Google Scholar] [CrossRef] [PubMed]
- Liakos, D.G.; Sparta, M.; Kesharwani, M.K.; Martin, J.M.L.; Neese, F. Exploring the accuracy limits of local pair natural orbital coupled-cluster theory. J. Chem. Theory Comput. 2015, 11, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, A.; Hattig, C.; Hofener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Karton, A.; Martin, J.M.L. Estimating the Hartree–Fock limit from finite basis set calculations. Theor. Chem. Acc. 2006, 115, 330–333. [Google Scholar] [CrossRef]
- Truhlar, D.G. Basis-set extrapolation. Chem. Phys. Lett. 1998, 294, 45–48. [Google Scholar] [CrossRef]
- Neese, F.; Valeev, E.F. Revisiting the atomic natural orbital approach for basis sets: Robust systematic basis sets for explicitly correlated and conventional correlated ab initio methods? J. Chem. Theory. Comput. 2011, 7, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.L.; Sundermann, A.; Fast, P.L.; Truhlar, D.G. Thermochemical analysis of core correlation and scalar relativistic effects on molecular atomization energies. J. Chem. Phys. 2000, 113, 1348–1358. [Google Scholar] [CrossRef]
- Chan, B.; Radom, L. W1X-1 and W1X-2: W1-quality accuracy with an order of magnitude reduction in computational cost. J. Chem. Theory Comput. 2012, 8, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Pinski, P.; Riplinger, C.; Valeev, E.F.; Neese, F. Sparse maps—A systematic infrastructure for reduced-scaling electronic structure methods. I. An efficient and simple linear scaling local MP2 method that uses an intermediate basis of pair natural orbitals. J. Chem. Phys. 2015, 143, 34108. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A.; Dunning, T.H., Jr. Accurate correlation consistent basis sets for molecular core–valence correlation effects: The second row atoms Al–Ar, and the first row atoms B–Ne revisited. J. Chem. Phys. 2002, 117, 10548–10560. [Google Scholar] [CrossRef]
- Balabanov, N.B.; Peterson, K.A. Systematically convergent basis sets for transition metals. I. All-electron correlation consistent basis sets for the 3d elements Sc–Zn. J. Chem. Phys. 2005, 123, 64107. [Google Scholar] [CrossRef] [PubMed]
- Basis Sets Contracted for Relativistic DKH Calculations were Obtained from the Molpro Basis Set Library. Available online: http://www.molpro.net/info/basis.php (accessed on 21 September 2018).
- Moore, C.E. Atomic Energy Levels; U.S. Department of Commerce: Washington, DC, USA, 1971. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).