Chemical Profile and Multicomponent Quantitative Analysis for the Quality Evaluation of Toad Venom from Different Origins
Abstract
1. Introduction
2. Results and Discussion
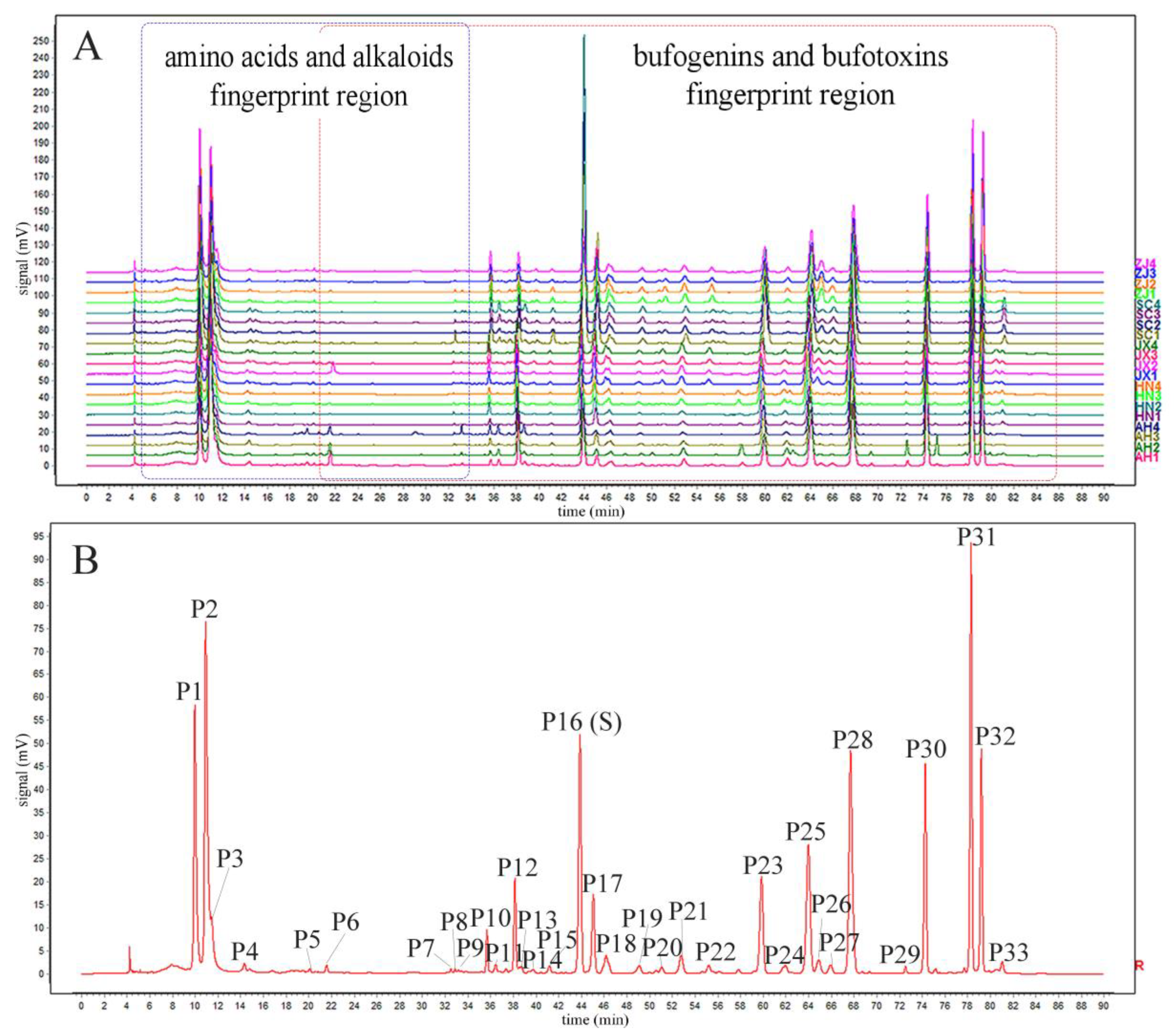
2.1. Optimization of Chromatographic Conditions for HPLC Fingerprint
2.2. Method Validation for HPLC Fingerprint Analysis
2.3. Method Validation for Multicomponent Quantitative Analysis of HPLC-DAD
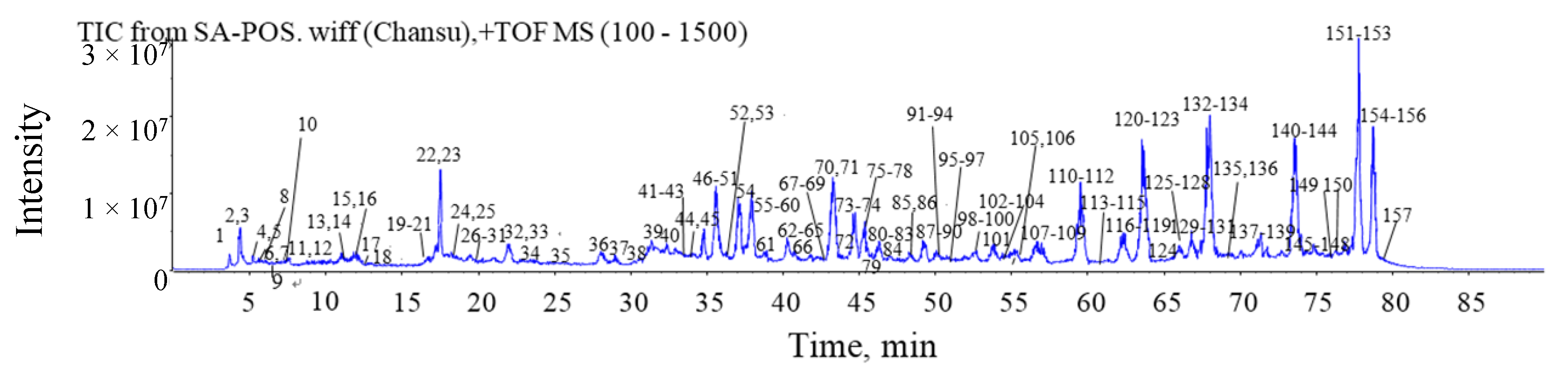
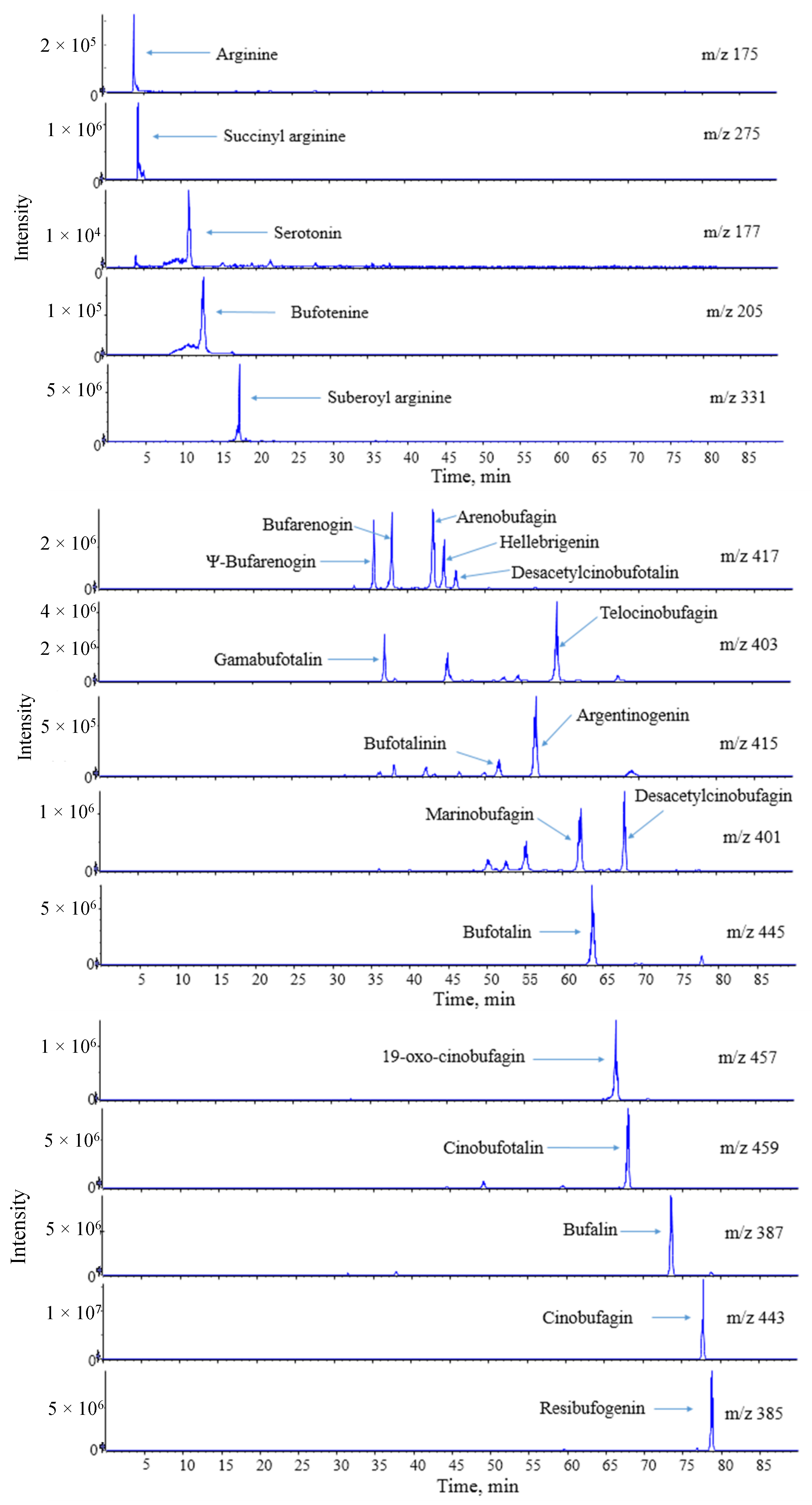
2.4. HPLC-ESI-Q-TOF-MS/MS Analysis for Chansu
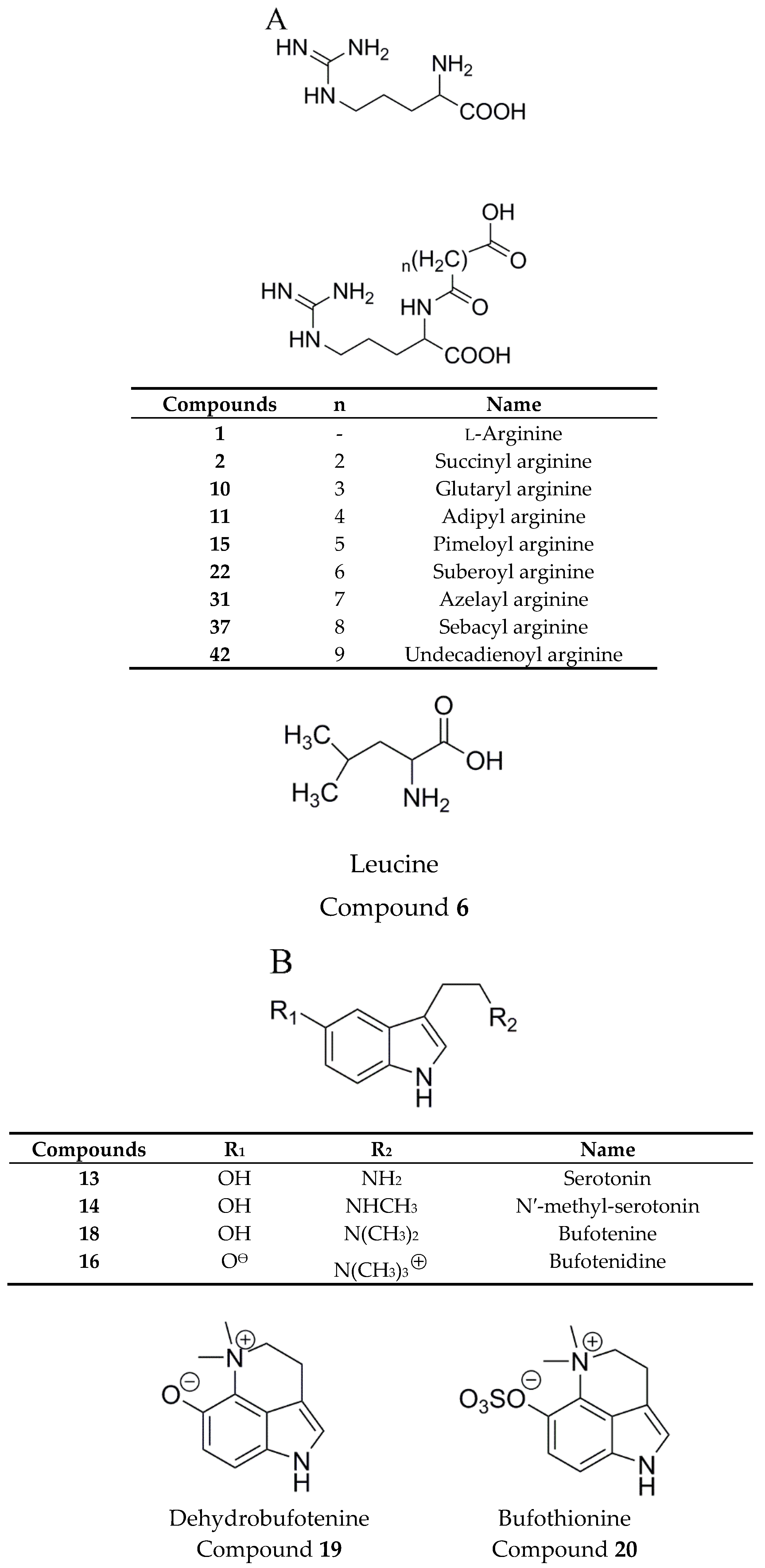
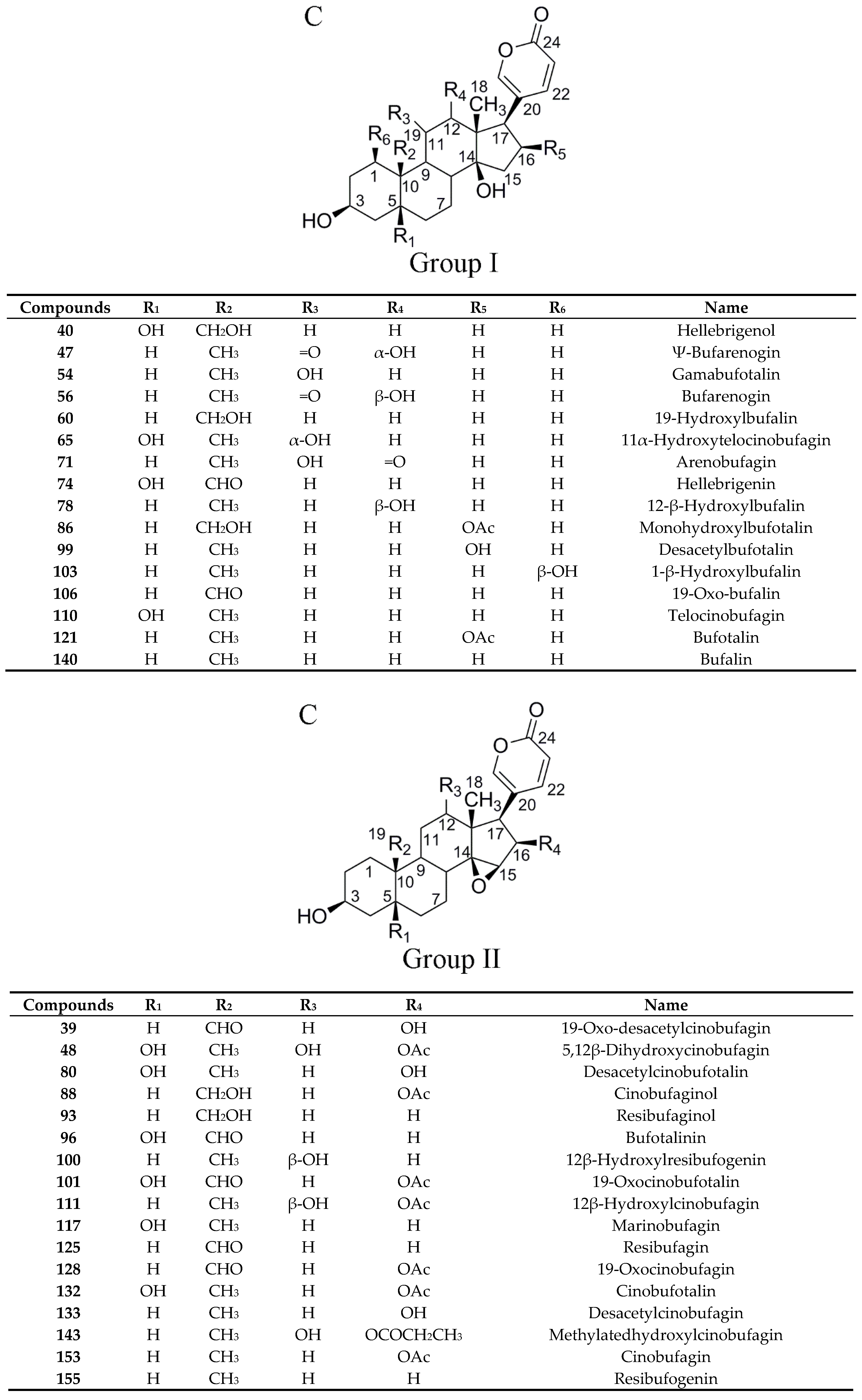
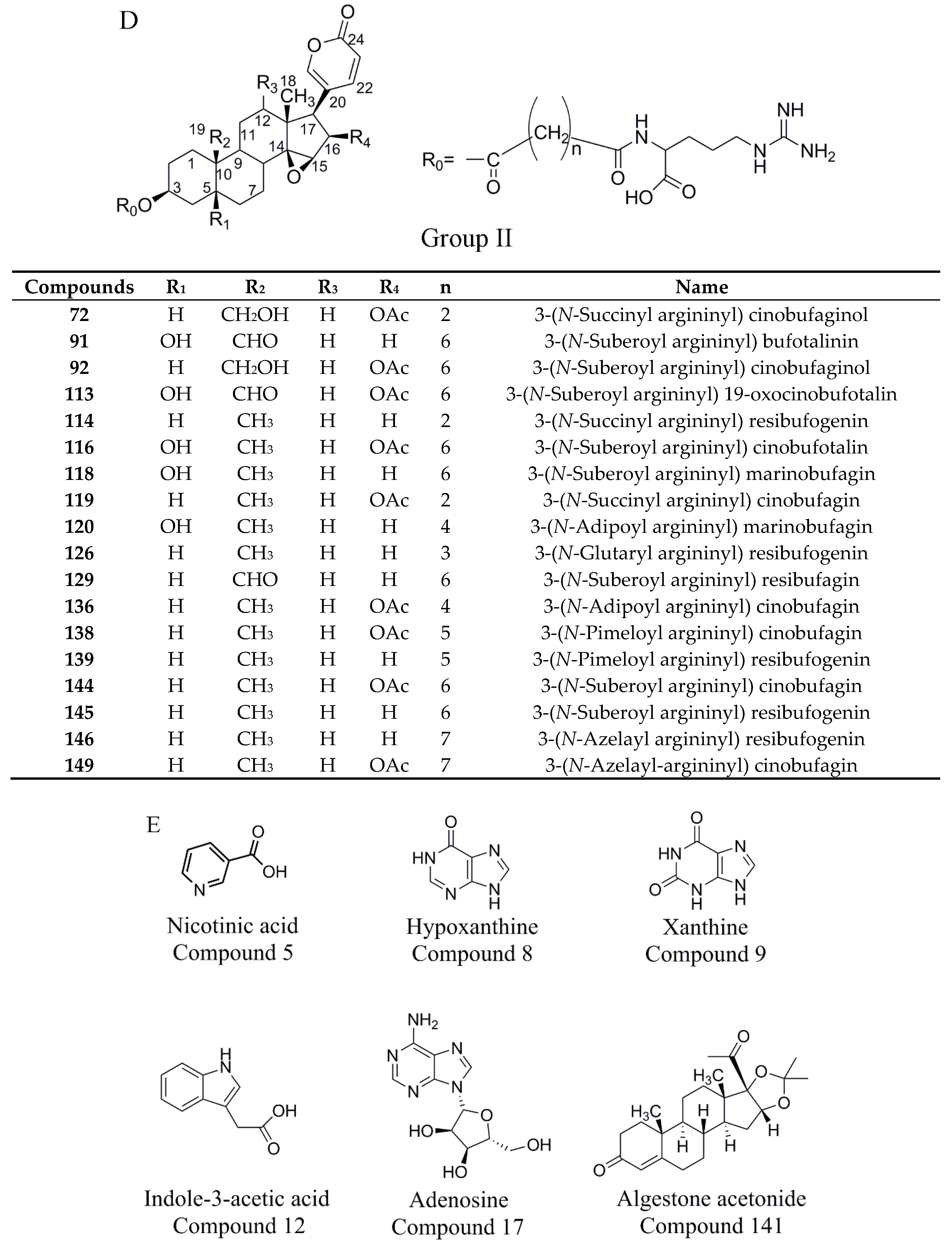
2.4.1. Establishment of Chemical Compositions Database for Chansu
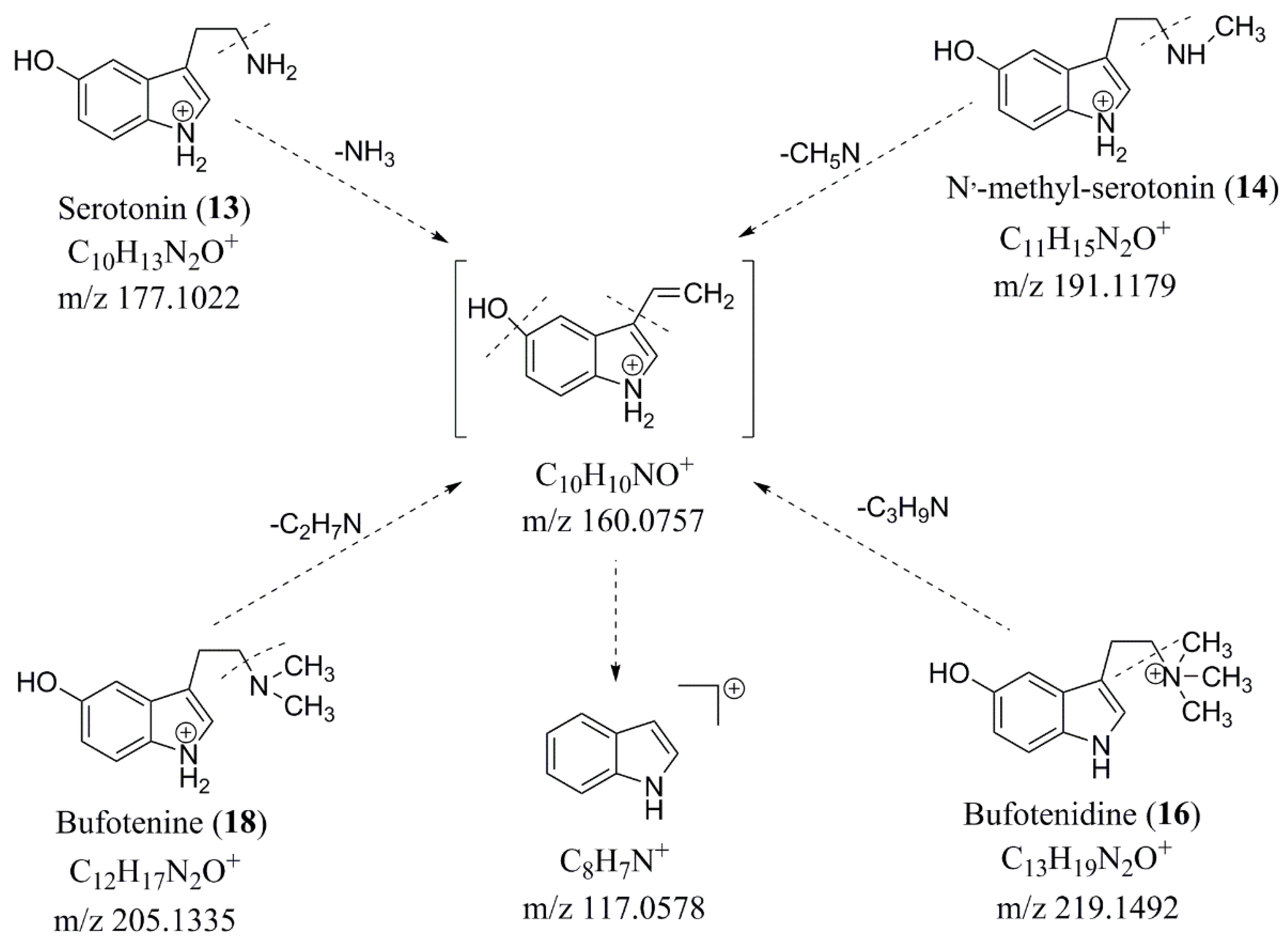
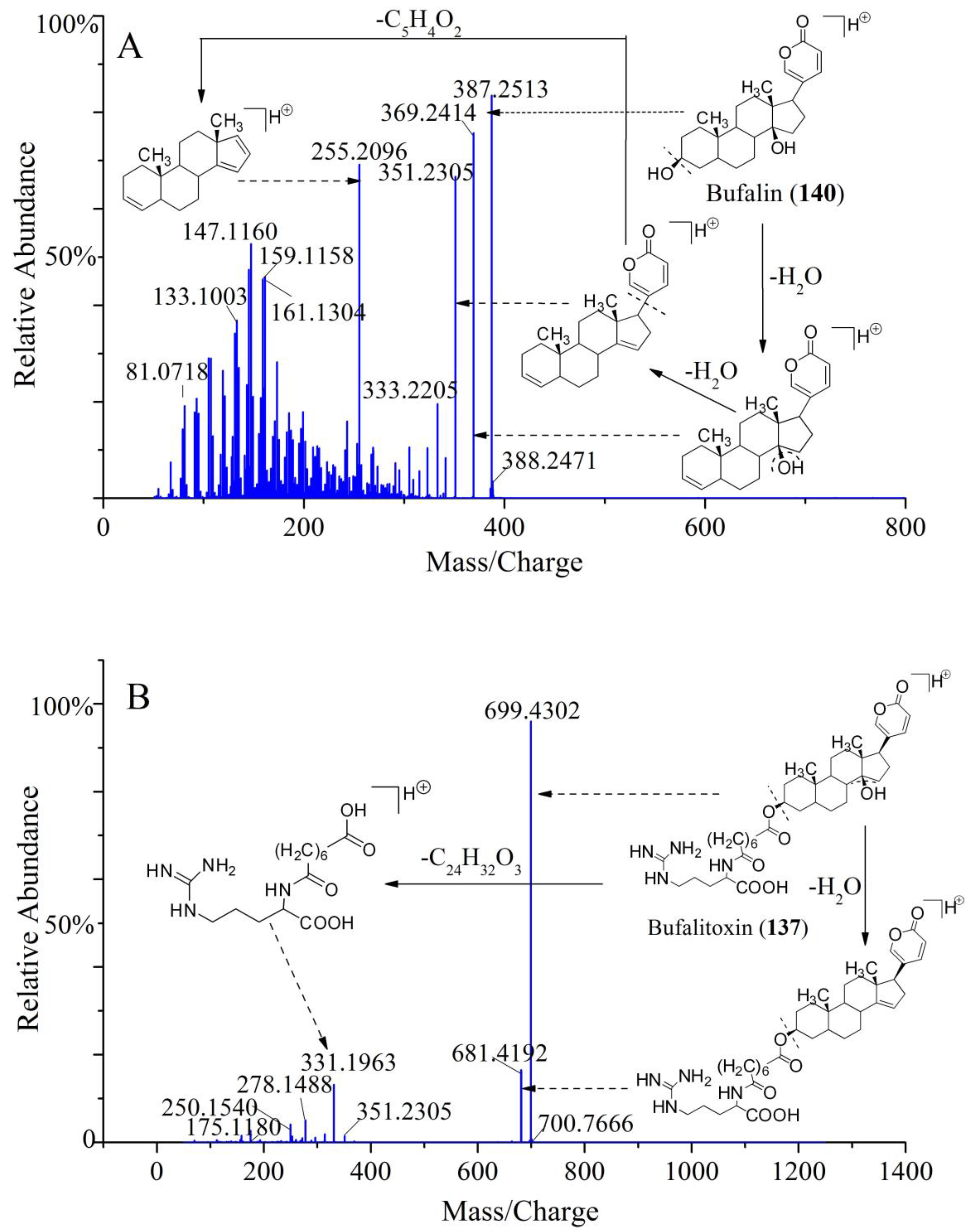
2.4.2. Identification of Chemical Constituents in Methanol Extract of Chansu
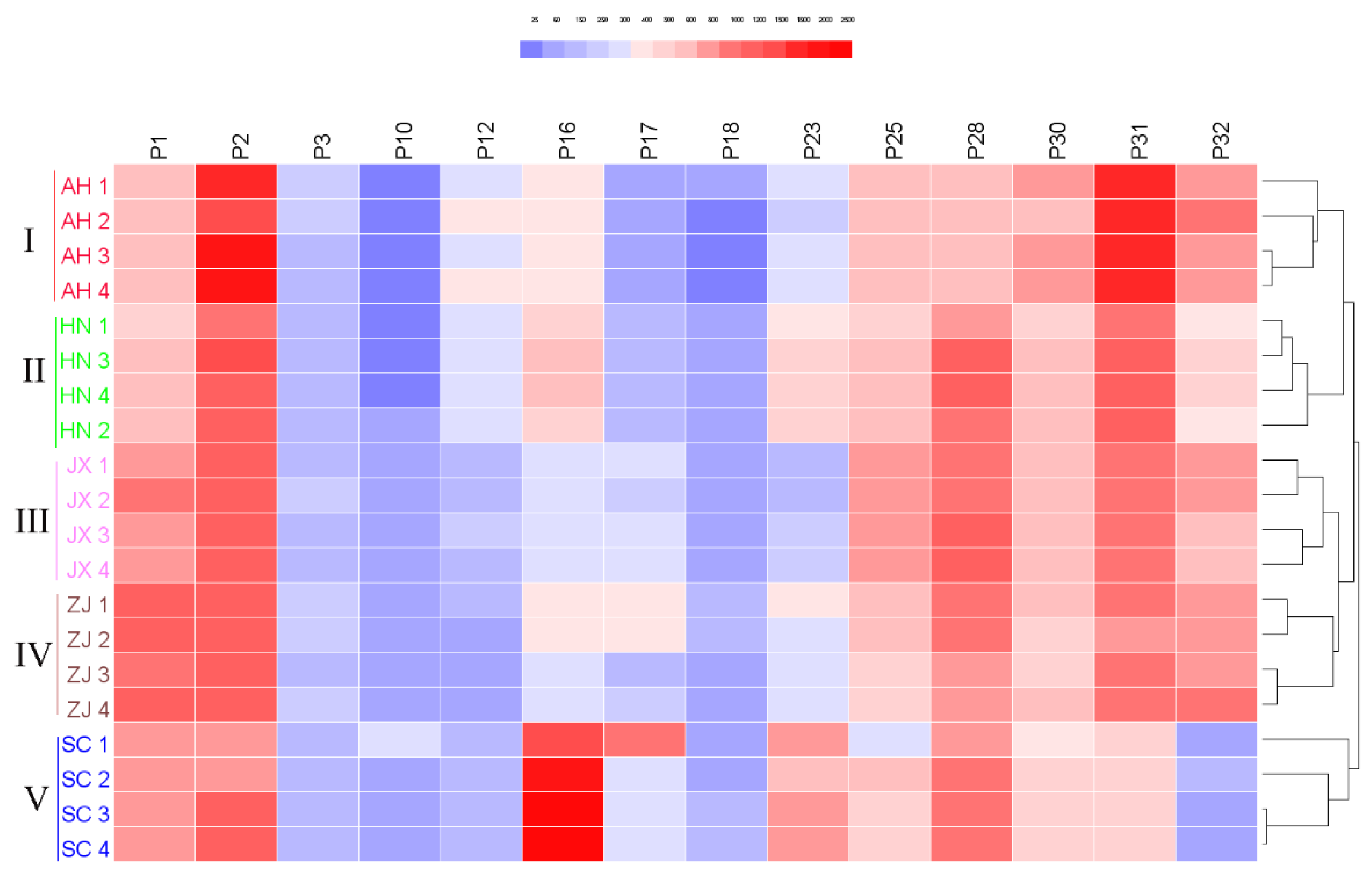
2.5. HPLC Fingerprint and Chemical Similarity Analysis of Chansu
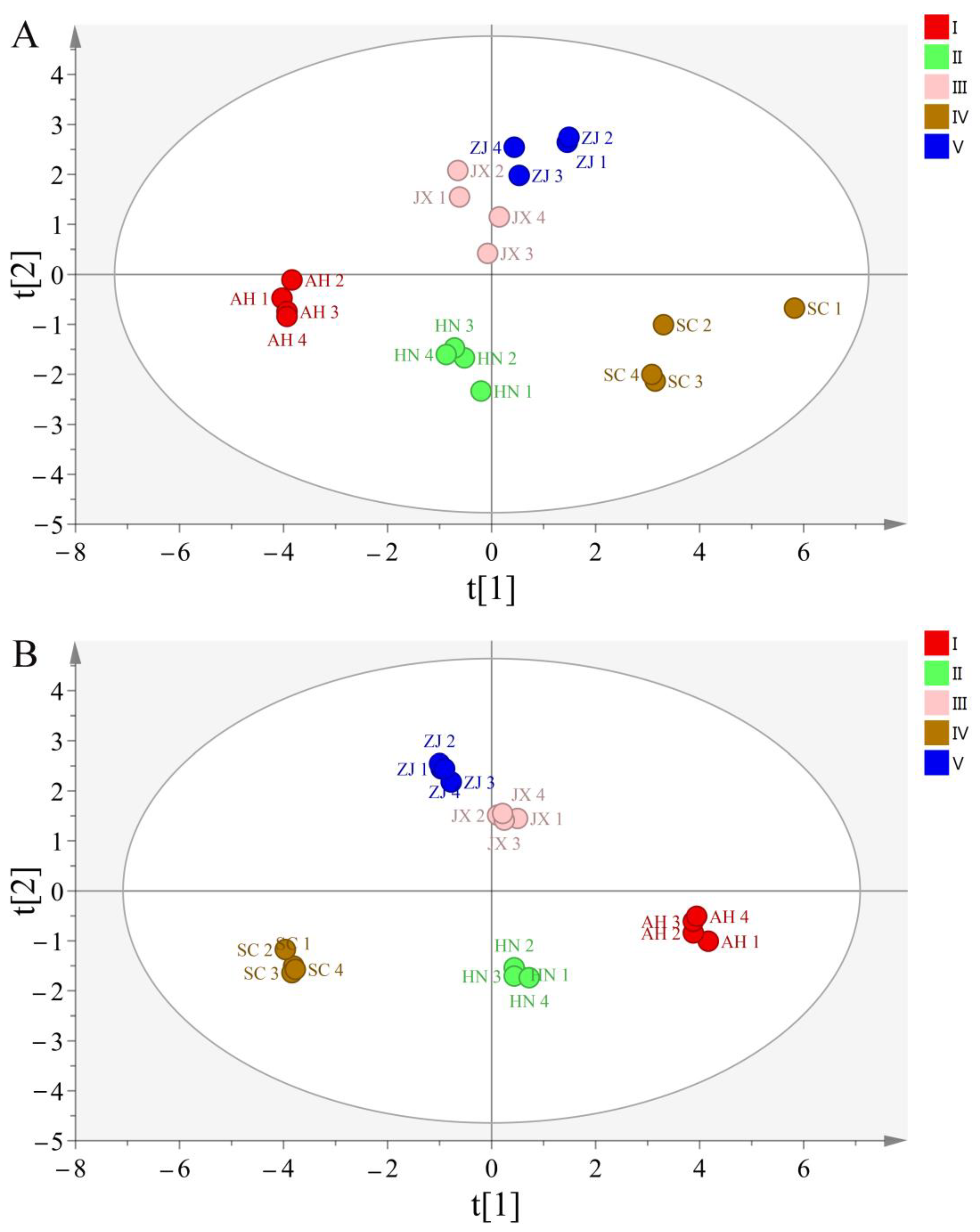
2.6. The Origin Characteristics Analysis of the Chansu from Different Geographical Origins
2.7. Quantitative Analysis of Seven Components in Chansu by HPLC-DAD
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Sample Solutions and Standard Solutions
3.3. Instrumentation and Chromatographic Conditions
3.4. Method Validation for HPLC Fingerprinting
3.5. Method Validation for the Quantitative Analysis of HPLC-DAD
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ye, M.; Guo, D.A. Analysis of bufadienolides in the Chinese drug ChanSu by high-performance liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.L.; Jin, H.Z.; Yan, S.K.; Zhang, W.D. Analysis of the bioactive constituents of ChanSu in rat plasma by high performance liquid chromatography with mass spectrometric detection. J. Pharm. Biomed. Anal. 2010, 53, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Kopp, B. Bufadienolides from animal and plant sources. Phytochemistry 1998, 48, 1–29. [Google Scholar] [CrossRef]
- Hu, Y.M.; Yu, Z.L.; Yang, Z.J. Comprehensive chemical analysis of Venenum Bufonis by using liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.H.; Li, A.Y.; Inagaki, Y. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int. Immunopharmacol. 2011, 11, 342–349. [Google Scholar] [CrossRef]
- Yu, Z.L.; Guo, W.; Ma, X.C. Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKK β/NF-κB signaling pathway in lung cancer cells 1–14. Mol. Cancer 2014, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Wang, L.H.; Peng, C.K. Fibroblast Activation Protein α Activated Tripeptide Bufadienolide Antitumor Prodrug with Reduced Cardiotoxicity. J. Med. Chem. 2017, 60, 5320–5333. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yau, L.F.; Lu, J.G.; Wu, Z.Z.; Zhang, B.X.; Wang, J.R.; Jiang, Z.H. Chemical profiling and cytotoxicity assay of bufadienolides in toad venom and toad skin. J. Ethnopharmacol. 2016, 187, 74–82. [Google Scholar] [CrossRef]
- Ma, H.Y.; Zhou, J.; Jiang, J.J.; Duan, J.A.; Xu, H.Q.; Tang, Y.P.; Lv, G.H.; Zhang, J.F.; Zhan, Z.; Ding, A.W. The novel antidote Bezoar Bovis prevents the cardiotoxicity of Toad (Bufo bufo gargarizans Canto) Venom in mice. Exp. Toxicol. Pathol. 2012, 64, 417–423. [Google Scholar] [CrossRef]
- Ye, M.; Guo, H.; Guo, H.Z. Simultaneous determination of cytotoxic bufadienolides in the Chinese medicine ChanSu by high-performance liquid chromatography coupled with photodiode array and mass spectrometry detections. J. Chromatogr. B. 2006, 838, 86–95. [Google Scholar] [CrossRef]
- Xing, J.F.; Chen, L.; Song, J. Separation and determination of resibufogenin and cinobufagin in Chansu using reversed-phase liquid chromatography with γ-cyclodextrin as mobile-phase modifier. J. Sep. Sci. 2012, 35, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Z.; Xie, P.S.; Chan, K. Perspective of Chemical Fingerprinting of Chinese Herbs. Planta Med. 2010, 76, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.H.; Cheng, Y.Y.; Ye, Z.L. Multiple chromatographic fingerprinting and its application to the quality control of herbal medicines. Anal. Chim. Acta 2006, 555, 217–224. [Google Scholar] [CrossRef]
- Jing, J.; Ren, W.C.; Chen, S.B. Advances in analytical technologies to evaluate the quality of traditional Chinese medicines. Trac.-Trends Anal. Chem. 2013, 44, 39–45. [Google Scholar] [CrossRef]
- Wang, C.Q.; Jia, X.H.; Zhu, S. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta 2015, 134, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopeia Commission. Pharmacopeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume 8, p. 76. [Google Scholar]
- Wang, N.; Li, Z.Y.; Zheng, X.L. Quality Assessment of Kumu Injection, a Traditional Chinese Medicine Preparation, Using HPLC Combined with Chemometric Methods and Qualitative and Quantitative Analysis of Multiple Alkaloids by Single Marker. Molecules 2018, 23, 856. [Google Scholar] [CrossRef]
- Xie, G.Y.; Xu, Q.H.; Li, R. Chemical profiles and quality evaluation of Buddleja officinalis flowers by HPLC-DAD and HPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 164, 283–295. [Google Scholar] [CrossRef]
- Huang, W.P.; Tan, T.; Li, Z.F. Structural characterization and discrimination of Chimonanthus nitens Oliv. leaf from different geographical origins based on multiple chromatographic analysis combined with chemometric methods. J. Pharm. Biomed. Anal. 2018, 154, 236–244. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, C.; Yang, F.L. UHPLC-ESI-Q-TOF-MS/MS analysis, antioxidant activity combined fingerprints for quality consistency evaluation of compound liquorice tablets. RSC Adv. 2018, 8, 27661–27673. [Google Scholar] [CrossRef]
- Ren, W.; Han, L.Y.; Luo, M.Y. Multi-component identification and target cell-based screening of potential bioactive compounds in toad venom by UPLC coupled with high-resolution LTQ-Orbitrap MS and high-sensitivity Qtrap MS. Anal. Bioanal. Chem. 2018, 410, 4419–4435. [Google Scholar] [CrossRef]
- Hirschmann, G.S.; Quispe, C.; Arana, G.V. Antiproliferative activity and chemical composition of the venom from the Amazonian toad Rhinella marina (Anura: Bufonidae). Toxicon 2016, 121, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.S.; Ferreira, J.M.; Predes, D.; Abreu, J.G.; Noël, F.; Eduardo, L.M. QuintasTelocinobufagin and Marinobufagin Produce Different Effects in LLC-PK1 Cells: A Case of Functional Selectivity of Bufadienolides. Int. J. Mol. Sci. 2018, 19, 2769. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Compound | Regressive Equation | Linear Range (μg/mL) | R2 | LOD (μg/mL) | LOQ (μg/mL) | Precision | Repeatability RSD% (n = 6) | Recovery | Stability RSD% | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intraday RSD% (n = 6) | Interday RSD% (n = 6) | Mean | RSD% (n = 6) | ||||||||
| GB | y = 8.5450x + 0.4726 | 2.632–263.2 | 0.9995 | 0.1053 | 0.3290 | 0.86 | 1.73 | 1.76 | 99.50 | 1.44 | 1.19 |
| TBG | y = 8.4668x − 2.6368 | 3.380–338.0 | 0.9995 | 0.2253 | 0.6760 | 0.74 | 1.19 | 1.30 | 101.18 | 1.68 | 0.97 |
| BFL | y = 7.6345x − 1.2852 | 2.548–254.8 | 0.9995 | 0.0849 | 0.2548 | 0.37 | 2.92 | 1.47 | 100.08 | 1.53 | 1.45 |
| CFL | y = 7.8537x − 2.4578 | 3.852–385.2 | 0.9995 | 0.2568 | 0.7704 | 0.37 | 2.45 | 1.51 | 98.91 | 1.52 | 0.90 |
| BL | y = 8.7099x − 2.5159 | 2.560–256.0 | 0.9995 | 0.1024 | 0.3012 | 0.74 | 1.48 | 1.41 | 99.83 | 2.11 | 1.81 |
| CBG | y = 7.9887x − 3.5450 | 4.208–420.8 | 0.9994 | 0.2805 | 0.8416 | 0.57 | 2.07 | 1.40 | 98.18 | 1.82 | 0.58 |
| RBG | y = 8.5660x − 2.4699 | 3.424–342.4 | 0.9995 | 0.2283 | 0.6848 | 2.36 | 2.73 | 2.70 | 100.90 | 1.83 | 2.54 |
| No. | GB | TBG | BFL | CFL | BL | CBG | RBG |
|---|---|---|---|---|---|---|---|
| AH 1 | 10.99 ± 0.26 | 10.33 ± 0.16 | 19.11 ± 0.14 | 19.72 ± 0.35 | 27.23 ± 0.20 | 56.35 ± 0.62 | 25.50 ± 0.21 |
| AH 2 | 12.80 ± 0.46 | 8.14 ± 0.14 | 18.89 ± 0.25 | 17.92 ± 0.45 | 23.27 ± 0.27 | 54.10 ± 0.67 | 28.90 ± 0.25 |
| AH 3 | 11.48 ± 0.21 | 9.53 ± 0.04 | 19.07 ± 0.20 | 18.93 ± 0.04 | 25.64 ± 0.14 | 55.44 ± 0.45 | 26.99 ± 0.24 |
| AH 4 | 11.56 ± 0.26 | 9.22 ± 0.08 | 18.99 ± 0.03 | 18.78 ± 0.07 | 24.28 ± 1.25 | 55.47 ± 0.47 | 27.29 ± 0.41 |
| HN 1 | 9.51 ± 0.48 | 17.05 ± 0.69 | 19.33 ± 0.51 | 31.13 ± 0.83 | 17.39 ± 0.72 | 35.17 ± 0.74 | 14.78 ± 0.99 |
| HN 2 | 10.48 ± 0.53 | 15.16 ± 0.70 | 19.57 ± 0.96 | 27.54 ± 0.85 | 18.77 ± 0.71 | 37.31 ± 1.84 | 12.97 ± 0.90 |
| HN 3 | 9.56 ± 0.38 | 16.26 ± 0.78 | 18.88 ± 0.78 | 30.28 ± 1.42 | 18.04 ± 0.70 | 40.78 ± 0.56 | 14.17 ± 0.31 |
| HN 4 | 9.72 ± 0.57 | 15.74 ± 0.38 | 18.47 ± 0.84 | 30.57 ± 0.46 | 17.99 ± 0.72 | 39.35 ± 1.80 | 15.07 ± 0.61 |
| JX 1 | 6.24 ± 0.37 | 6.35 ± 0.36 | 25.19 ± 0.86 | 26.27 ± 0.78 | 20.64 ± 0.47 | 32.23 ± 0.55 | 23.72 ± 0.49 |
| JX 2 | 6.26 ± 0.18 | 6.81 ± 0.33 | 24.14 ± 1.02 | 26.02 ± 0.59 | 18.94 ± 1.07 | 32.32 ± 1.52 | 23.09 ± 1.19 |
| JX 3 | 8.12 ± 0.09 | 8.46 ± 0.26 | 27.23 ± 0.27 | 29.74 ± 0.22 | 18.53 ± 0.98 | 28.99 ± 0.49 | 17.38 ± 1.19 |
| JX 4 | 7.17 ± 0.10 | 7.94 ± 0.14 | 25.29 ± 0.77 | 29.54 ± 0.48 | 18.87 ± 0.14 | 30.84 ± 0.36 | 18.62 ± 0.26 |
| SC 1 | 7.21 ± 0.20 | 25.56 ± 0.65 | 11.11 ± 0.30 | 26.25 ± 0.74 | 12.71 ± 0.43 | 14.64 ± 0.42 | 2.92 ± 0.18 |
| SC 2 | 5.89 ± 0.36 | 21.97 ± 0.28 | 19.00 ± 0.23 | 30.56 ± 0.39 | 15.43 ± 0.06 | 16.22 ± 0.20 | 6.71 ± 0.16 |
| SC 3 | 6.69 ± 0.43 | 24.57 ± 0.51 | 18.05 ± 0.48 | 31.35 ± 0.56 | 16.36 ± 0.39 | 16.06 ± 0.37 | 2.91 ± 0.19 |
| SC 4 | 6.58 ± 0.29 | 24.23 ± 0.38 | 17.74 ± 0.25 | 31.39 ± 0.66 | 16.13 ± 0.27 | 15.81 ± 0.06 | 2.90 ± 0.13 |
| ZJ 1 | 5.34 ± 0.11 | 11.86 ± 0.28 | 19.84 ± 0.48 | 22.08 ± 0.84 | 18.68 ± 0.58 | 30.02 ± 0.72 | 23.92 ± 0.46 |
| ZJ 2 | 5.10 ± 0.14 | 11.41 ± 0.26 | 19.17 ± 0.48 | 21.73 ± 0.50 | 17.97 ± 0.41 | 29.17 ± 0.77 | 23.49 ± 0.47 |
| ZJ 3 | 4.28 ± 0.09 | 9.03 ± 0.20 | 16.45 ± 0.19 | 19.08 ± 0.68 | 18.07 ± 0.15 | 29.56 ± 0.38 | 26.98 ± 0.19 |
| ZJ 4 | 4.54 ± 0.13 | 9.65 ± 0.27 | 17.33 ± 0.25 | 19.67 ± 1.07 | 18.91 ± 0.28 | 30.99 ± 0.40 | 28.50 ± 0.43 |
| RSD (%) | 32.46 | 46.66 | 18.06 | 20.07 | 18.30 | 39.08 | 48.85 |
| No. | Source | No. | Source |
|---|---|---|---|
| AH 1 | Anhui | JX 3 | Jiangxi |
| AH 2 | Anhui | JX 4 | Jiangxi |
| AH 3 | Anhui | SC 1 | Sichuan |
| AH 4 | Anhui | SC 2 | Sichuan |
| HN 1 | Henan | SC 3 | Sichuan |
| HN 2 | Henan | SC 4 | Sichuan |
| HN 3 | Henan | ZJ 1 | Zhejiang |
| HN 4 | Henan | ZJ 2 | Zhejiang |
| JX 1 | Jiangxi | ZJ 3 | Zhejiang |
| JX2 | Jiangxi | ZJ4 | Zhejiang |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Wu, J.; Pan, H.; Wang, L. Chemical Profile and Multicomponent Quantitative Analysis for the Quality Evaluation of Toad Venom from Different Origins. Molecules 2019, 24, 3595. https://doi.org/10.3390/molecules24193595
Cao Y, Wu J, Pan H, Wang L. Chemical Profile and Multicomponent Quantitative Analysis for the Quality Evaluation of Toad Venom from Different Origins. Molecules. 2019; 24(19):3595. https://doi.org/10.3390/molecules24193595
Chicago/Turabian StyleCao, Yueting, Jiheng Wu, Hongye Pan, and Longhu Wang. 2019. "Chemical Profile and Multicomponent Quantitative Analysis for the Quality Evaluation of Toad Venom from Different Origins" Molecules 24, no. 19: 3595. https://doi.org/10.3390/molecules24193595
APA StyleCao, Y., Wu, J., Pan, H., & Wang, L. (2019). Chemical Profile and Multicomponent Quantitative Analysis for the Quality Evaluation of Toad Venom from Different Origins. Molecules, 24(19), 3595. https://doi.org/10.3390/molecules24193595







