2. Results
Compound
1 was obtained as white powder and its positive HRESIMS displayed a protonated molecular ion peak at
m/
z 482.2643 ([M + H]
+ which corresponds to a molecular formula of C
27H
35N
3O
5 (calcd for C
27H
36N
3O
5+, 482.2649). The IR spectrum exhibited two characteristic stretches (1589 and 1353 cm
−1) of a nitro group [
3,
4]. The UV maxima (λmax 206, 232, 262, 270 sh, 349 nm) of
1 were very similar to those observed in clopiamine C (
3), a nitro-containing indole alkaloid derivative isolated from a species of the same genus
Penicillium (
Penicillium sp.) [
3]. The
1H-NMR spectrum displayed four upfielded singlet methyls at δ 0.82, 0.95, 1.40 and 1.7 ppm (each corresponds to 3H), an aromatic methoxyl group (δ 3.80, s, 3H), two methine protons at δ 2.87 (m) and 3.17 (m), three sets of diastereotopic methylene protons at: δ 2.50 and 2.97 (each doublet, H-5ab); δ 3.48 and 3.15 (each doublet, H-20ab); and δ 2.60 and 2.94 (each doublet, H-24ab), five pairs of methylene protons at δ 1.33 (m, H-15a) and 2.77 (dt, H-15b), 1.22 (m, H-19a) and 1.99 (dq, H-19b), δ 1.25 (m, H-18a) and 1.68 (qt, H-18b), δ 1.58 (qt, H-17a) and 1.92 (brd, H-17b), δ 2.83 (brd, H-20a) and 2.86 (m, H-20b); and two
ortho coupled aromatic protons (δ 6.65 and δ 7.30, each doublet). The
13C-NMR spectrum displayed 27 carbon signals corresponding to five methyls (23.5, C-29; 23.7, C-26; 24.4, C-28; 26.8, C-25; and 56.5, C-27), six carbons assignable to a tetrasubstituted aromatic ring (108.4, C-7; 160.2, C-8; 105.2, CH-9; 133.6, CH-10; 122.0, C-11 and 149.9, C-12), four quaternary carbons (62.3, 57.9, 48.8, and 97.2), one of which assignable to a nitro group bearing quaternary carbon, two methines (δ 48.8 and 57.0), eight
sp3 hybridized methylenes (δ 21.1, C-18; 26.1, C-19; 26.9, C-17; 27.2, C-15; 44.7, C-24; 54.1, C-5; 56.0, C-20; and 60.1, C-22), and two carbonyls ascribable to a ketone (192.9, C-6) and an amide carbonyl (δ 183.3, C-2). The
1H and
13C-NMR data of compound
1 were very similar to those of
3 except for the presence of an additional set of methylene signals arising from the A-ring of
1. Interpretation of the Correlation Spectroscopy (COSY) spectroscopic data concluded that the additional methylene was part of the spin network of a partial structure –CH-CH
2-CH-CH
2-CH
2-CH
2-CH
2- (from H-14 to H-20). In addition, the three observed methylene protons (δ 3.48 and 3.15, each doublet,
J = 11.9 Hz, H-22ab; δ 2.60, and 2.94, d,
J = 16.1 Hz, H-24ab; and δ 2.50 and 2.97, d,
J = 15.6 Hz, H-5ab) of C-22, C24, and C-5 respectively were concluded to be isolated. The allocation of all functionalities present in
1 was carried out by comparison of its spectroscopic data with those of clopiamine C (
3) as well as interpretation of its HMBC spectroscopic data.
The presence of a 2,2,-dimethyl-2,3-dihydroquinolin-4(1H)-one was confirmed by the HMBC long-range correlations from H-25 and H-26 to C-4 and C-5, H-5 to C-6 and C-7, OCH3 to C-8, the ortho-coupled methine proton at δ.65 (H-9) to C-7, C-8, and C-11. The presence of 8,8-dimethyldecahydro-1H-cyclopenta[f]quinolizine in 1 instead of 8,8-dimethyldecahydro-1H-cyclopenta[f]indolizine (3) was substantiated by the above-mentioned spin network deduced from the COSY spectrum and the HMBC correlations from H-28/29 to C-1, C-13 and C-14, from H-20 to C-22, from H-22 to C-24, C-14, and C-23. The above data together with the HMBC correlation observed from H-10 to the quaternary carbon at δ 62.3 (C-1) and the presence of 12 degree of unsaturation in 1 allowed us to conclude that as in 3, the structure of 1 must also contain a spiro-oxindole moiety.
The relative configurations at C-1, C-14, C-16 and C-23 of
1 were determined by NOE experiment while the absolute configurations of
1 were established by comparison of its optical rotation and CD spectroscopic data with those of
3, the absolute stereostructure of which was deduced by NMR, CD and X-ray diffraction crystallography analyses. The CD spectrum of
1 which displayed negative and positive Cotton effects [(Δε
356 − 0.92) and (Δε
242 − 6.08), and (Δε
265 + 2.82) and (Δε
229 + 2.8), respectively) is superimposable with those of
3 [
3].
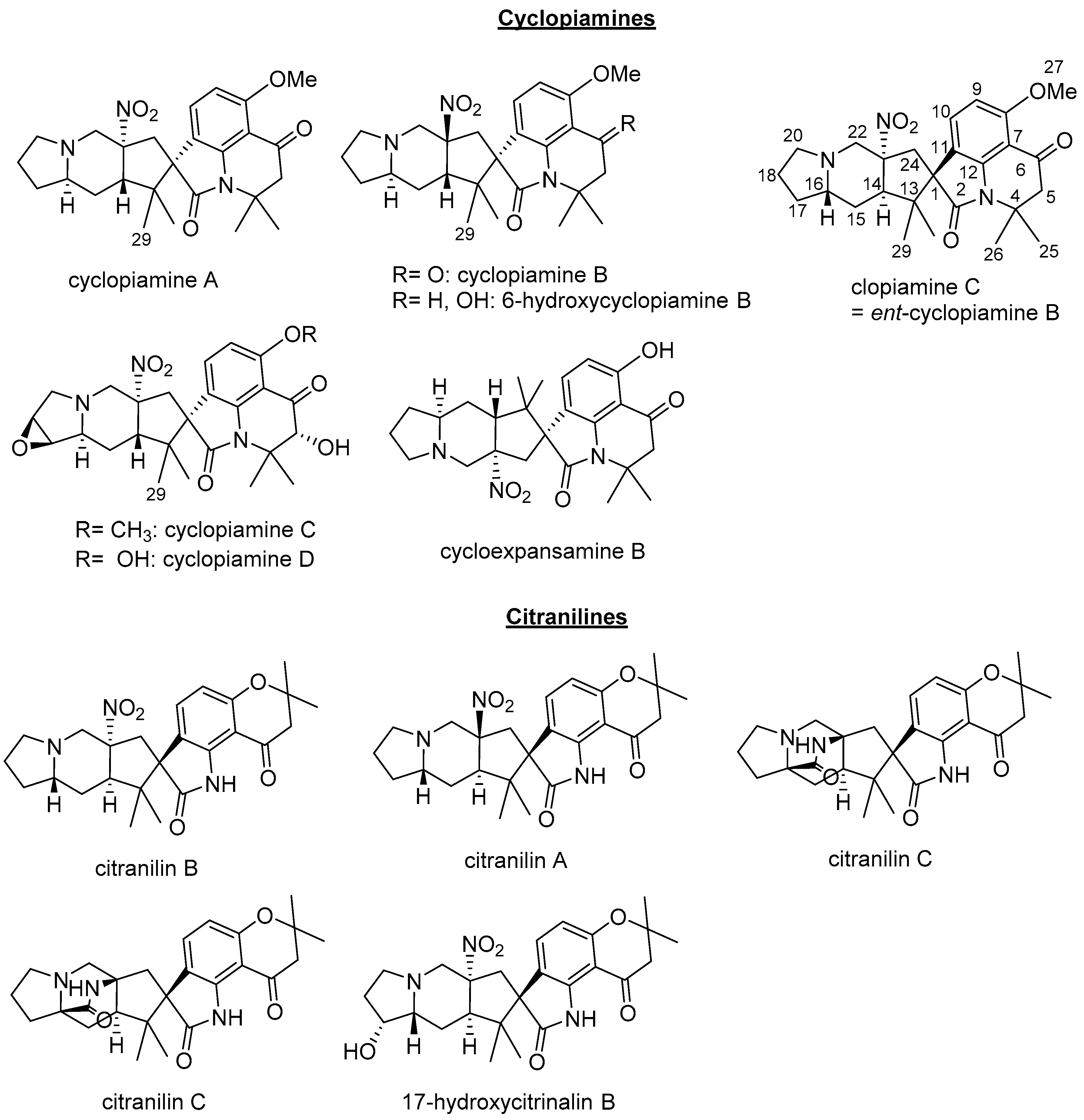
Clopiamine C (
3) belongs to the rare alkyl nitro group-bearing prenylated spirooxindole alkaloids of
Penicillium species. Of this group of compounds, only cyclopiamine and citranilin-type have been reported (
Figure 1) [
3,
4,
5,
6]. It is worthy to note that clopiamine C (
3) and cyclopiamine B (
Figure 1) are enantiomers. Their NMR spectroscopic data (although measured in different solvent) are superimposable while their reported optical rotations ([α]
D) have opposite sign (−97.9° and +117, respectively) [
3,
4]. From these data, the absolute configurations at C-1, C-14, C-16, and C-22 were thus determined to be
S,
R,
S, and
R, respectively and the structure of
1 was deduced to be
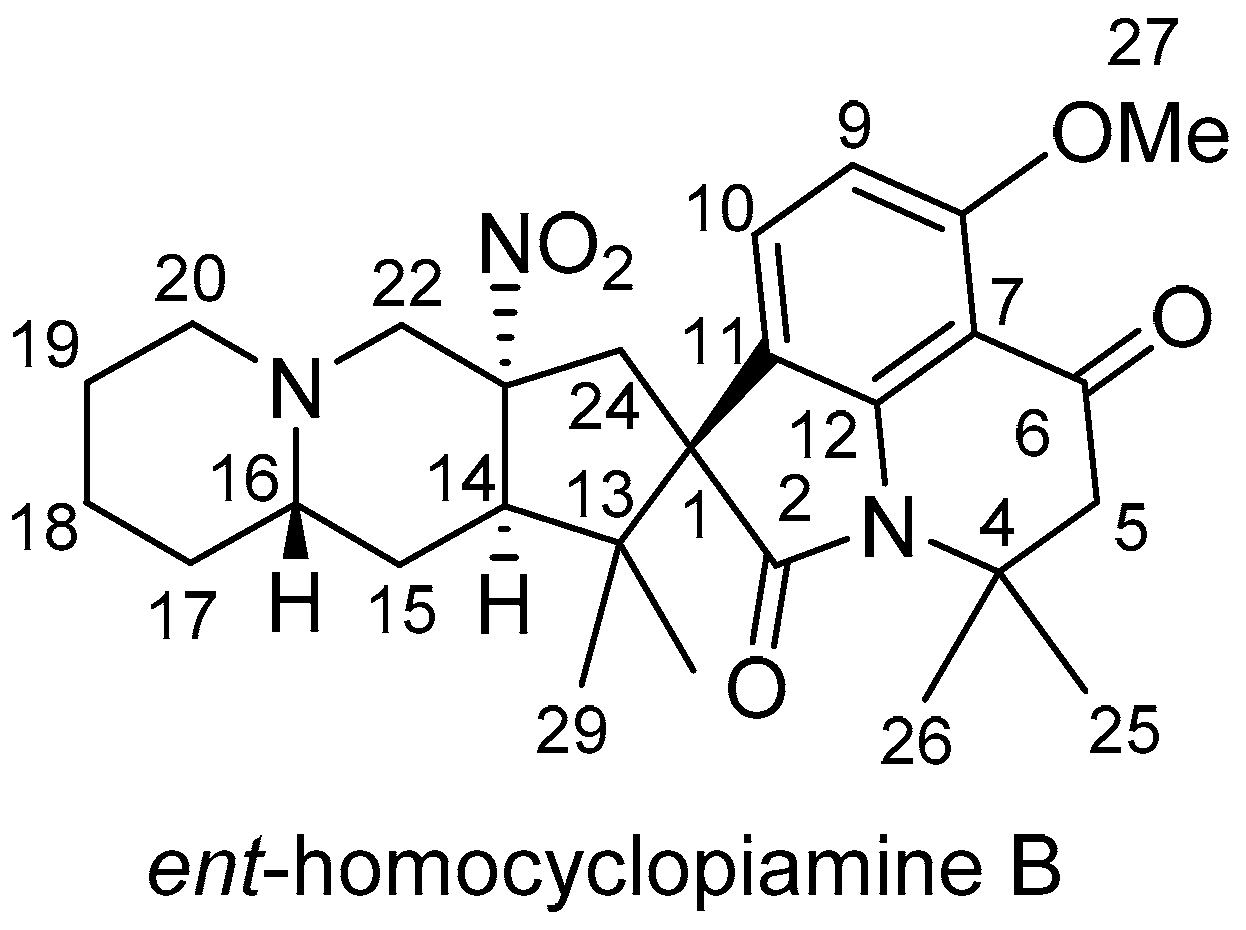
ent-homocyclopiamine B as depicted in
Figure 2.
The isolation of
1 from
Penicillium concentricum adds to the list of
Penicillium strains that produce cyclopiamines. To determine if supplementation of sodium monofluoroacetate to the rice medium affects production of
1, an ethyl acetate extract of
P. concentricum grown on brown rice medium and an ethyl acetate extract of the fungus fermented on brown rice supplemented with sodium monofluoroacetate were analyzed using LC-MS.
Ent-homocyclopiamine B and its derivatives have been detected in the LCMS spectra of both extracts showing that the supplementation did not influence the production of compound
1. This was confirmed by positive ion electrospray LC-MS screening of the two extracts, which both displayed the peak at
m/
z 482.2650 (compound
1). Additionally, LC-MS analyses (
Figure S11) of the extracts also showed a peak corresponding to the characteristic loss of a nitro-radical (
m/
z 435.2642) from the molecular ion of
1 [
3].
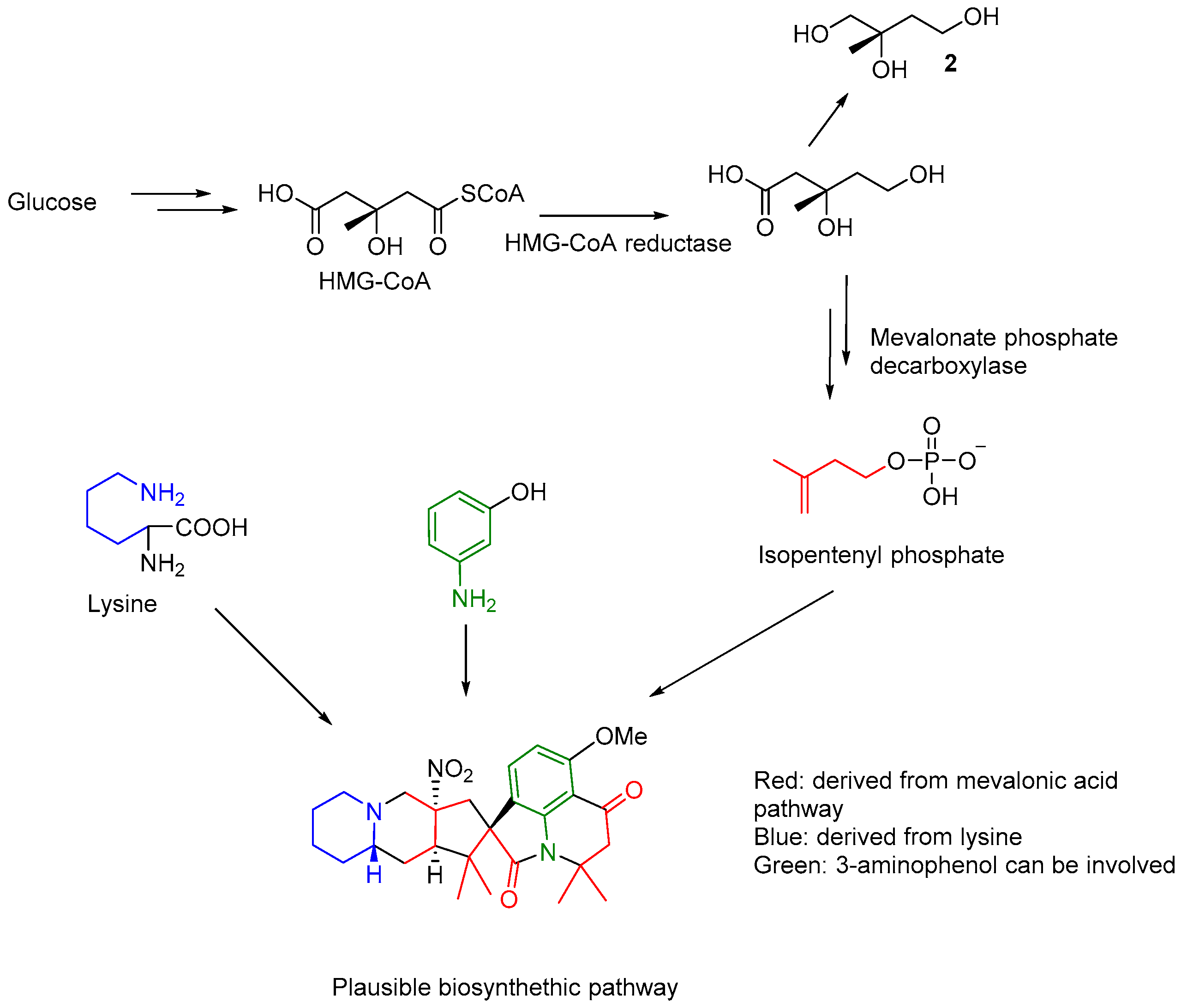
The possible of biosynthesis of citranilins and cyclopiamines have been studied using stable isotope (
13C) labelling experiments to conclude glucose, anthranilic acid and ornithine as proposed precursors [
6]. The present investigation led to the isolation of mevalonic acid derived compound 2-methylbutane-1,2,3-triol (
2), suggesting that the prenyl group in
1 may be introduced from the catabolism of glucose through mevalonic pathway. Lysine instead ornithine was may be involved in the biosynthesis of the indolizidine component of
1, analogous to ornithine in clopiamine C. The clear observation of three units of isoprene and a lysine residue in the structure of
1 suggests that further investigation is needed to understand if an aminophenol related compound (apart from the reported anthranilic acid) may be involved in its biosynthesis (
Figure 3).
Next, the ethyl acetate fraction was subjected to fluorine NMR experiments (1H coupled and decoupled experiments). As results, the presence of fluorinated compounds was clearly observed in the two experiments performed (Data not shown). However, fluorinated compounds were not isolated during this study due to their apparent instability of during the isolation process.
Antibacterial Activity of Ent-homocyclopiamine B
Prenylated indole alkaloids have been reported to wide-ranging bioactivities including antibacterial, cytotoxic, and insecticidal properties. The antibacterial activity of
1 was tested against a panel of Gram-positive and -negative strains. Although
1 inhibited growth of
Bacillus subtilis ATCC 6633 and
Mycobacterium smegmatis NRRL B-14646 on agar plates, zones were significantly smaller than from an equivalent amount of kanamycin (
Figure S11). Compound
1 showed no activity against
Escherichia coli K12,
Salmonella LT2,
Micrococcus luteus ATCC 4698,
Pseudomonas putida PRS2000, and
Serratia marcescens NRRL B-2544 in this assay.
B. subtilis ATCC 6633, Rhodococcus jhostii RHA1, and Corynebacterium glutamicum NRRL B-2784 were partially inhibited in microbroth dilution assays with 100 μM of 1 (30% inhibition compared with background controls), but not with any concentrations below. No detectable activity was observed against any of the other tested strains.
In summary, inhibition was observed against some (but not all) of the tested Gram-positive strains. None of the tested Gram-negative bacteria were susceptible to 1.
3. Materials and Methods
1H and 13C-NMR spectra were recorded at 25 °C with a Bruker Avance III 400 HD NMR spectrometer and Bruker Avance III HD Ascend 700 MHz. High-resolution mass spectra were acquired with a Thermo LTQ Orbitrap, specifications; analyzer: ITMS and FTMS, mass range: 50–4000 m/z, resolution: 7500–100,000. Optical rotations were determined on an Anton Paar MCP 150 polarimeter. Ultraviolet spectra (UV) were recorded using a Hitachi U-2910 UV/Vis double-beam spectrophotometer (Hitachi High- Technologies America, Schaumburg, IL, USA). Circular dichroism was measured on a Jasco J-810 spectropolarimeter. Thermo LTQ Orbitrap and Agilent 1100 HPLC were used to measure LC-MS spectra.
3.1. Fungal Source
Penicillium concentricum, the endophytic fungus used in this study was isolated from healthy liverwort
Trichocolea tomentella and identified as previously described [
1].
3.2. Solvent Extraction and Partition of Fungal Material Fermented on Rice Medium Supplemented with Sodiummonofluoroacetate
Eleven-days fungal culture in rice medium supplemented with sodium monofluoroacetate was soaked with (2 × 3 L) EtOAc and left for overnight at room temperature. The EtOAc extract was evaporated under vacuum to yield a greenish residue (2.3 g). The extract residue was dissolved in methanol and partitioned with hexane (3 × 250 mL) to afford 1.1 g of hexanes and 367 mg of methanol fractions. The MeOH fraction was fractionated on C18 reversed-phase silica gel liquid chromatography using 40% aqueous methanol (150 mL) followed by 70% aqueous methanol (150 mL) and later 100% methanol to yield three fractions (B2-1, 127 mg), (B2-2, 78 mg) and (B2-3, 51 mg). B2-1 (127 mg) was subjected to Silica gel column chromatography (2.2 cm × 28 cm; solvent system: 15:6:1 (CHCl3: MeOH: H2O)) to give six sub-fractions B2-1-1 through B2-1-6. Fraction B2-1-1 and B2-1-4 yielded compound 1 (0.93 mg) and compound 2 (3.7 mg), respectively.
3.3. LC-MS Method
LC-MS experiment was performed using Agilent 1100 HPLC binary and Thermo LTQ Orbitrap Mass spectrometer. For the HPLC, Beckman ODS column (5 μm, 4.6 mm, 25 cm, Part#235329) heated at 25 °C (oven temperature). Sample was injected to a water (containing 0.1% formate)-Acetonitrile (containing 0.1% formate) gradient from 5% to 95% (
v/
v) over 20 min with a 5 min hold at 95% (
v/
v) for 5 min. The column was then reequilibrated to 5% (
v/
v) for 3 min. The flow rate was kept at 1 mL/min. Mass spectrometry aquisitions were performed using instrument settings as described in
SI.
Ent-homocyclopiamine B (
1): White powder, [α]
D25 −22.7 (c 0.1, MeOH); CD (c 0.8, MeOH) ∆ε (nm) +2.8 (229), −6.08 (242), +2.82 (265), −0.92 (356); IR (NaCl, thin film) ν
max: 2990, 2932, 1706, 1614, 1589, 1353, 1221, 1139;
1H-NMR and
13C-NMR spectral data, see
Table 1; positive HRESIMS
m/
z 482.2643 ([M + H]
+ which corresponds to a molecular formula of C
27H
35N
3O
5 (calcd for C
27H
36N
3O
5+, 482.2649).
2-methyl-1,2,4-butanetriol (2): Colorless oil; 1H-NMR (400 MHz, CD3OD) δ 1.19 (3H, s, H-5), 1.76 (2H, dddd, J = 6.5 Hz, 13.9 Hz, 6.8 Hz, H-3), 3.39 (2H, dd, J = 15.5 Hz, H-1), 3.39 (2H, dddd, J = 15.5 Hz, 6.8 Hz, 6.5 Hz, H-4), HRESIMS m/z 143.0678 (calcd for C5H12NaO3+, 143.0684).
3.4. Antibacterial Testing
Assayed strains included Escherichia coli K12, Salmonella enterica serovar Typhimurium LT2, Pseudomonas putida PRS2000, Serratia marcescens NRRL B-2544, Bacillus subtilis ATCC 6633, Micrococcus luteus ATCC 4698, Mycobacterium smegmatis NRRL B-14616, Corynebacterium glutamicum NRRL B-2784, and Rhodococcus jhostii RHA1. Strains PRS2000, ATCC 4698 and RHA1 were grown on nutrient broth (5 g peptone, 3 g meat extract, per L) at 30 °C and all others on LB (5 g NaCl, 5 g yeast extract, 10 g tryptone, per L) at 37 °C. Bacto agar (16 g L−1) was added for plates.
Growth inhibition was evaluated on plates inoculated with lawns of the test strains. 50 μL of an overnight culture of bacteria was added to 5 mL of broth and grown until the culture reached a turbidity equal to 1 × 107–2 × 108 CFU/mL (based on a previously determined calibration curve). 100 μL was then evenly spread onto plates using sterile glass beads to dryness. 50 nmoles ent-homocyclopiamine B (dissolved in 5 μL 25% DMSO), 50 nmoles kanamycin (dissolved in 5 μL water), or diluent controls (5 μL of sterile water or 5 μL 25% DMSO) were applied, allowed to dry, and then incubated for 16–20 h at the growth temperature appropriate for each strain.
The broth microdilution assay was performed as outlined by the Clinical and Laboratory Standards Institute (CLSI) [
7]. Briefly, 2 μL of a 50X stock of
ent-homocyclopiamine B (dissolved in 25% DMSO) was added to wells of a 96-well plate. Live and background controls received 2 μL of diluent. 88 μL of broth was added to all wells except background control wells which received 98 μL. 50 μL of an overnight culture of bacteria was added to 5 mL of broth and grown until the culture reached a turbidity equal to 1 × 10
7–2 × 10
8 CFU/mL (based on a previously determined calibration curve). The culture was diluted to 5 × 10
6 CFU/mL and 10 μL added to each well except background controls to a final concentration of 5 × 10
5 CFU/mL. Plates were incubated at 30 °C or 37 °C (as appropriate for each strain) for 16–20 h. Absorbance was then recorded on a Bio-Rad xMark microplate spectrophotometer at λ = 600nm. Minimal inhibitory concentration (MIC) values were defined as the lowest concentration resulting in ≥ 90% growth inhibition.