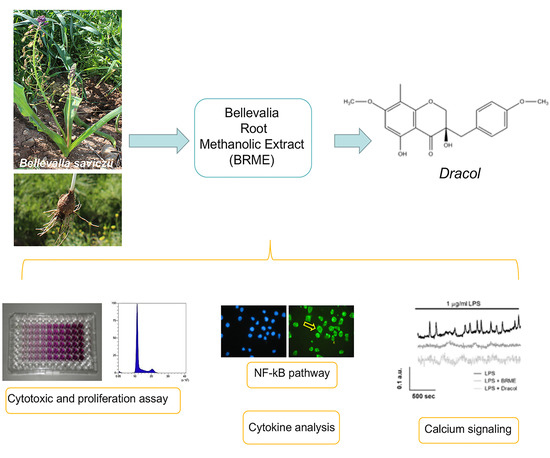
Anti-Inflammatory Properties of Bellevalia saviczii Root Extract and Its Isolated Homoisoflavonoid (Dracol) Are Mediated by Modification on Calcium Signaling
Abstract
1. Introduction
2. Results
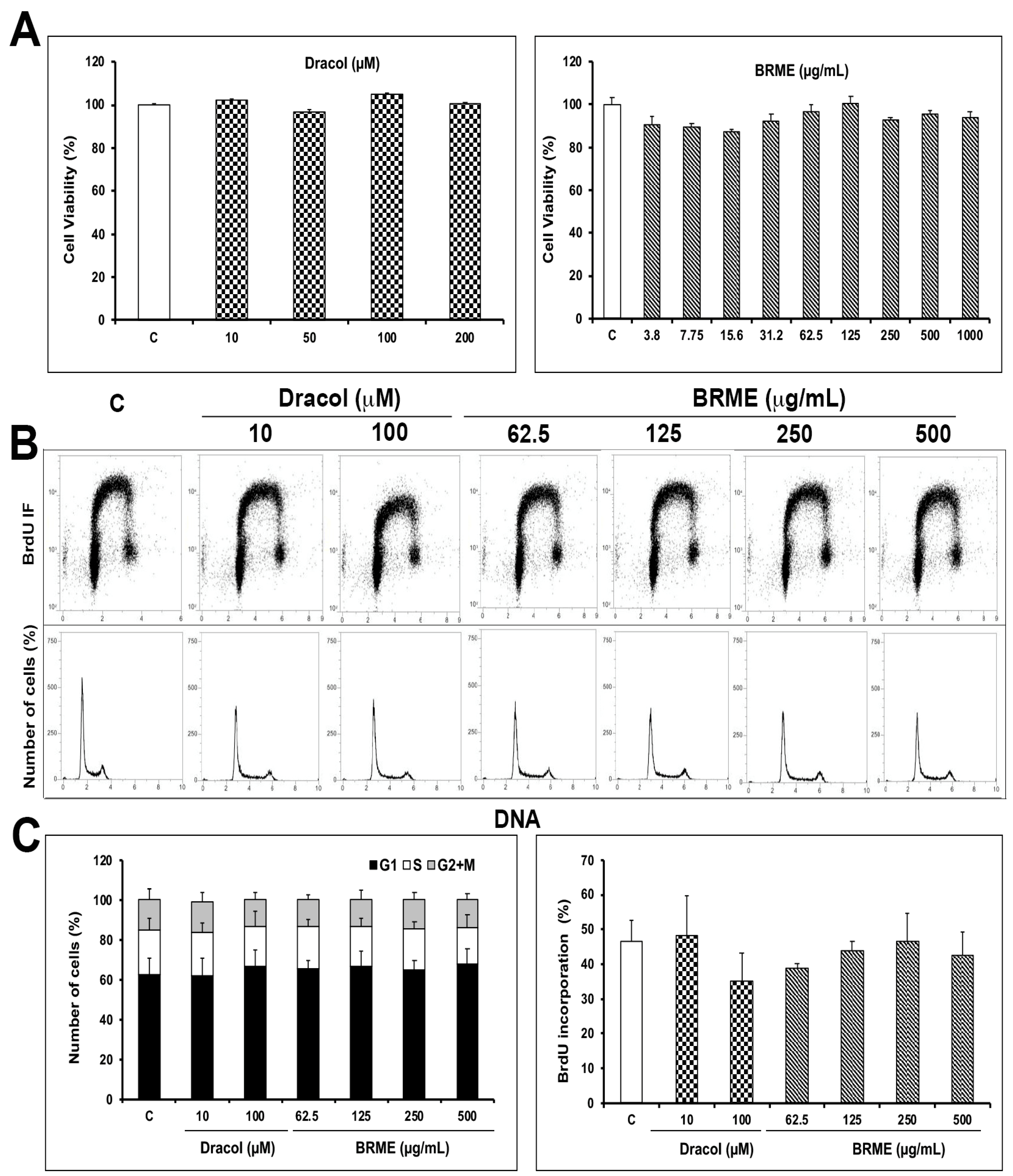
2.1. Cell Viability and Proliferation Assays
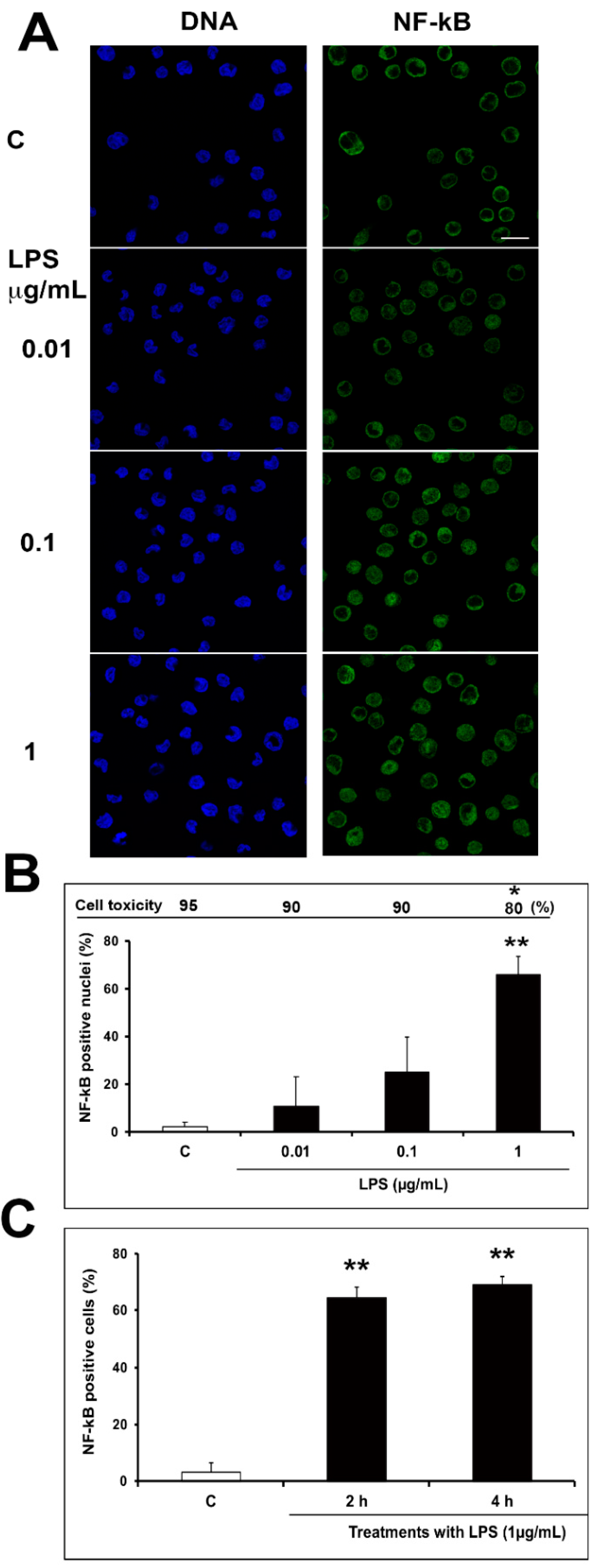
2.2. Optimization of Inflammatory Model: Preliminary Evaluation
2.3. Effect of BRME and Dracol on LPS-Activated THP1
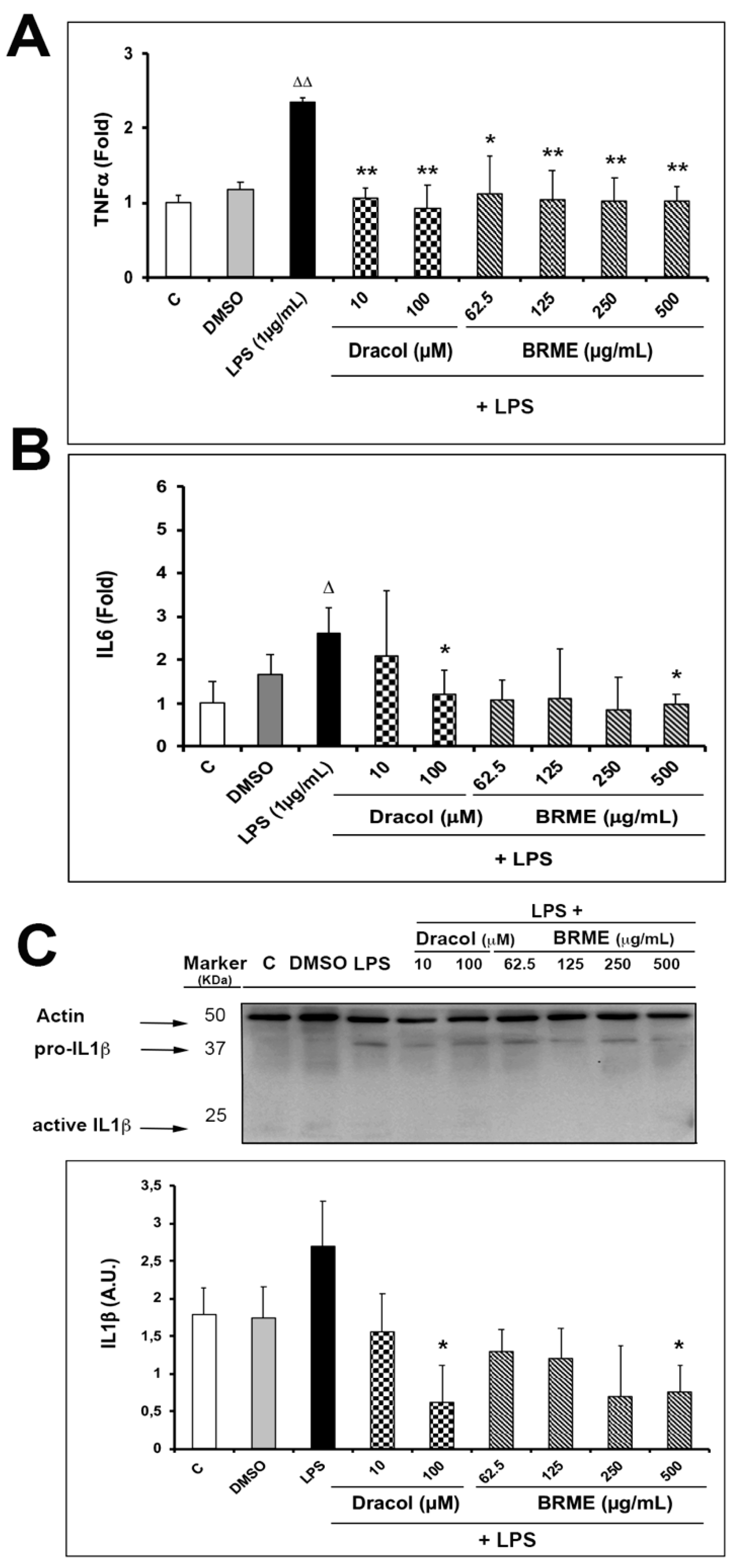
2.4. Cytokine Analysis
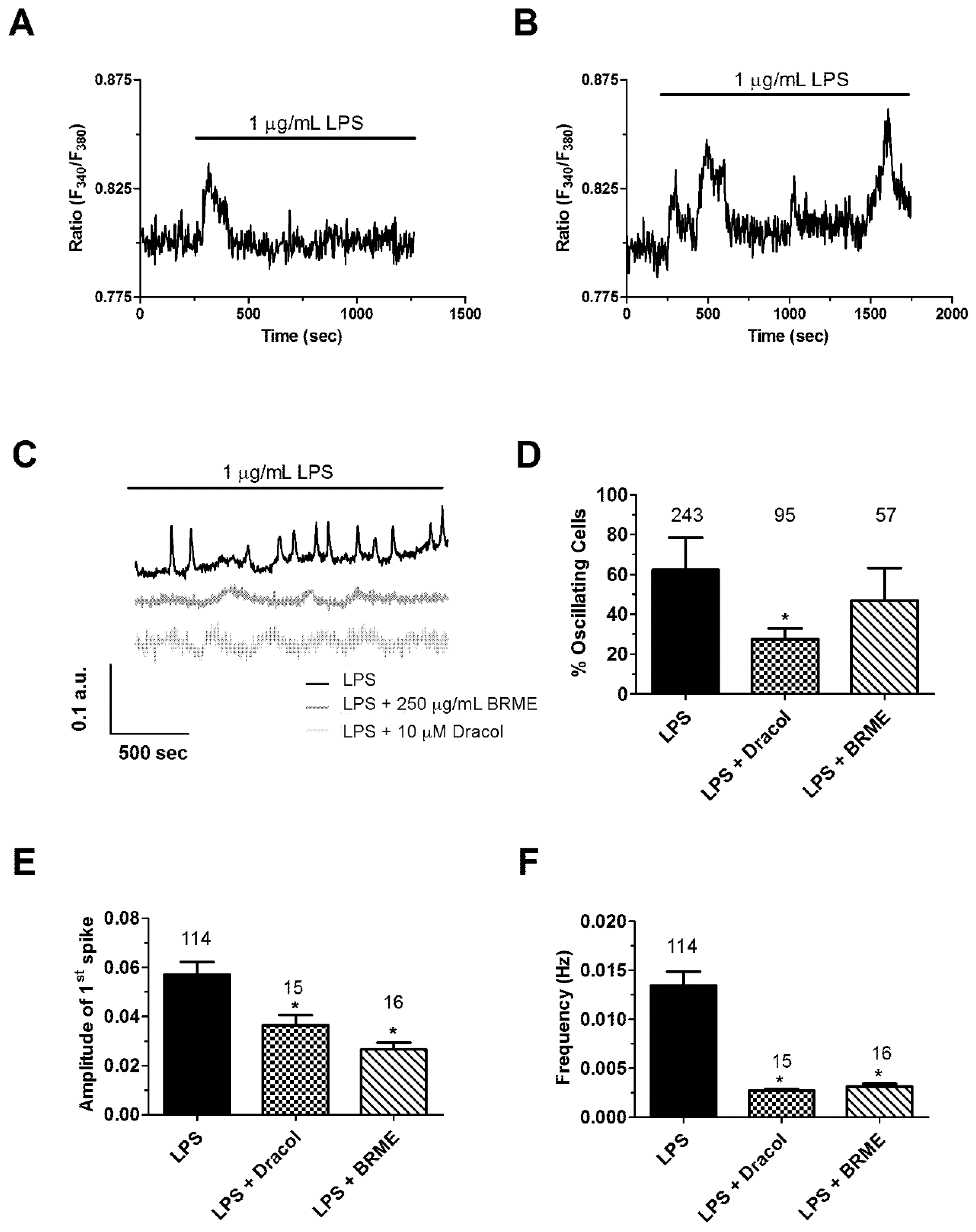
2.5. Calcium Signalling
3. Materials and Methods
3.1. Reagents
3.2. Plant Materials
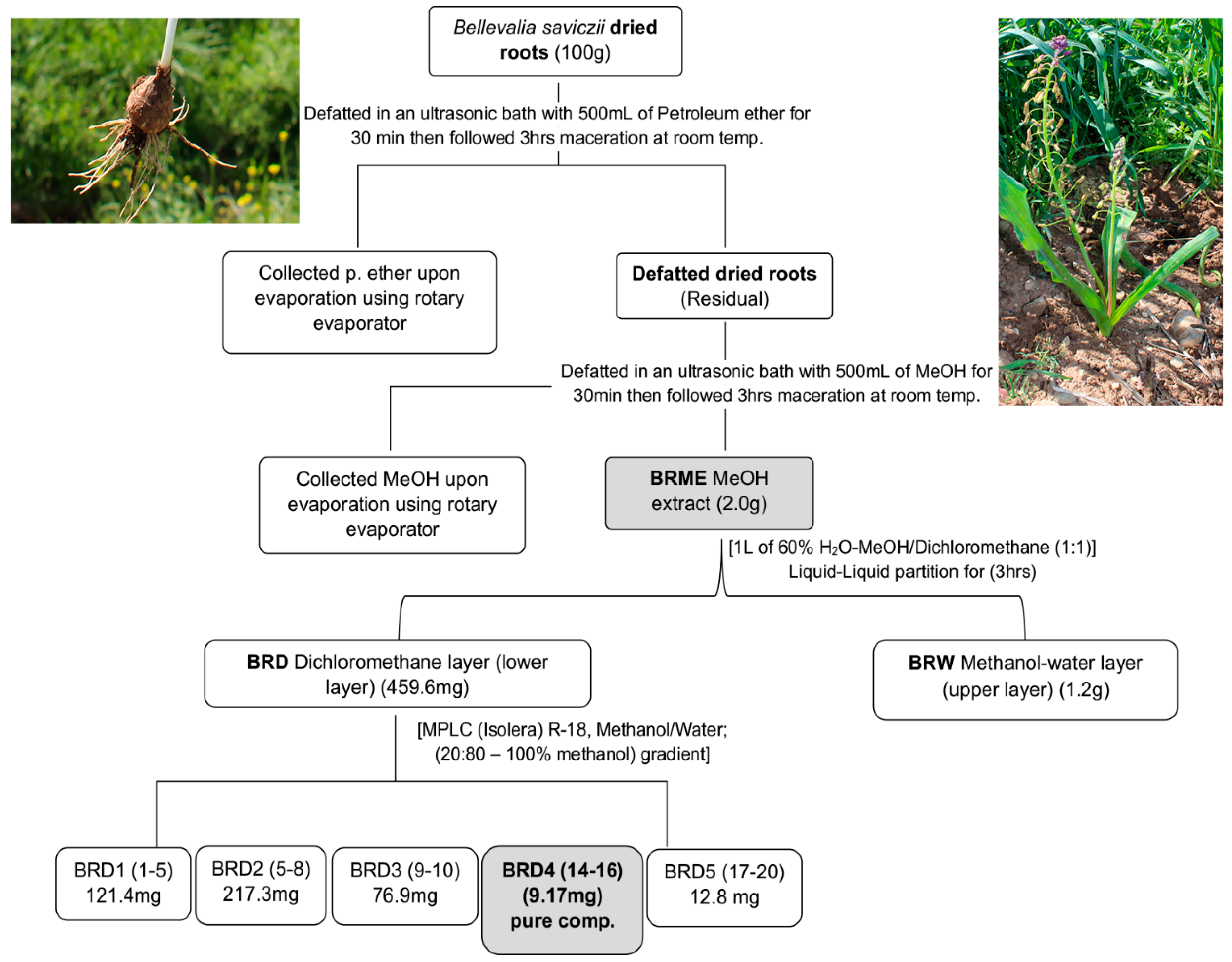
3.3. Root Extraction and Compound Isolation
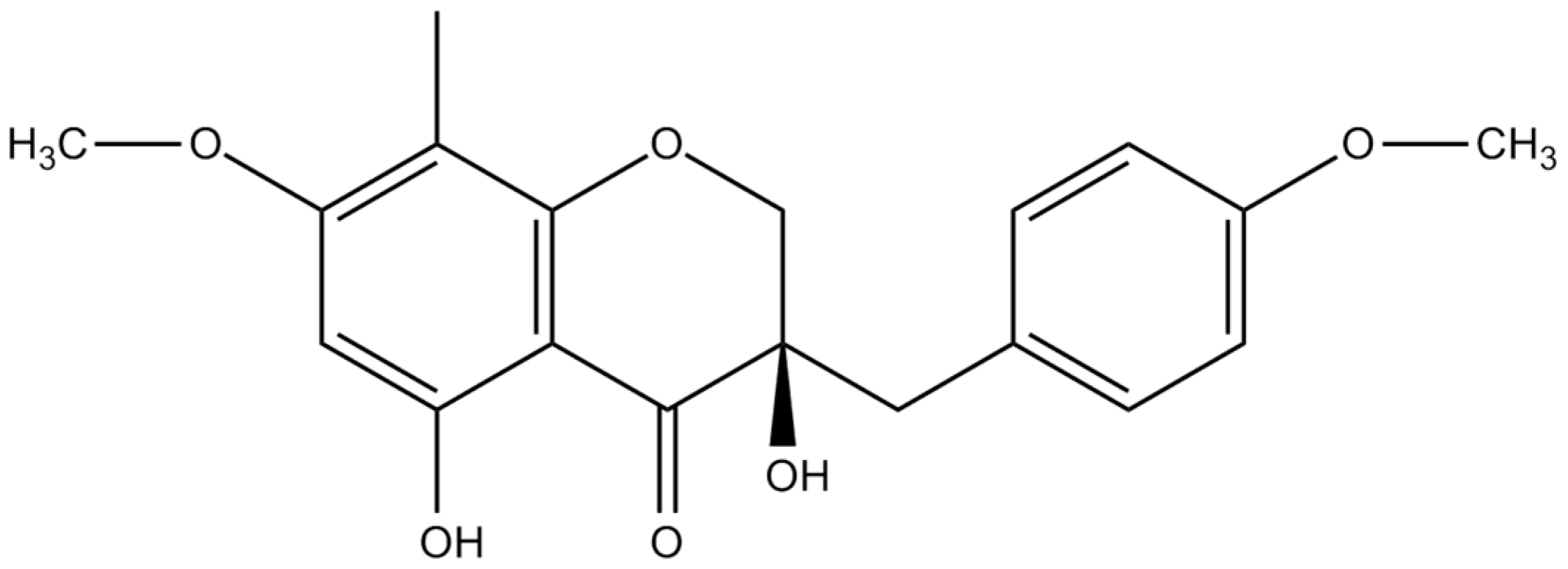
3.4. Spectral Data of Isolated Compound
3.5. In Vitro Cell Culture and Treatments
3.6. Cytotoxicity, Proliferation and Trypan Blue Exclusion Assay
3.7. NF-kB Staining
3.8. Cytokine Analysis
3.9. [Ca2+]i Measurements
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, L.G.; Liu, Q.Y.; Ye, Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014, 80, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Alali, F.; El-Elimat, T.; Albataineh, H.; Al-Balas, Q.; Al-Gharaibeh, M.; Falkinham, J.O.; Chen, W.L.; Swanson, S.M.; Oberlies, N.H. Cytotoxic Homoisoflavones from the Bulbs of Bellevalia eigii. J. Nat. Prod. 2015, 78, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.C.; León, F.; Estévez, F.; Quintana, J.; Bermejo, J. A homo-isoflavonoid and a cytotoxic saponin from Dracaena draco. Chem. Biodivers 2006, 3, 62–68. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Saeidnia, S.; Babaei, E.; Kiuchi, F.; Hussain, H. Scillapersicene: A new homoisoflavonoid with cytotoxic activity from the bulbs of Scilla persica HAUSSKN. Nat. Prod. Res. 2016, 30, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuping, Z.; Zhao, H.; Liang, J.; Zhang, Y.; Shi, S. Antioxidant homoisoflavonoids from Polygonatum odoratum. Food Chem. 2015, 186, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Liang, Z.; Peng, L.; Wang, B.; Yu, J.; Su, Y.; Ma, C. Homoisoflavonoids and the Antioxidant Activity of Ophiopogon japonicus Root. Iran J. Pharm. Res. 2017, 16, 357–365. [Google Scholar]
- Roy, S.K.; Agrahari, U.C.; Gautam, R.; Srivastava, A.; Jachak, S.M. Isointricatinol, a new antioxidant homoisoflavonoid from the roots of Caesalpinia digyna Rottler. Nat. Prod. Res. 2012, 26, 690–695. [Google Scholar] [CrossRef]
- Hung, T.M.; Thu, C.V.; Dat, N.T.; Ryoo, S.W.; Lee, J.H.; Kim, J.C.; Na, M.; Jung, H.J.; Bae, K.; Min, B.S. Homoisoflavonoid derivatives from the roots of Ophiopogon japonicus and their in vitro anti-inflammation activity. Bioorg. Med. Chem. Lett. 2010, 20, 2412–2416. [Google Scholar] [CrossRef]
- Famuyiwa, S.O.; Ntumy, A.N.; Andrae-Marobela, K.; Yeboah, S.O. A new homoisoflavonoid and the bioactivities of some selected homoisoflavonoids from the inter-bulb surfaces of Scilla nervosa subsp. rigidifolia. South Afr. J. Bot. 2013, 88, 17–22. [Google Scholar] [CrossRef]
- Adinolfi, M. Homoisoflavanones from Bellevalia romana. Phytochemistry 1989, 28, 3244–3246. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Hund, A.C.; Lockmann, A.; Schön, M.P. Mutually enhancing anti-inflammatory activities of dimethyl fumarate and NF-κB inhibitors--implications for dose-sparing combination therapies. Exp. Derm. 2016, 25, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Du, J.; Liu, A.D.; Holmberg, L.; Chen, S.Y.; Bu, D.; Tang, C.; Jin, H. L-cystathionine inhibits oxidized low density lipoprotein-induced THP-1-derived macrophage inflammatory cytokine monocyte chemoattractant protein-1 generation via the NF-κB pathway. Sci. Rep. 2015, 5, 10453. [Google Scholar] [CrossRef] [PubMed]
- Frantz, B.; Nordby, E.C.; Bren, G.; Steffan, N.; Paya, C.V.; Kincaid, R.L.; Tocci, M.J.; O’Keefe, S.J.; O’Neill, E.A. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J. 1994, 13, 861–870. [Google Scholar] [CrossRef]
- Hughes, K.; Antonsson, A.; Grundstrøm, T. Calmodulin dependence of NFkappaB activation. FEBS Lett. 1998, 441, 132–136. [Google Scholar] [CrossRef]
- Dolmetsch, R.E.; Xu, K.; Lewis, R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 1998, 392, 933–936. [Google Scholar] [CrossRef]
- Dragoni, S.; Laforenza, U.; Bonetti, E.; Lodola, F.; Bottino, C.; Berra-Romani, R.; Carlo Bongio, G.; Cinelli, M.P.; Guerra, G.; Pedrazzoli, P.; et al. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem. Cells 2011, 29, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, K.; Bezavada, L.; Escue, R.B.; Parthasarathi, K. Lipopolysaccharide induces endoplasmic store Ca2+-dependent inflammatory responses in lung microvessels. PLoS ONE 2013, 8, e63465. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, R.; Yan, H.; Yin, H.; Wu, X.; Tan, Y.; Li, L. Metabotropic glutamate receptor 5 modulates calcium oscillation and innate immune response induced by lipopolysaccharide in microglial cell. Neuroscience 2014, 281, 24–34. [Google Scholar] [CrossRef]
- Smedler, E.; Uhlén, P. Frequency decoding of calcium oscillations. Biochim. Biophys. Acta 2014, 1840, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Guedes de Pinho, P.; Henrique, R.; Pereira, J.A.; Carvalho, M. Further insights into chemical characterization through GC–MS and evaluation for anticancer potential of Dracaena draco leaf and fruit extracts. Food Chem. Toxicol. 2012, 50, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, II-2–II-10. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Gandhirajan, R.K.; Meng, S.; Chandramoorthy, H.C.; Mallilankaraman, K.; Mancarella, S.; Gao, H.; Razmpour, R.; Yang, X.F.; Houser, S.R.; Chen, J.; et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Investig. 2013, 123, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Jembrek, M.J.; Vlainić, J.; Radovanović, V.; Erhardt, J.; Oršolic, N. Effects of copper overload in P19 neurons: Impairment of glutathione redox homeostasis and crosstalk between caspase and calpain protease systems in ROS-induced apoptosis. Biometals 2014, 27, 1303–1322. [Google Scholar] [CrossRef]
- Nakayama, T.; Mihara, K.; Kawata, J.; Kimura, H.; Saitoh, H. Adhesion of suspension cells on a coverslip in serum-free conditions. Anal. Biochem. 2014, 466, 1–3. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, P.; Chen, Z.L.; Zhang, S.J.; Wang, Y.Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway. Front. Pharm. 2018, 9, 962. [Google Scholar] [CrossRef]
- Maccario, C.; Savio, M.; Ferraro, D.; Bianchi, L.; Pizzala, R.; Pretali, L.; Forti, L.; Stivala, L.A. The resveratrol analog 4,4’-dihydroxy-trans-stilbene suppresses transformation in normal mouse fibroblasts and inhibits proliferation and invasion of human breast cancer cells. Carcinogenesis 2012, 33, 2172–2180. [Google Scholar] [CrossRef]
- Savio, M.; Coppa, T.; Bianchi, L.; Vannini, V.; Maga, G.; Forti, L.; Cazzalini, O.; Lazzè, M.C.; Perucca, P.; Prosperi, E.; et al. The resveratrol analogue 4,4’-dihydroxy-trans-stilbene inhibits cell proliferation with higher efficiency but different mechanism from resveratrol. Int. J. Biochem. Cell Biol. 2009, 41, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savio, M.; Ibrahim, M.F.; Scarlata, C.; Orgiu, M.; Accardo, G.; Sardar, A.S.; Moccia, F.; Stivala, L.A.; Brusotti, G. Anti-Inflammatory Properties of Bellevalia saviczii Root Extract and Its Isolated Homoisoflavonoid (Dracol) Are Mediated by Modification on Calcium Signaling. Molecules 2019, 24, 3376. https://doi.org/10.3390/molecules24183376
Savio M, Ibrahim MF, Scarlata C, Orgiu M, Accardo G, Sardar AS, Moccia F, Stivala LA, Brusotti G. Anti-Inflammatory Properties of Bellevalia saviczii Root Extract and Its Isolated Homoisoflavonoid (Dracol) Are Mediated by Modification on Calcium Signaling. Molecules. 2019; 24(18):3376. https://doi.org/10.3390/molecules24183376
Chicago/Turabian StyleSavio, Monica, Mohammed Farhad Ibrahim, Chiara Scarlata, Matteo Orgiu, Giuseppe Accardo, Abdullah Shakur Sardar, Francesco Moccia, Lucia Anna Stivala, and Gloria Brusotti. 2019. "Anti-Inflammatory Properties of Bellevalia saviczii Root Extract and Its Isolated Homoisoflavonoid (Dracol) Are Mediated by Modification on Calcium Signaling" Molecules 24, no. 18: 3376. https://doi.org/10.3390/molecules24183376
APA StyleSavio, M., Ibrahim, M. F., Scarlata, C., Orgiu, M., Accardo, G., Sardar, A. S., Moccia, F., Stivala, L. A., & Brusotti, G. (2019). Anti-Inflammatory Properties of Bellevalia saviczii Root Extract and Its Isolated Homoisoflavonoid (Dracol) Are Mediated by Modification on Calcium Signaling. Molecules, 24(18), 3376. https://doi.org/10.3390/molecules24183376