Chemical Composition, In Vitro Antioxidant Potential, and Antimicrobial Activities of Essential Oils and Hydrosols from Native American Muscadine Grapes
Abstract
1. Introduction
2. Results and Discussion
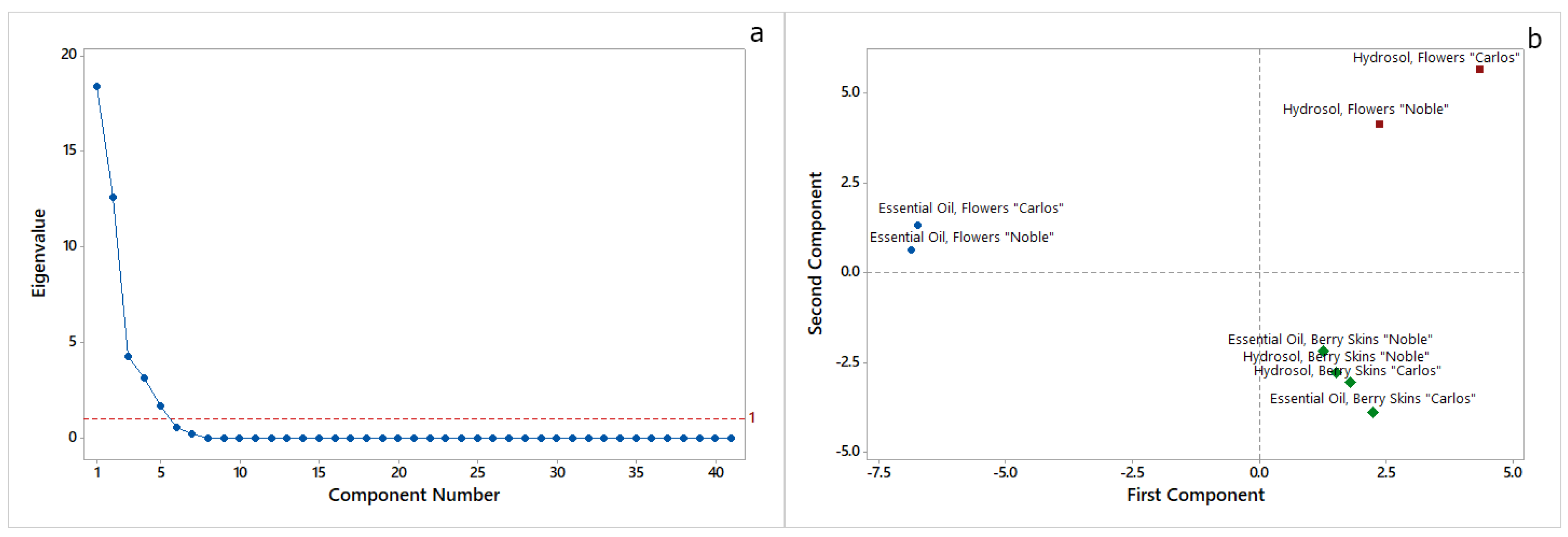
2.1. Essential Oil and Hydrosol Compositions
2.2. Evaluation of Antioxidant Potential
2.3. Evaluation of Antimicrobial Activity
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oils and Hydrosols
3.3. GC-MS Analyses
3.4. Antioxidant Activity Assays
3.5. Antimicrobial Activity Evaluation
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, W.J. Aromatherapy, botanicals, and essential oils in acne. Clin. Dermatol. 2018, 36, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Taghavi, T.; Kim, C.; Rahemi, A. Role of natural volatiles and essential oils in extending shelf life and controlling postharvest microorganisms of small fruits. Microorganisms 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Adwan, L.; K’aibni, S.; Zaid, A.N.; Shtaya, M.J.Y.; Shraim, N.; Assali, M. Variability of chemical compositions and antimicrobial and antioxidant activities of ruta chalepensis leaf essential oils from three palestinian regions. BioMed Res. Int. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Buchbauer, G.; Jirovetz, L.; Wasicky, M.; Nikiforov, A. Headspace analysis of vitis vinifera (vitaceae) flowers. J. Essent. Oil Res. 1994, 6, 311–314. [Google Scholar] [CrossRef]
- Jalali Heravi, M.; Sereshti, H. Determination of essential oil components of artemisia haussknechtii boiss. Using simultaneous hydrodistillation-static headspace liquid phase microextraction-gas chromatography mass spectrometry. J. Chromatogr. A 2007, 1160, 81–89. [Google Scholar] [CrossRef]
- Edris, A.E. Identification and absolute quantification of the major water-soluble aroma components isolated from the hydrosols of some aromatic plants. J. Essent. Oil Bear. Plants 2009, 12, 155–161. [Google Scholar] [CrossRef]
- Jentzsch, P.; Ramos, L.; Ciobotă, V. Handheld raman spectroscopy for the distinction of essential oils used in the cosmetics industry. Cosmetics 2015, 2, 162. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Coutinho, P.; Barros, A.; Delgadillo, I.; Coimbra, M.A. Establishment of the varietal volatile profile of musts from white vitis vinifera l. Varieties. J. Sci. Food Agric. 2007, 87, 1667–1676. [Google Scholar] [CrossRef]
- Augustyn, P.; Rapp, A.; Van Wyk, C. Some volatile aroma components of vitis vinifera l. Cv. Sauvignon blanc. South. Afr. J. Enol. Vitic. 1982, 3, 52–60. [Google Scholar] [CrossRef][Green Version]
- Gil, M.; Bottini, R.; Berli, F.; Pontin, M.; Silva, M.F.; Piccoli, P. Volatile organic compounds characterized from grapevine (vitis vinifera l. Cv. Malbec) berries increase at pre-harvest and in response to uv-b radiation. Phytochemistry 2013, 96, 148–157. [Google Scholar] [CrossRef]
- Kaula, C.M.; Boss, P.K. Comparison of major volatile compounds from riesling and cabernet sauvignon grapes (vitis vinifera l.) from fruitset to harvest. Aust. J. Grape Wine Res. 2010, 16, 337–348. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (vitis rotundifolia michx.) grown in united states. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Zhuang, J.; Peng, R.-H.; Cheng, Z.-M.; Zhang, J.; Cai, B.; Zhang, Z.; Gao, F.; Zhu, B.; Fu, X.-Y.; Jin, X.-F.; et al. Genome-wide analysis of the putative ap2/erf family genes in vitis vinifera. Sci. Hortic. 2009, 123, 73–81. [Google Scholar] [CrossRef]
- A Marshall, D.; J Stringer, S.; D Spiers, J. Stilbene, ellagic acid, flavonol, and phenolic content of muscadine grape (vitis rotundifolia michx.) cultivars. Pharm. Crops 2012, 3, 69–77. [Google Scholar] [CrossRef]
- Horvat, R.J.; Senter, S.D. Identification of the volatile constituents from scuppernong berries (vitis rotundifolia). J. Food Sci. 1984, 49, 64–66. [Google Scholar] [CrossRef]
- Lamikanra, O. Aroma constituents of muscadine wines1. J. Food Qual. 1987, 10, 57–66. [Google Scholar] [CrossRef]
- Baek, H.H.; Cadwallader, K.R.; Marroquin, E.; Silva, J.L. Identification of predominant aroma compounds in muscadine grape juice. J. Food Sci. 1997, 62, 249–252. [Google Scholar] [CrossRef]
- Lücker, J.; Bowen, P.; Bohlmann, J. Vitis vinifera terpenoid cyclases: Functional identification of two sesquiterpene synthase cdnas encoding (+)-valencene synthase and (−)-germacrene d synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 2004, 65, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Rocha, S.M.; Delgadillo, I.; Coimbra, M.A. Headspace-spme applied to varietal volatile components evolution during vitis vinifera l. Cv. ‘Baga’ ripening. Anal. Chim. Acta 2006, 563, 204–214. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Analysis of defensive responses activated by volatile allo-ocimene treatment in arabidopsis thaliana. Phytochemistry 2006, 67, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Dimitrić Marković, J.M.; Pejin, B.; Milenković, D.; Amić, D.; Begović, N.; Mojović, M.; Marković, Z.S. Antiradical activity of delphinidin, pelargonidin and malvin towards hydroxyl and nitric oxide radicals: The energy requirements calculations as a prediction of the possible antiradical mechanisms. Food Chem. 2017, 218, 440–446. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Cals-Grierson, M.M.; Ormerod, A.D. Nitric oxide function in the skin. Nitric Oxide 2004, 10, 179–193. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Rivero, A. Nitric oxide: An antiparasitic molecule of invertebrates. Trends Parasitol. 2006, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Petrović, S.; Pavlović, M.; Maksimović, Z.; Milenković, M.; Couladis, M.; Tzakou, O.; Niketić, M. Composition and antimicrobial activity of marrubium incanum desr.(lamiaceae) essential oil. Nat. Prod. Commun. 2009, 4, 1934578X0900400324. [Google Scholar] [CrossRef]
- Powers, C.N.; Osier, J.L.; McFeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Satyal, P.; Setzer, W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Marchev, A.; Nikolova, M.; Ivanov, I.; Gochev, V.; Stoyanova, A.; Pavlov, A. Chemical compositions of essential oils from leaves and flowers of salvia ringens sibth. Et sm. Growing wild in bulgaria. J. Essent. Oil Bear. Plants 2013, 16, 624–629. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Marchev, A.; Ivanov, I.; Denev, P.; Nikolova, M.; Gochev, V.; Stoyanova, A.; Pavlov, A.; Georgiev, V. Acetylcholinesterase inhibitory, antioxidant, and antimicrobial activities of salvia tomentosa mill. Essential oil. J. Biosci. Biotechnol. 2015, 4, 219–229. [Google Scholar]
- Kumar, R.; Phani, K.G.; Chaurasia, O.P. In vitro antioxidant activity of methanolic extract of rhodiola imbricata edgew. Pharmacogn. J. 2010, 2, 157–161. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility test. In Approved Standard-9th Edition M2-A9; Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In Approved Standard-7th Edition M7-A7; Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference method for antifungal disk diffusion susceptibility testing of yeasts. In Approved Standard-2nd Edition M44-A2; Wayne, PA, USA, 2009. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. In Approved Standard-3rd Edition M27-A3; Wayne, PA, USA, 2008. [Google Scholar]
Sample Availability: Not available. |
| Compound | Calculated Retention Index (Kovats) | % of TIC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EO *, Car-F | EO, Nob-F | EO, Car-B | EO, Nob-B | HY **, Car-F | HY, Nob-F | HY, Car-B | HY, Nob-B | ||
| Eucalyptol | 1031 | - | - | 1.28 | 0.15 | - | - | - | - |
| β-Linalool | 1097 | 0.29 | 0.11 | 8.69 | 5.95 | 12.44 | 10.73 | 7.62 | 6.50 |
| Myrcenol | 1117 | - | - | 5.10 | 0.08 | - | - | - | - |
| allo-Ocimene | 1130 | - | - | 14.05 | 0.23 | - | - | - | - |
| β-Terpineol | 1145 | - | - | 1.17 | - | - | - | 5.33 | 6.29 |
| p-Cymen-8-ol | 1184 | - | - | - | - | - | - | 13.38 | 7.08 |
| α-Terpineol | 1190 | 0.18 | 0.07 | 45.42 | 59.43 | 10.39 | 12.22 | 65.41 | 72.83 |
| cis-Geraniol | 1227 | - | - | 0.86 | 0.10 | 1.21 | 0.15 | 1.29 | 1.20 |
| trans-Geraniol | 1256 | - | - | 1.38 | 0.96 | 2.97 | 1.58 | 1.17 | 0.88 |
| 4-Hydroxy-3-methyl acetophenone | 1322 | - | - | - | - | 6.56 | 18.94 | - | - |
| α-Cubebene | 1349 | 0.13 | 0.15 | - | - | - | - | - | - |
| Eugenol | 1355 | - | - | 1.69 | 0.08 | - | - | 1.03 | - |
| Ylangene | 1371 | 0.27 | 0.69 | - | - | - | - | - | - |
| α-Copaene | 1376 | 0.41 | 0.35 | - | - | - | - | - | - |
| trans-β-Damascenone | 1381 | - | - | 0.87 | 0.09 | - | - | - | - |
| 3,4,5-Trimethoxy toluene | 1398 | - | - | - | - | 4.65 | 7.42 | - | - |
| 1,3,5-Trimethyoxy benzene | 1416 | - | - | - | - | 3.09 | 5.04 | - | - |
| β-Caryophyllene | 1419 | 3.48 | 3.72 | - | - | - | - | - | - |
| (+)-Aromadendrene | 1439 | 3.01 | 3.49 | - | - | - | - | - | - |
| β-Farnesene | 1444 | 2.79 | 3.72 | - | - | - | - | - | - |
| α-Humulene | 1455 | 1.64 | 1.45 | - | - | - | - | - | - |
| allo-Aromadendrene | 1462 | 2.74 | 3.11 | - | - | - | - | - | - |
| Germacrene D | 1479 | 6.94 | 4.48 | - | - | - | - | - | - |
| β-Selinene | 1486 | 3.43 | 3.75 | - | - | - | - | - | - |
| Valencene | 1490 | 34.32 | 39.71 | - | - | - | - | - | - |
| α-Selinene | 1495 | 4.29 | 3.28 | - | - | - | - | - | - |
| α-Farnesene | 1505 | 2.26 | 1.29 | - | - | - | - | - | - |
| α-Selinene, 7-epi | 1517 | 3.28 | 2.03 | - | - | 0.35 | 2.69 | - | - |
| α-Cadinene | 1539 | 2.16 | 2.88 | - | - | - | - | - | - |
| Elemicin | 1555 | - | - | - | - | 1.23 | 0.09 | - | - |
| Nerolidol | 1564 | - | - | - | - | 0.35 | 0.12 | - | - |
| Ledol | 1565 | - | - | - | - | 0.46 | 0.25 | - | - |
| Globulol | 1585 | 1.53 | 0.82 | - | - | 3.92 | 0.60 | - | - |
| Veridiflorol | 1589 | - | - | 0.29 | 0.65 | 0.22 | 0.16 | - | - |
| Humulene epoxide II | 1606 | 2.21 | 1.42 | - | - | 0.29 | 2.97 | - | - |
| Asarone | 1623 | - | - | - | - | 2.32 | 0.10 | - | - |
| epi-α-Cadinol | 1641 | 1.03 | 0.68 | - | - | 2.85 | 4.36 | - | - |
| epi-α-Muurolol | 1643 | 1.45 | 1.08 | - | - | 8.77 | 6.18 | - | - |
| Torreyol | 1646 | 1.64 | 0.54 | - | - | 3.00 | 2.74 | - | - |
| α-Cadinol | 1654 | 4.30 | 2.86 | - | - | 22.56 | 14.26 | - | - |
| Juniper camphor | 1690 | - | - | - | - | 5.83 | 3.19 | - | - |
| Sample | DPPH, µM Trolox Eq./g Oil | TEAC, µM Trolox Eq./g Oil | FRAP, µM Trolox Eq./g Oil | CUPRAC, µM Trolox Eq./g Oil | NO, EC50 **, mg Oil; µL Hydrosol |
|---|---|---|---|---|---|
| EO, Car-F | 3173.7 ± 326.4 *, a | 112,986.5 ± 742.3 *, a | 56,286.3 ± 466.5 *, a | 1,141,694.4 ± 2455.9 *, a | 20.0 ± 0.1 *, d |
| EO, Nob-F | 2549.3 ± 308.8 *, a,b | 79,276.3 ± 431.7 *, b | 43,884.9 ± 336.9 *, b | 878,509.6 ± 1901.9 *, b | 20.0 ± 0.1 *, d |
| EO, Car-B | 2964.7 ± 116.5 *, a | 4239.0 ± 162.9 *, c | 12,400.8 ± 160.0 *, c | 649,043.4 ± 1753.3 *, c | 10.0 ± 0.2 *, d |
| EO, Nob-B | 1712.0 ± 256.8 *, b | 1382.3 ± 108.9 *, d | 7964.2 ± 352.5 *, d | 575,580.6 ± 2160.2 *, d | 10.0 ± 0.0 *, d |
| HY, Car-F | 33.5 ± 0.7 *, c | 71.8 ± 2.3 *, e | 22.1 ± 0.6 *, e | 1.2 ± 0.0 *, e | 1720.0 ± 0.1 *, c |
| HY, Nob-F | 39.6 ± 2.2 *, c | 81.0 ± 0.8 *, e | 28.6 ± 1.0 *, e | 14.3 ± 2.6 *, e | 2030.0 ± 0.2 *, b |
| HY, Car-B | 21.0 ± 1.5 *, c | 20.2 ± 1.3 *, e | 19.9 ± 1.0 *, e | 1.2 ± 0.0 *, e | 1700.0 ± 0.2 *, c |
| HY, Nob-B | 11.6 ± 0.7 *, c | 10.5 ± 0.8 *, e | 17.9 ± 1.0 *, e | 2.1 ± 1.5 *, e | 2220.0 ± 0.2 *, a |
| Positive Control (Gallic Acid) | 15,004.9 ± 43.1 | 23,297.7 ± 25.3 | 14,850.4 ± 77.4 | 13,418.2 ± 160.4 | 210.0 ± 0.4 |
| Test Microorganism | Essential Oil, Flowers, Carlos | Essential Oil, Flowers, Noble | Positive Control | |||
|---|---|---|---|---|---|---|
| IZ ± SD *, mm | MIC **, % (w/v) | IZ ± SD, mm | MIC, % (w/v) | IZ ± SD, mm | MBC/MFC *** µg/mL | |
| Staphylococcus aureus ATCC 6538 | 10.06 ± 0.12 | 1.00 | 9.23 ± 0.25 | 1.00 | 31.30 ± 0.29 | 0.125 |
| Bacillus cereus ATCC 11778 | 8.23 ± 0.23 | 1.00 | 8.06 ± 0.12 | 1.00 | 28.30 ± 0.30 | 0.125 |
| Escherichia coli ATCC 8739 | 8.06 ± 0.12 | 2.00 | 8.06 ± 0.12 | 2.00 | 21.00 ± 0.28 | 0.25 |
| Pseudomonas aeruginosa ATCC 9027 | - | >2.00 | - | >2.00 | 9.60 ± 0.17 | 1.00 |
| Candida albicans ATTC 10231 | 14.20 ± 0.32 | 0.125 | 12.30 ± 0.26 | 0.25 | 16.60 ± 0.29 | 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, V.; Ananga, A.; Dincheva, I.; Badjakov, I.; Gochev, V.; Tsolova, V. Chemical Composition, In Vitro Antioxidant Potential, and Antimicrobial Activities of Essential Oils and Hydrosols from Native American Muscadine Grapes. Molecules 2019, 24, 3355. https://doi.org/10.3390/molecules24183355
Georgiev V, Ananga A, Dincheva I, Badjakov I, Gochev V, Tsolova V. Chemical Composition, In Vitro Antioxidant Potential, and Antimicrobial Activities of Essential Oils and Hydrosols from Native American Muscadine Grapes. Molecules. 2019; 24(18):3355. https://doi.org/10.3390/molecules24183355
Chicago/Turabian StyleGeorgiev, Vasil, Anthony Ananga, Ivayla Dincheva, Ilian Badjakov, Velizar Gochev, and Violeta Tsolova. 2019. "Chemical Composition, In Vitro Antioxidant Potential, and Antimicrobial Activities of Essential Oils and Hydrosols from Native American Muscadine Grapes" Molecules 24, no. 18: 3355. https://doi.org/10.3390/molecules24183355
APA StyleGeorgiev, V., Ananga, A., Dincheva, I., Badjakov, I., Gochev, V., & Tsolova, V. (2019). Chemical Composition, In Vitro Antioxidant Potential, and Antimicrobial Activities of Essential Oils and Hydrosols from Native American Muscadine Grapes. Molecules, 24(18), 3355. https://doi.org/10.3390/molecules24183355







