Castanea sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-Apoptotic Effects
Abstract
1. Introduction
2. Results
2.1. Characterization of the Phenolic Compounds from CSDE
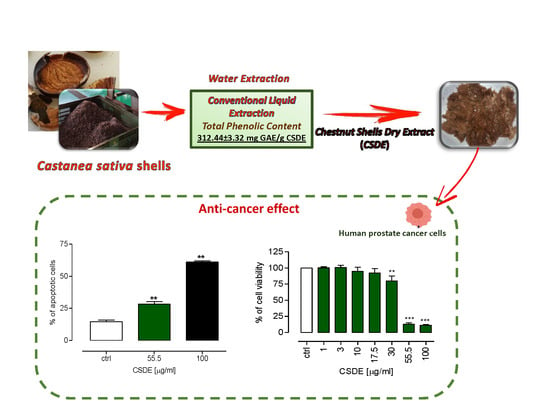
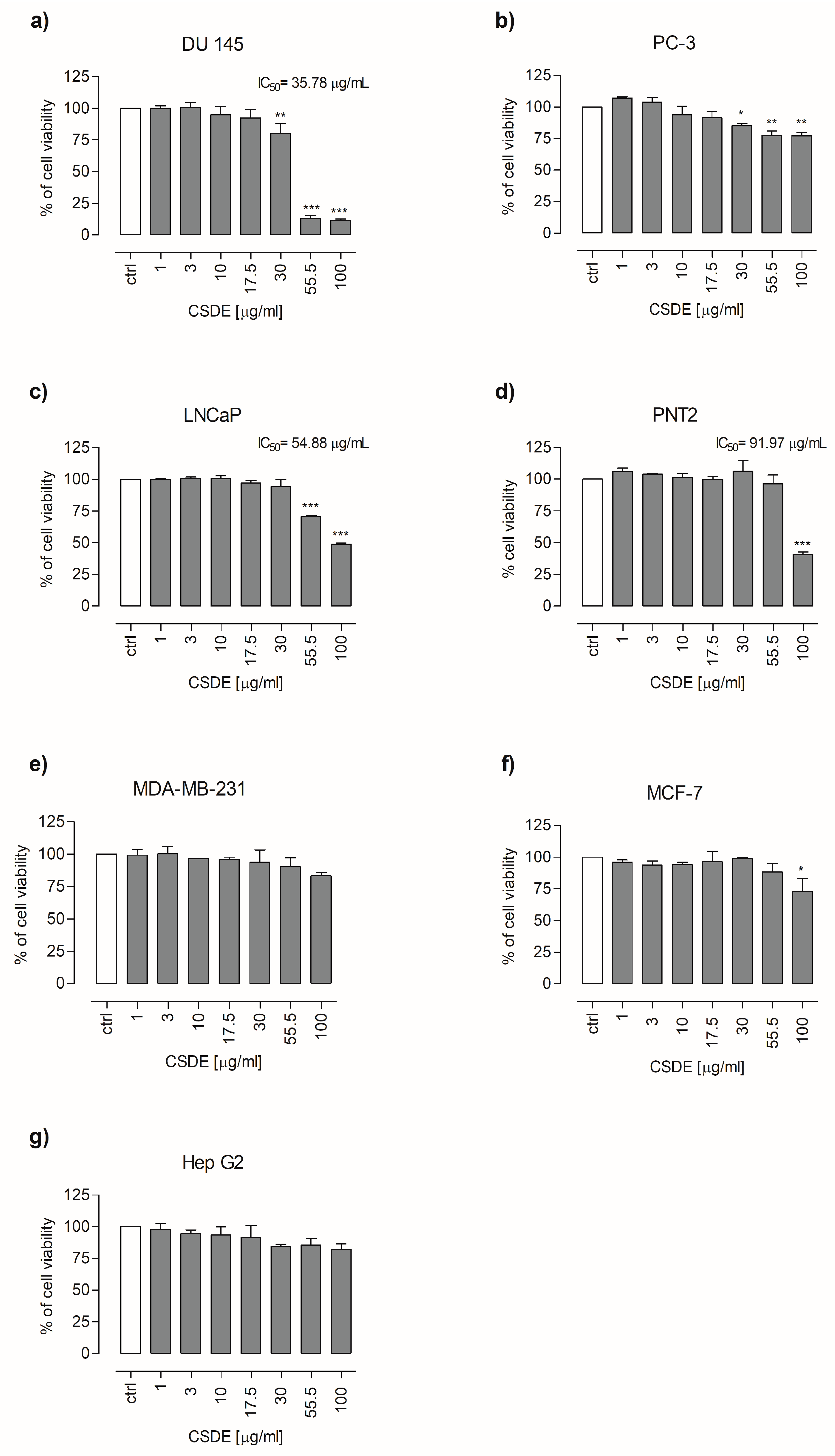
2.2. CSDE Exerts Cytotoxic Effects on Human Cancer Cell Lines
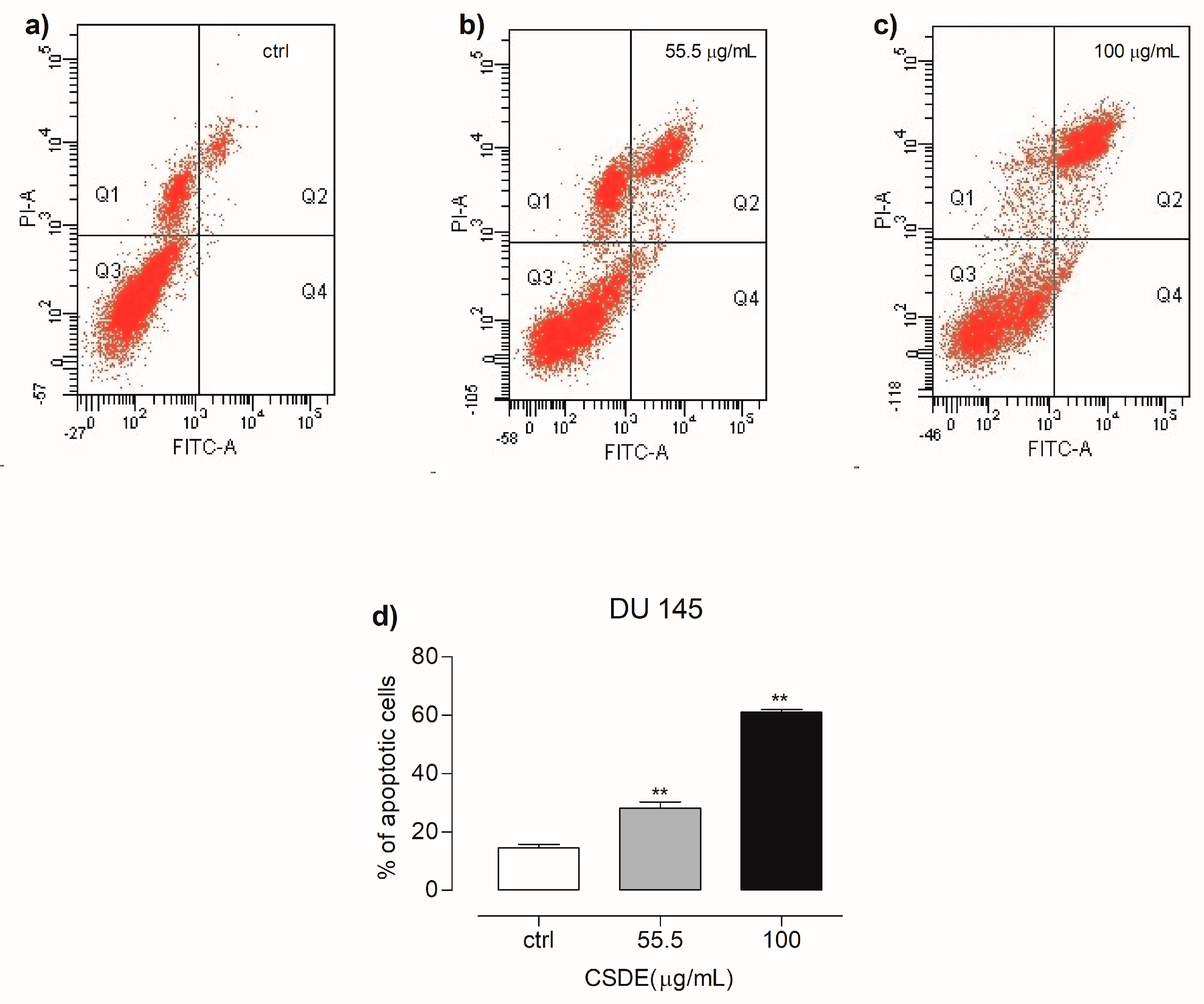
2.3. CSDE Induces Apoptosis in DU 145 Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Extraction of Phenolic Compounds from Chestnut Shells
4.3. Preparation of CSDE for Analyses
4.4. Total Phenolic Content
4.5. Total Ortho-Diphenolic Content
4.6. Total Flavonoid Content
4.7. Total Tannin Content
4.8. Reversed-Phase (RP)-HPLC Analysis
4.9. Cell Cultures
4.10. Assessment of Cell Viability Assay
4.11. Evaluation of Apoptosis by Flow Cytometry
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beart, J.E.; Lilley, T.H.; Haslam, E. Plant Polyphenols-Secondary Metabolism and Chemical Defense-Some Observations. Phytochemistry 1985, 24, 33–38. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Croft, K.D. Dietary flavonoids: Effects on endothelial function and blood pressure. J. Sci. Food Agr. 2006, 86, 2492–2498. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Wolffram, S.; de Vos, R.; Bovy, A.; Gibbins, J.M.; Lovegrove, J.A. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: A pilot study. Br. J. Nutr. 2006, 96, 482–488. [Google Scholar]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Kong, A.N. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef]
- Sorice, A.; Guerriero, E.; Volpe, M.G.; Capone, F.; La Cara, F.; Ciliberto, G.; Colonna, G.; Costantini, S. Differential Response of Two Human Breast Cancer Cell Lines to the Phenolic Extract from Flaxseed Oil. Molecules 2016, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, H.I.; Ballesteros Vivas, D.; Ortega Barbosa, J.; Morantes Medina, S.J.; Aristizabal Gutierrez, F.; Parada-Alfonso, F. Bioactive Phenolic Compounds from the Agroindustrial Waste of Colombian Mango Cultivars ‘Sugar Mango’ and ‘Tommy Atkins’-An Alternative for Their Use and Valorization. Antioxidants 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Oluyori, A.P.; Shaw, A.K.; Olatunji, G.A.; Rastogi, P.; Meena, S.; Datta, D.; Arora, A.; Reddy, S.; Puli, S. Sweet Potato Peels and Cancer Prevention. Nutr. Cancer 2016, 68, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Kim, D.H.; Keum, Y.S.; Seok, P.G.; Sharma, K. Valorization of onion solid waste and their flavonols for assessment of cytotoxicity, enzyme inhibitory and antioxidant activities. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 119, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Barros, L.; Bento, A.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C. Castanea sativa Mill. Flowers amongst the most powerful antioxidant matrices: A phytochemical approach in decoctions and infusions. BioMed Res. Int. 2014, 2014, 232956. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.Y.; Chen, S.Y.; Shi, L.L.; Liu, Y.J.; Ma, C. Antioxidant potential of polyphenols and tannins from burs of Castanea mollissima Blume. Molecules 2011, 16, 8590–8600. [Google Scholar] [CrossRef] [PubMed]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; De Lucia, A.; Colucci, G.; La Cara, F.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Vazquez, G.; Gonzalez-Alvarez, J.; Santos, J.; Freire, M.S.; Antorrena, G. Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crop. Prod. 2009, 29, 364–370. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef]
- Morana, A.; Squillaci, G.; Paixao, S.M.; Alves, L.; La Cara, F.; Moura, P. Development of an Energy Biorefinery Model for Chestnut (Castanea sativa Mill.) Shells. Energies 2017, 10, 1504. [Google Scholar] [CrossRef]
- Zhang, H.; Ke, J.; Shao, T.; Li, J.; Duan, Y.; He, Y.; Zhang, C.; Chen, G.; Sun, G.; Sun, X. Cytotoxic effects of procyanidins from Castanea mollissima Bl. shell on human hepatoma G2 cells in vitro. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 64, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.S.; Lee, N.K.; Na, D.S.; Yu, H.H.; Paik, H.D. Comparative analysis of the antioxidant and anticancer activities of chestnut inner shell extracts prepared with various solvents. J. Sci. Food Agric. 2016, 96, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z. Antioxidative and antiradical properties of plant phenolics. Z. Fur. Naturforschung. C J. Biosci. 2005, 60, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Everson, R.J.; Joshi, B.; Bulsara, P.A.; Upasani, R.; Clarke, M.J. Structure-function relationship of phenolic antioxidants in topical skin health products. Int. J. Cosmet. Sci. 2017, 39, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Baghdikian, B.; Filly, A.; Fabiano-Tixier, A.S.; Petitcolas, E.; Mabrouki, F.; Chemat, F.; Ollivier, E. Extraction by solvent using microwave and ultrasound-assisted techniques followed by HPLC analysis of Harpagoside from Harpagophytum procumbens and comparison with conventional solvent extraction methods. C. R. Chim. 2016, 19, 692–698. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Tech. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Fernandez-Agullo, A.; Freire, M.S.; Antorrena, G.; Pereira, J.A.; Gonzalez-Alvarez, J. Effect of the Extraction Technique and Operational Conditions on the Recovery of Bioactive Compounds from Chestnut (Castanea sativa) Bur and Shell. Sep. Sci. Technol. 2014, 49, 267–277. [Google Scholar] [CrossRef]
- Sushma, R.D.; Sadiya, T.; Krishna Murthy, T.P.; Bhavya, S.G.; Manjunath, D. Extraction of Polyphenols from Decalepis hamiltonii Root: Optimization of Batch Extraction Process Parameters. Res. J. Pharm. Biol. Chem. Sci. 2016, 5, 624–632. [Google Scholar]
- Jahangiri, Y.G.H.; Abedini Torghabeh, J.; Ataye Salehi, E. Effect of temperature and solvent on the total phenolic compounds extraction from leaves of Ficus carica. J. Chem. Pharm. Res. 2011, 3, 253–259. [Google Scholar]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.S.; Silva, N.J.; Soares, N.C.; Monteiro, M.C.; Teodoro, A.J. Anticancer Properties of Phenolic Acids in Colon Cancer–A Review. J. Nutr. Food Sci. 2016, 6. [Google Scholar]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Arnow, L.E. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Peri, C.; Pompei, C. Estimation of Different Phenolic Groups in Vegetable Extracts. Phytochemistry 1971, 10, 2187–2189. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Extraction Method | Extraction Yield (w/w%) a | Total Phenols (mg GAE/g CSDE) a,b,c | ortho-Diphenols (mg CAE/g CSDE) a,d,c | Flavonoids (mg CE/g CSDE) a,e,c | Tannins (mg GAE/gCS DE) a,b,c |
|---|---|---|---|---|---|
| CLE | 5.2 ± 0.1 | 312.44 ± 3.32 | 148.72 ± 2.61 | 62.18 ± 1.19 | 205.99 ± 1.95 |
| UAE | 2.2 ± 0.1 | 190.12 ± 1.16 | 73.90 ± 1.01 | 47.75 ± 2.32 | 118.97 ± 2.12 |
| MAE | 3.8 ± 0.1 | 247.63 ± 3.42 | 104.20 ± 2.67 | 58.19 ± 1.18 | 175.91 ± 3.75 |
| Compound | CLE (mg/g CSDE) a,b | UAE (mg/g CSDE) a,b | MAE (mg/g CSDE) a,b |
|---|---|---|---|
| Gallic acid | 86.97 ± 1.32 | 150.09 ± 2.16 | 117.58 ± 1.93 |
| Protocatechuic acid | 11.20 ± 0.30 | 21.57 ± 1.57 | 16.8 ± 0.09 |
| Chlorogenic acid | 0.67 ± 0.01 | 0.79 ± 0.05 | 1.18 ± 0.04 |
| Epicatechin | 0.71 ± 0.05 | 0.79 ± 0.04 | 1.28 ± 0.02 |
| Syringic acid | 0.2 0± 0.01 | 0.14 ± 0.01 | 0.21 ± 0.01 |
| Ellagic acid | 0.81 ± 0.06 | 0.58 ± 0.01 | 1.09 ± 0.03 |
| p-Coumaric acid | 0.22 ± 0.01 | 0.52 ± 0.02 | 0.43 ± 0.01 |
| Sinapic acid | 0.16 ± 0.02 | 0.48 ± 0.03 | 0.27 ± 0.01 |
| Ferulic acid | 0.03 ± 0.01 | 0.31 ± 0.05 | 0.09 ± 0.01 |
| Scopoletin | 0.11 ± 0.01 | 0.41 ± 0.03 | 0.20 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciola, N.A.; Squillaci, G.; D’Apolito, M.; Petillo, O.; Veraldi, F.; La Cara, F.; Peluso, G.; Margarucci, S.; Morana, A. Castanea sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-Apoptotic Effects. Molecules 2019, 24, 3401. https://doi.org/10.3390/molecules24183401
Cacciola NA, Squillaci G, D’Apolito M, Petillo O, Veraldi F, La Cara F, Peluso G, Margarucci S, Morana A. Castanea sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-Apoptotic Effects. Molecules. 2019; 24(18):3401. https://doi.org/10.3390/molecules24183401
Chicago/Turabian StyleCacciola, Nunzio Antonio, Giuseppe Squillaci, Mariella D’Apolito, Orsolina Petillo, Francesco Veraldi, Francesco La Cara, Gianfranco Peluso, Sabrina Margarucci, and Alessandra Morana. 2019. "Castanea sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-Apoptotic Effects" Molecules 24, no. 18: 3401. https://doi.org/10.3390/molecules24183401
APA StyleCacciola, N. A., Squillaci, G., D’Apolito, M., Petillo, O., Veraldi, F., La Cara, F., Peluso, G., Margarucci, S., & Morana, A. (2019). Castanea sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-Apoptotic Effects. Molecules, 24(18), 3401. https://doi.org/10.3390/molecules24183401