Michaelis-Arbuzov-Type Reaction of 1-Imidoalkyltriarylphosphonium Salts with Selected Phosphorus Nucleophiles
Abstract
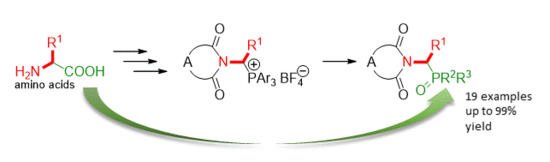
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Substrate Synthesis
3.2.2. General Procedure for the Reaction of 1-Imidoalkyltriarylphosphonium Salts 2 with Phosphorus Nucleophiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kukhar, V.; Hudson, H. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Kafarski, P.; Lejczak, B. Aminophosphonic Acids of Potential Medical Importance. Curr. Med. Chem. Anti Cancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, M.; Rojas-Cabrera, H.; Cativiela, C. An overview of stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2009, 65, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic acids and derivatives. Synthesis and biological applications. Curr. Med. Chem. 2010, 17, 264–289. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids-phosphorus analogues of natural Amino Acids. Part 1: Syntheses of α-aminophosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable Potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821–12835. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, M.; Viveros-Ceballos, J.L.; Cativiela, C.; Sayago, F.J. An update on the stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2015, 71, 1745–1784. [Google Scholar] [CrossRef]
- Lewkowski, J.; Rodriguez Moya, M.; Chmielak, M.; Rogacz, D.; Lewicka, K.; Rychter, P. Synthesis, Spectral Characterization of Several Novel Pyrene-Derived Aminophosphonates and Their Ecotoxicological Evaluation Using Heterocypris incongruens and Vibrio fisheri Tests. Molecules 2016, 21, 936. [Google Scholar] [CrossRef]
- Cypryk, M.; Drabowicz, J.; Gostynski, B.; Kudzin, M.H.; Kudzin, Z.H.; Urbaniak, P. 1-(Acylamino)alkylphosphonic Acids—Alkaline Deacylation. Molecules 2018, 23, 859. [Google Scholar] [CrossRef]
- Huang, J.; Chen, R. An Overview of Recent Advances on the Synthesis and Biological Activity of α-Aminophosphonic Acid Derivatives. Heteroat. Chem. 2000, 11, 480–492. [Google Scholar] [CrossRef]
- Lavielle, G.; Hautefaye, P.; Schaeffer, C.; Boutin, J.A.; Cudennec, C.A.; Pierre, A. New alpha-amino phosphonic acid derivatives of vinblastine: Chemistry and antitumor activity. J. Med. Chem. 1991, 34, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-X.; Li, K.; Shi, D.-Q. A Convenient Synthesis and Herbicidal Activity of N-phosphonoalkylpyrazolo[4,3-e][1,2,4]-triazolo[1,5-d]pyrimidines. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 3156–3165. [Google Scholar] [CrossRef]
- Malachowski, W.P.; Coward, J.K. The Chemistry of Phosphapeptides: Formation of Functionalized Phosphonochloridates under Mild Conditions and Their Reaction with Alcohols and Amines. J. Org. Chem. 1994, 59, 7616–7624. [Google Scholar] [CrossRef]
- Pradhan, B.S.; Dhokiya, K.V. Pyrrole Derivatives. U.K. Patent GB2479830, 26 October 2011. [Google Scholar]
- Fathi, R.; Huang, Q.; Syi, J.-L.; Delaney, W.; Cook, A.F. (Aminomethyl)phosphonate derivatives of oligonucleotides. Bioconjug. Chem. 1994, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Nahrwold, M.; Bogner, T.; Eissler, S.; Verma, S.; Sewald, N. “Clicktophycin-52”: A Bioactive Cryptophycin-52 Triazole Analogue. Org. Lett. 2010, 12, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Plenat, F.; Cassagne, M.; Cristau, J.H. Synthesis of new phosphorus 2,4,5-imidazolidinetriones. Tetrahedron 1995, 51, 9551–9558. [Google Scholar] [CrossRef]
- Kálmán, F.K.; Woods, M.; Caravan, P.; Jurek, P.; Spiller, M.; Tircsó, G.; Király, R.; Brülcher, E.; Sherry, A.D. Potentiometric and Relaxometric Properties of a Gadolinium-Based MRI Contrast Agent for Sensing Tissue pH. Inorg. Chem. 2007, 46, 5260–5270. [Google Scholar] [CrossRef] [PubMed]
- Leygue, N.; De Heredia, A.P.; Galaup, C.; Benoist, E.; Lamarque, L.; Picard, C. New advances in the synthesis of tripyridinophane macrocycles suitable to enhance the luminescence of Ln(III) ions in aqueous solution. Tetrahedron 2018, 74, 4272–4287. [Google Scholar] [CrossRef]
- Abdou, W.M.; Khidre, R.E. Efficient synthesis routes for various phthalimido phosphor esters as antimicrobial agents in terms of structure–activity relationship. Monatsh. Chem. 2010, 141, 214–228. [Google Scholar] [CrossRef]
- Davidsen, S.K.; Phllips, G.W.; Martin, S.F. Geminal Acylation-Alkylation at a Carbonyl Center Using Diethyl N-Benzylideneaminomethylphosphonate: 2-Methyl-2-Phenyl-4-Pentenal. Org. Synth. 1987, 65, 119–134. [Google Scholar] [CrossRef]
- Hirschmann, R.; Yager, K.M.; Taylor, C.M.; Witherington, J.; Sprengeler, P.A.; Phillips, B.W.; Moore, W.; Smith, A.B. Phosphonate Diester and Phosphonamide Synthesis. Reaction Coordinate Analysis by 31P NMR Spectroscopy: Identification of Pyrophosphonate Anhydrides and Highly Reactive Phosphonylammonium Salts. J. Am. Chem. Soc. 1997, 119, 8177–8190. [Google Scholar] [CrossRef]
- Seyferth, D.; Marmor, R.S.; Hilbert, P. Reactions of dimethylphosphono-substituted diazoalkanes. (MeO)2P(O)CR transfer to olefins and 1,3-dipolar additions of (MeO)2P(O)C(N2)R. J. Org. Chem. 1971, 36, 1379–1386. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kinoshita, M.; Imoto, M. Peptides Containing Aminophosphonic Acids. II. The Synthesis of Tripeptide Analogs. Bull. Chem. Soc. Jpn. 1972, 45, 2531–2534. [Google Scholar] [CrossRef]
- Ösapay, G.; Szilagyi, I.; Seres, J. Conversion of amino acids and dipeptides into their phosphonic analogs: Aminoalkylphosphonic acids and peptides II. Tetrahedron 1987, 43, 2977–2983. [Google Scholar] [CrossRef]
- Elliott, R.L.; Marks, N.; Berg, M.J.; Portoghese, P.S. Synthesis and Biological Evaluation of Phosphonamidate Peptide Inhibitors of Enkephalinase and Angiotensin-Converting Enzyme. J. Med. Chem. 1985, 28, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Popoff, I.C.; Peter, B.B.; Huber, L.K. Aminoalkylphosphinic Acids. U.S. Patent 3,332,987, 25 July 1967. [Google Scholar]
- Popoff, I.C.; Huber, L.K.; Block, B.P.; Morton, P.D.; Riordan, R.P. α-Aminophosphinic Acids and α-Aminophosphine Oxides. I. Alkyl-α-aminoalkylphosphinic Acids, α-Aminoalkyl(aryl)phosphinic Acids, and α-Aminoalkyl(diaryl)phosphine Oxides. J. Org. Chem. 1963, 28, 2898–2900. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Guarneri, M.; Moroder, F.; Pollini, G.P.; Simoni, D. Synthesis of 1-Phthalimidoalkanephosphonates. Synthesis 1982, 653–655. [Google Scholar] [CrossRef]
- Chun, Y.-J.; Park, J.-N.; Oh, G.-M.; Hong, S.-I.; Kim, Y.-J. Synthesis of ω-Phthalimidoalkylphosphonates. Synthesis 1994, 909–910. [Google Scholar] [CrossRef]
- Adamek, J.; Mazurkiewicz, R.; Węgrzyk, A.; Erfurt, K. 1-Imidoalkylphosphonium salts with modulated Cα-P+ bond strength: Synthesis and application as new active α-imidoalkylating agents. Beilstein J. Org. Chem. 2017, 13, 1446–1455. [Google Scholar] [CrossRef]
- Adamek, J.; Węgrzyk, A. Catalyst-free Friedel-Crafts reaction of 1-(N-acylamino)- and 1-imidoalkyltriarylphosphonium salts with arenes. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 351–352. [Google Scholar] [CrossRef]
- Adamek, J.; Październiok-Holewa, A.; Zielińska, K.; Mazurkiewicz, R. Comparative Studies on the Amidoalkylating Properties of N-(1-Methoxyalkyl)Amides and 1-(N-Acylamino)Alkyltriphenylphosphonium Salts in the Michaelis–Arbuzov-Like Reaction: A New One-Pot Transformation of N-(1-Methoxyalkyl)Amides into Phosphonic or Phosphinic Analogs of N-Acyl-α-Amino Acids. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 967–980. [Google Scholar] [CrossRef]
- Adamek, J.; Węgrzyk, A.; Kończewicz, J.; Walczak, K.; Erfurt, K. 1-(N-Acylamino)alkyltriarylphosphonium Salts with Weakened Cα-P+ Bond Strength—Synthetic Applications. Molecules 2018, 23, 2453. [Google Scholar] [CrossRef] [PubMed]
- Październiok-Holewa, A.; Adamek, J.; Mazurkiewicz, R.; Zielińska, K. Amidoalkylating Properties of 1-(N-Acylamino)Alkyltriphenylphosphonium Salts. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 205–212. [Google Scholar] [CrossRef]
- Hoffmann, M.; Wasielewski, C. Amino phosphonic acids. Part VI. Diazomethane as a reagent in the synthesis of N-acylated 1-aminophosphonic acid esters. Roczniki Chem. 1976, 50, 139–146. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kinoshita, M.; Imoto, M. Peptides Containing Aminophosphonic Acids. I. Reactivity of α-Aminobenzylphosphonic Acid. Bull. Chem. Soc. Jpn. 1972, 45, 2528–2531. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 are available from the authors. |




| Entry | 2 | A | R1 | Ar | PN a (PR2R3OR) | Solvent | Molar Ratio of 2:PN:Catalyst | T, °C | Time, h | 1 | Yield, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OMe)3 | CHCl3 | 1:1.5:0.25 | 100 | 2 | 1a | 36 b |
| 2 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OMe)3 | CHCl3 | 1:5:0.25 | 100 | 2 | 1a | 70 b |
| 3 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OMe)3 | CHCl3 | 1:10:0.25 | 100 | 2 | 1a | 85 b |
| 4 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OMe)3 | CH3CN | 1:10:0.25 | 100 | 2 | 1a | 50 b |
| 5 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OMe)3 | C6H5Cl | 1:10:0.25 | 100 | 2 | 1a | 51 b |
| 6 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OMe)3 | CHCl3 | 1:10:0.25 | 100 | 2 | 1a | 76 c |
| 7 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OMe)3 | CHCl3 | 1:10:- | 100 | 2 | 1a | 52 c |
| 8 | 2b | o-C6H4 | Me | m-C6H4Cl | P(OMe)3 | CHCl3 | 1:10:0.25 | 120 | 2 | 1a | 70 c |
| 9 | 2c | o-C6H4 | Me | Ph | P(OMe)3 | CHCl3 | 1:10:0.25 | 150 | 0.5 | 1a | 22 c,d |
| 10 | 2d | o-C6H4 | H | m-C6H4Cl | P(OMe)3 | CHCl3 | 1:10:0.25 | 170 | 2 | 1b | 95 c |
| 11 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OEt)3 | CHCl3 | 1:10:0.25 | 100 | 2 | 1c | 90 c |
| 12 | 2a | o-C6H4 | Me | p-C6H4CF3 | P(OEt)3 | CHCl3 | 1:10:- | 100 | 2 | 1c | 86 c |
| 13 | 2e | o-C6H4 | Ph | p-C6H4CF3 | P(OEt)3 | CHCl3 | 1:10:0.25 | 80 | 0.5 | 1d | 99 c |
| 14 | 2e | o-C6H4 | Ph | p-C6H4CF3 | P(OEt)3 | CHCl3 | 1:10:- | 80 | 0.5 | 1d | 91 c |
| 15 | 2f | o-C6H4 | i-Bu | m-C6H4Cl | P(OEt)3 | CHCl3 | 1:10:0.25 | 120 | 2 | 1e | 94 c |
| 16 | 2f | o-C6H4 | i-Bu | m-C6H4Cl | P(OEt)3 | CHCl3 | 1:10:- | 120 | 2 | 1e | 83 c |
| 17 | 2g | (CH2)2 | H | p-C6H4CF3 | P(OEt)3 | CH3CN | 1:10:0.25 | 180 | 2 | 1f | nr e |
| 18 | 2h | (CH2)2 | Me | p-C6H4CF3 | P(OEt)3 | CH3CN | 1:30:0.25 | 150 | 2 | 1g | 65 c |
| 19 | 2a | o-C6H4 | Me | p-C6H4CF3 | PhP(OMe)2 | CHCl3 | 1:10:0.25 | 100 | 0.5 | 1h | 59 c,f |
| 20 | 2a | o-C6H4 | Me | p-C6H4CF3 | PhP(OMe)2 | CHCl3 | 1:10:- | 100 | 0.5 | 1h | 37 c,g |
| 21 | 2e | o-C6H4 | Ph | p-C6H4CF3 | PhP(OMe)2 | CHCl3 | 1:10:0.25 | 80 | 0.5 | 1i | 94 c,h |
| 22 | 2a | o-C6H4 | Me | p-C6H4CF3 | Ph2P(OMe) | CHCl3 | 1:10:0.25 | 100 | 0.5 | 1j | 47 c |
| 23 | 2a | o-C6H4 | Me | p-C6H4CF3 | Ph2P(OMe) | CHCl3 | 1:10:- | 100 | 0.5 | 1j | 35 c |
| 24 | 2e | o-C6H4 | Ph | p-C6H4CF3 | Ph2P(OMe) | CHCl3 | 1:10:0.25 | 80 | 0.5 | 1k | 88 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamek, J.; Węgrzyk-Schlieter, A.; Steć, K.; Walczak, K.; Erfurt, K. Michaelis-Arbuzov-Type Reaction of 1-Imidoalkyltriarylphosphonium Salts with Selected Phosphorus Nucleophiles. Molecules 2019, 24, 3405. https://doi.org/10.3390/molecules24183405
Adamek J, Węgrzyk-Schlieter A, Steć K, Walczak K, Erfurt K. Michaelis-Arbuzov-Type Reaction of 1-Imidoalkyltriarylphosphonium Salts with Selected Phosphorus Nucleophiles. Molecules. 2019; 24(18):3405. https://doi.org/10.3390/molecules24183405
Chicago/Turabian StyleAdamek, Jakub, Anna Węgrzyk-Schlieter, Klaudia Steć, Krzysztof Walczak, and Karol Erfurt. 2019. "Michaelis-Arbuzov-Type Reaction of 1-Imidoalkyltriarylphosphonium Salts with Selected Phosphorus Nucleophiles" Molecules 24, no. 18: 3405. https://doi.org/10.3390/molecules24183405
APA StyleAdamek, J., Węgrzyk-Schlieter, A., Steć, K., Walczak, K., & Erfurt, K. (2019). Michaelis-Arbuzov-Type Reaction of 1-Imidoalkyltriarylphosphonium Salts with Selected Phosphorus Nucleophiles. Molecules, 24(18), 3405. https://doi.org/10.3390/molecules24183405