Structural Characterization and Antioxidant Activity of Polysaccharides from Athyrium multidentatum (Doll.) Ching in d-Galactose-Induced Aging Mice via PI3K/AKT Pathway
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of AMC Polysaccharides
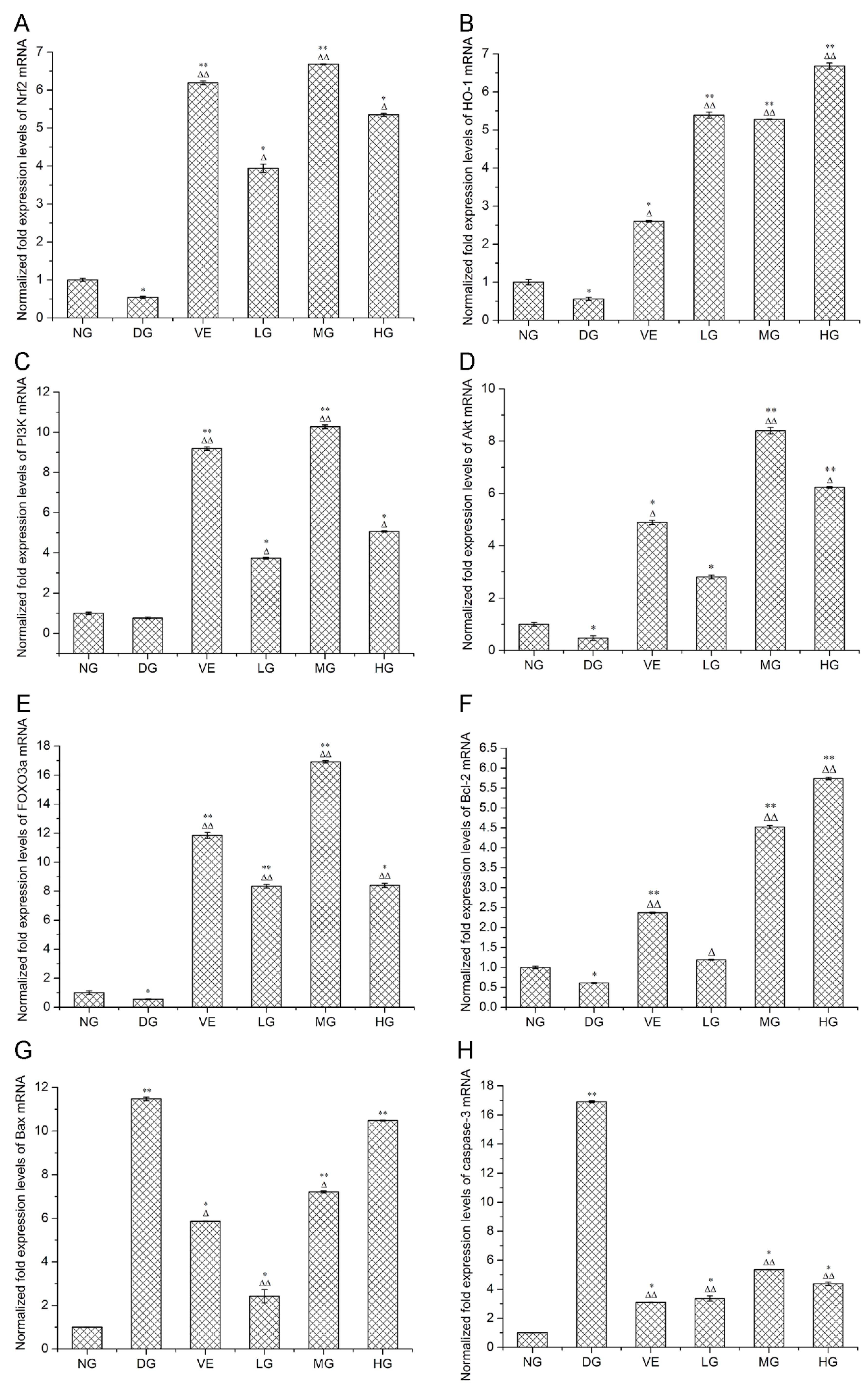
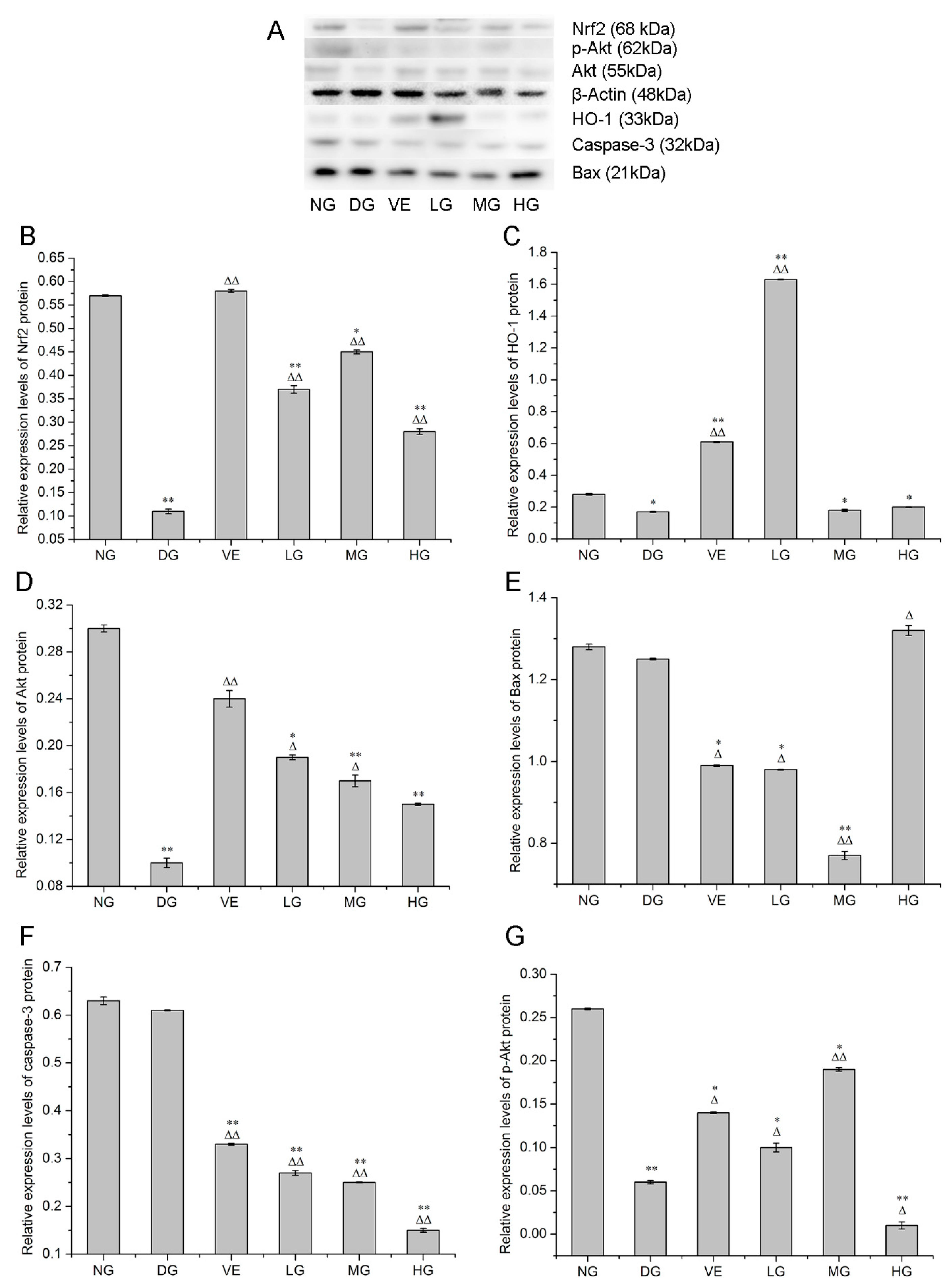
2.2. RT-qPCR and Western Blotting Analyses
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation and Characterization of AMC Polysaccharides
3.3. Animals and Experimental Protocol
3.4. RT-qPCR Analysis
3.5. Western Blotting Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.Q.; Zhang, M.L.; Zhang, D.W.; Wang, X.L.; Cao, H.J.; Zhang, Q.; Yan, C.Y. Structural elucidation and anti-osteoporosis activities of polysaccharides obtained from Curculigoorchioides. Carbohydr. Polym. 2019, 203, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Xu, Z.; Feng, S.; Wang, H.; Liu, J.; Zhou, L.; Yuan, M.; Huang, Y.; Ding, C. Structural elucidation and antiaging activity of polysaccharide from Paris polyphylla leaves. Int. J. Biol.Macromol. 2018, 107, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.H.; Xiao, D.M.; Chen, D.X.; Xiao, Y.; Liang, Z.Q.; Zhong, J.J. Polysaccharides from the Medicinal Mushroom Cordyceps taii Show Antioxidant and Immunoenhancing Activities in a d-Galactose-Induced Aging Mouse Model. Evid. Based. Complement. Alternat. Med. 2012, 2012, 273435. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.W.; Sun, Y.L. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr. Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.R.S.; Feitosa, J.P.A.; Freitas, A.L.P.; de Paula, R.C.M. Isolationand characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polym. 2002, 49, 491–498. [Google Scholar] [CrossRef]
- Mu, J.; Liu, D.M.; Sheng, J.W.; Li, Z.J.; Zhang, W.F.; Mao, S.M.; Jing, L.; Jiang, J.R.; Qi, L. Polysaccharides from Athyrium multidentatum (Doll.) Ching an Attenuated d-Galactose-Induced Mouse Aging via SIRT1-p53-p21 Pathway. J. Chem. Pharm. Res. 2018, 10, 40–51. [Google Scholar]
- Liu, D.M.; Sheng, J.W.; Li, Z.J.; Qi, H.M.; Sun, Y.L.; Duan, Y.; Zhang, W.F. Antioxidant activity of polysaccharide fractions extracted from Athyrium Multidentatum (Doll.) Ching. Int. J. Biol. Macromol. 2013, 56, 1–5. [Google Scholar] [CrossRef]
- Wang, S.A.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Harman, D. Origin and evolution of the free radical theory of aging: A brief personal history, 1954–2009. Biogerontology 2009, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Lang, S.Y.; Zuo, P.P.; Yang, N.; Wang, X.Q.; Xia, C. Effects of d-galactose on the expression of hippocampal peripheraltype benzodiazepine receptor and spatial memory performances in rats. Psychoneuroendocrinology 2006, 31, 805–811. [Google Scholar] [CrossRef]
- Xu, X.L.; Li, H.Q.; Hou, X.L.; Li, D.Y.; He, S.S.; Wan, C.R.; Yin, P.; Liu, M.J.; Liu, F.H.; Xu, J.Q. Punicalagin Induces Nrf2/HO-1 Expression via Upregulation of PI3K/AKT Pathway and Inhibits LPS-Induced Oxidative Stress in RAW264.7 Macrophages. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Nie, K.Y.; Wang, Z.J.; Luo, D.H. Quantitative structure activity relationship models for the antioxidant activity of polysaccharides. PLoS ONE 2016, 11, e0163536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, F.; Xu, Z.H.; Ding, Z.Y. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Chen, T.X.; Gao, T.; Miao, Z.G.; Jiang, A.L.; Shi, L.; Ren, A.; Zhao, M.W. UDP-glucose pyrophosphorylase influences polysaccharide synthesis, cell wall components, and hyphal branching in Ganoderma lucidum via regulation of the balance between glucose-1-phosphate and UDP-glucose. Fungal Genet. Biol. 2015, 82, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Koenig, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar]
- Barker, S.A.; Bourne, E.J.; Stacey, M.; Whiffen, D.H. Infra-redspectra of carbohydrates. Part I. Some derivatives of d-glucopyranose. J. Chem. Soc. 1954, 1, 171–176. [Google Scholar] [CrossRef]
- Cozzolino, R.; Malvagna, P.; Spina, E.; Giori, A.; Fuzzati, N.; Anelli, A.; Garozzo, D.; Impallomeni, G. Structural analysis of the polysaccharides from Echinacea angustifolia radix. Carbohydr. Polym. 2006, 65, 263–272. [Google Scholar] [CrossRef]
- Priyadarshini, D.K.S.; Kumar, M.T. Immunomodulatory and anti-cancer properties of pharmacologically relevant mushroom glycans. Recent Pat. Biotechnol. 2016, 10, 72–78. [Google Scholar] [CrossRef]
- Hsu, M.J.; Lee, S.S.; Lin, W.W. Polysaccharide purified from Ganoderma lucidum inhibits spontaneous and Fas-mediated apoptosis in human neutrophils through activation of the phosphatidylinositol 3 kinase/Akt signaling pathway. J. Leukocyte Biol. 2002, 72, 207–216. [Google Scholar]
- Bo-Htay, C.; Palee, S.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Effects of d-galactose-induced ageing on the heart and its potential interventions. J. Cell. Mol. Med. 2018, 22, 1392–1410. [Google Scholar] [CrossRef]
- Xu, L.; He, S.S.; Yin, P.; Li, D.Y.; Mei, C.; Yu, X.H.; Shi, Y.R.; Jiang, L.S.; Liu, F.H. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int. J. Hyperthermia. 2016, 32, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Jyrkkänen, H.K.; Levonen, A.L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012, 52, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Nakaso, K.; Yano, H.; Fukuhara, Y.; Takeshima, T.; WadaIsoe, K.; Nakashima, K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003, 546, 181–184. [Google Scholar] [CrossRef]
- Nakao, T.; Geddis, A.E.; Fox, N.E.; Kaushansky, K. PI3K/Akt/FOXO3a pathway contributes to thrombopoietin-induced proliferation of primary megakaryocytes in vitro and in vivo via modulation of p27Kip1. Cell Cycle 2008, 7, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–284. [Google Scholar] [CrossRef]
- Mooney, S.M.; Miller, M.W. Expression of bcl-2, bax, and caspase-3 in the brain of the developing rat. Dev. Brain Res. 2000, 123, 103–117. [Google Scholar] [CrossRef]
- Schavolt, K.L.; Pietenpol, J.A. p53 and Delta Np63 alpha differentially bind and regulate target genes involved in cell cycle arrest, DNA repair and apoptosis. Oncogene 2007, 26, 6125–6132. [Google Scholar] [CrossRef]
- Wu, W.; Hou, C.L.; Mu, X.P.; Sun, C.; Zhu, Y.C.; Wang, M.J.; Lv, Q.Z. H2S Donor NaHS changes the production of endogenous H2S and NO in d-galactose induced accelerated ageing. Oxid. Med. Cell. Longev. 2017, 2017, 5707830. [Google Scholar] [CrossRef]
Sample Availability: Polysaccharides from Athyrium multidentatum (Doll.) Ching are available from the authors. |
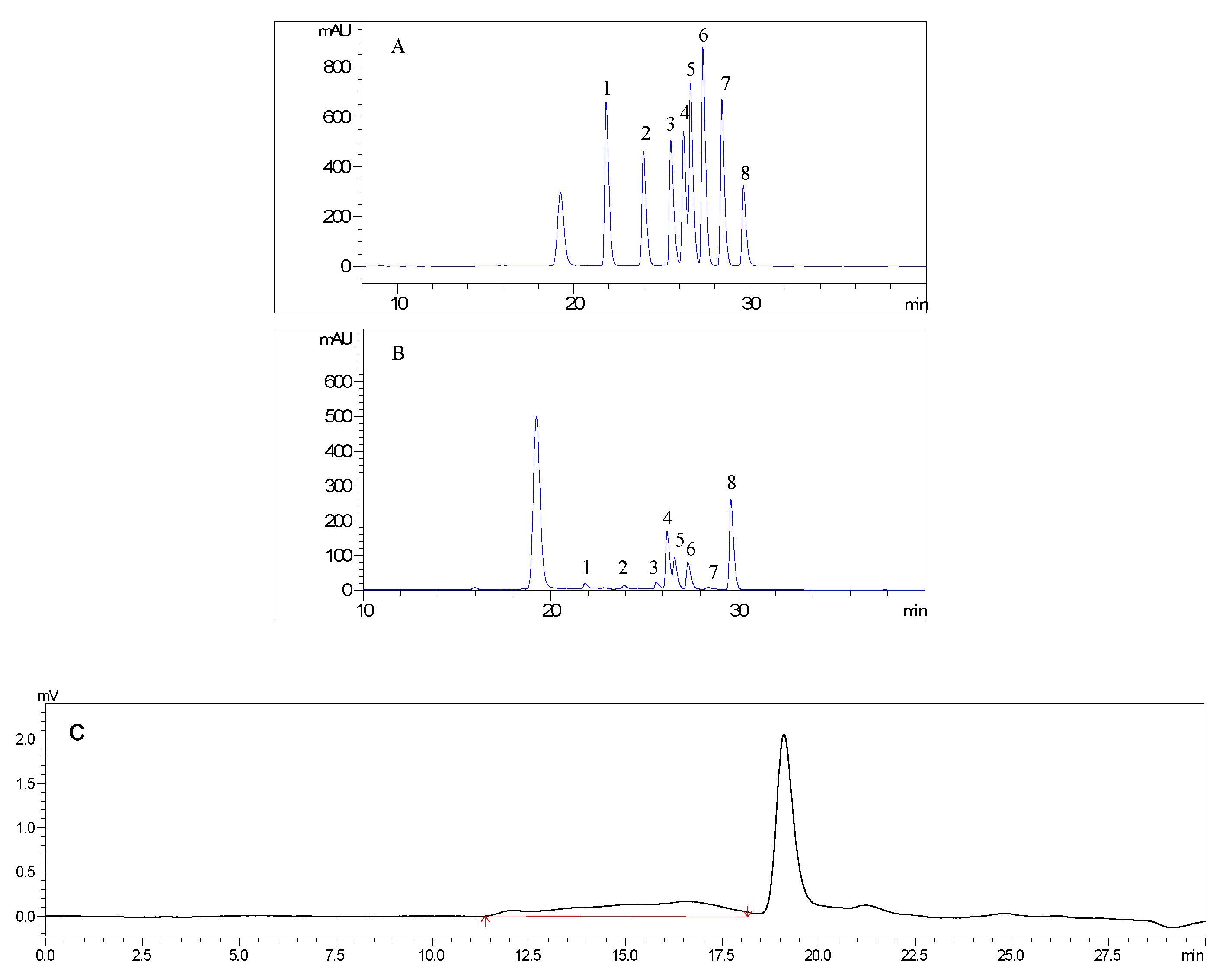
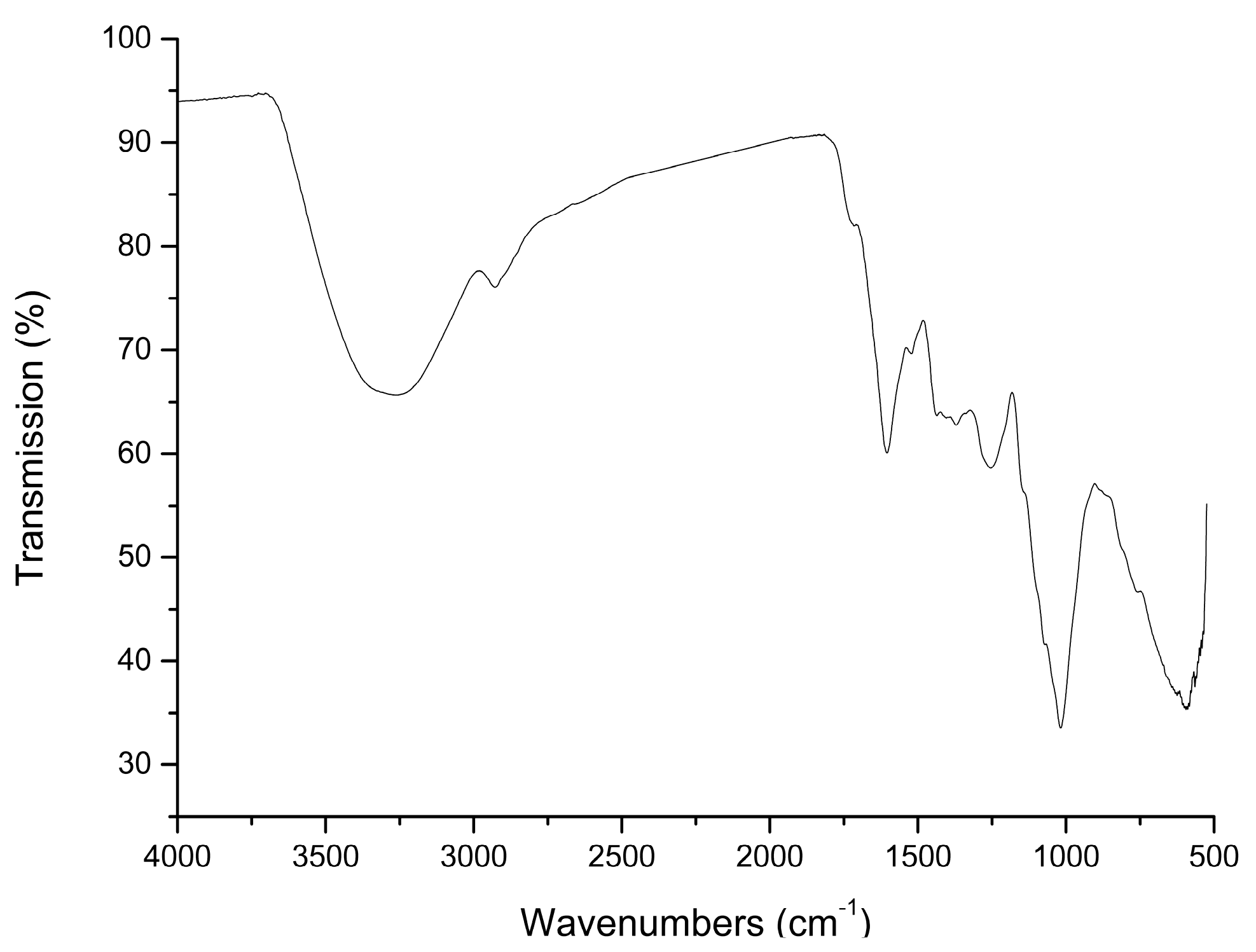
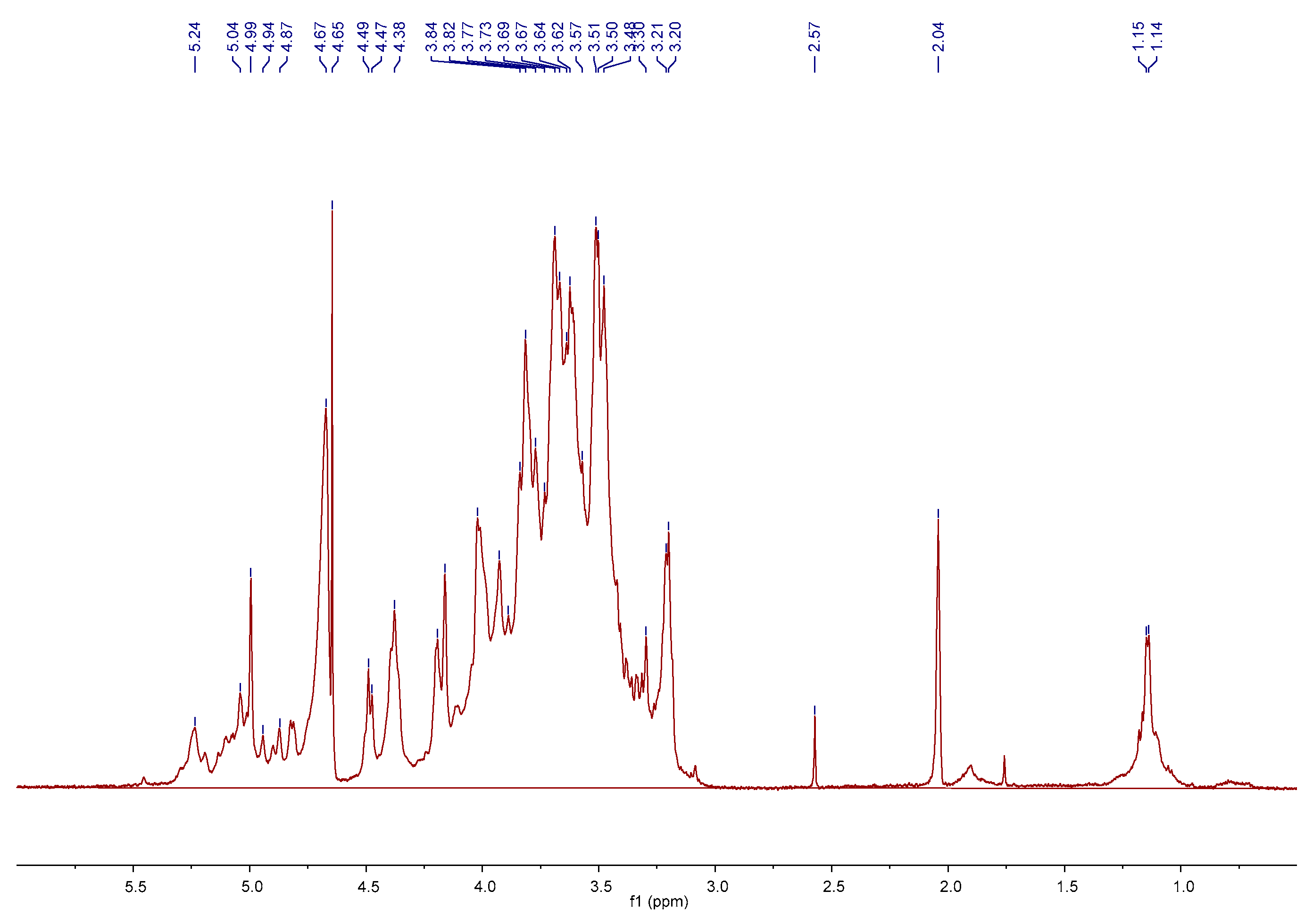
| Molecular Weight (Mw, Da) | Molar Ratio of Monosaccharide Composition | ||||||
|---|---|---|---|---|---|---|---|
| Man | Rha | Glc A | Glc | Gal | Ara | Fuc | |
| 33203 | 0.077 | 0.088 | 0.09 | 1 | 0.375 | 0.354 | 0.04 |
| Name | Primer Sequences (Forward/Reverse Primer) | Tm (°C) | GC (%) |
|---|---|---|---|
| FOXO3a | GGCGGCGTGCGGTCTCCATG | 65.65 | 75 |
| TGCTGGCGTTGGAATTCCTG | 57.45 | 55 | |
| PI3K | TAGGAGGAGGTTGGAAGAAG | 55.4 | 50 |
| CGTCAGCCACATCAAGTATT | 53.35 | 45 | |
| Akt | TGGCAGGATGTGTATGAG | 52.62 | 50 |
| CTGGCTGAGTAGGAGAAC | 54.9 | 55 | |
| Caspase-3 | AGCACTGGAATGTCATCTCG | 55.4 | 50 |
| TCCTTAGAAACACTATCCAT | 49.25 | 35 | |
| Bax | GCATCAGGGTTTCATCCAGG | 57.45 | 55 |
| AATCATCCTCTGCAGCTCCA | 55.4 | 50 | |
| Bcl-2 | GGACTTGAAGTGCCATTGGT | 55.4 | 50 |
| CGGTAGCGACGAGAGAAGTC | 59.5 | 60 | |
| HO-1 | CCCAGTCTATGCCCCACTCT | 59.5 | 90 |
| AGACGCTTTACATAGTGCTG | 53.35 | 45 | |
| Nrf2 | CAGCATAGAGCAGGACAT | 52.62 | 50 |
| GGAACAGCGGTAGTATCA | 52.62 | 50 | |
| β-actin | CACCACACCTTCTACAATGAG | 55.61 | 47.62 |
| TACGACCAGAGGCATACAG | 55.16 | 52.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, L.; Jiang, J.-R.; Liu, D.-M.; Sheng, J.-W.; Zhang, W.-F.; Li, Z.-J.; Wei, L.-Y. Structural Characterization and Antioxidant Activity of Polysaccharides from Athyrium multidentatum (Doll.) Ching in d-Galactose-Induced Aging Mice via PI3K/AKT Pathway. Molecules 2019, 24, 3364. https://doi.org/10.3390/molecules24183364
Jing L, Jiang J-R, Liu D-M, Sheng J-W, Zhang W-F, Li Z-J, Wei L-Y. Structural Characterization and Antioxidant Activity of Polysaccharides from Athyrium multidentatum (Doll.) Ching in d-Galactose-Induced Aging Mice via PI3K/AKT Pathway. Molecules. 2019; 24(18):3364. https://doi.org/10.3390/molecules24183364
Chicago/Turabian StyleJing, Liang, Jing-Ru Jiang, Dong-Mei Liu, Ji-Wen Sheng, Wei-Fen Zhang, Zhi-Jian Li, and Liu-Ya Wei. 2019. "Structural Characterization and Antioxidant Activity of Polysaccharides from Athyrium multidentatum (Doll.) Ching in d-Galactose-Induced Aging Mice via PI3K/AKT Pathway" Molecules 24, no. 18: 3364. https://doi.org/10.3390/molecules24183364
APA StyleJing, L., Jiang, J.-R., Liu, D.-M., Sheng, J.-W., Zhang, W.-F., Li, Z.-J., & Wei, L.-Y. (2019). Structural Characterization and Antioxidant Activity of Polysaccharides from Athyrium multidentatum (Doll.) Ching in d-Galactose-Induced Aging Mice via PI3K/AKT Pathway. Molecules, 24(18), 3364. https://doi.org/10.3390/molecules24183364




