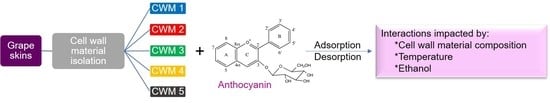
Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Cell Wall Material
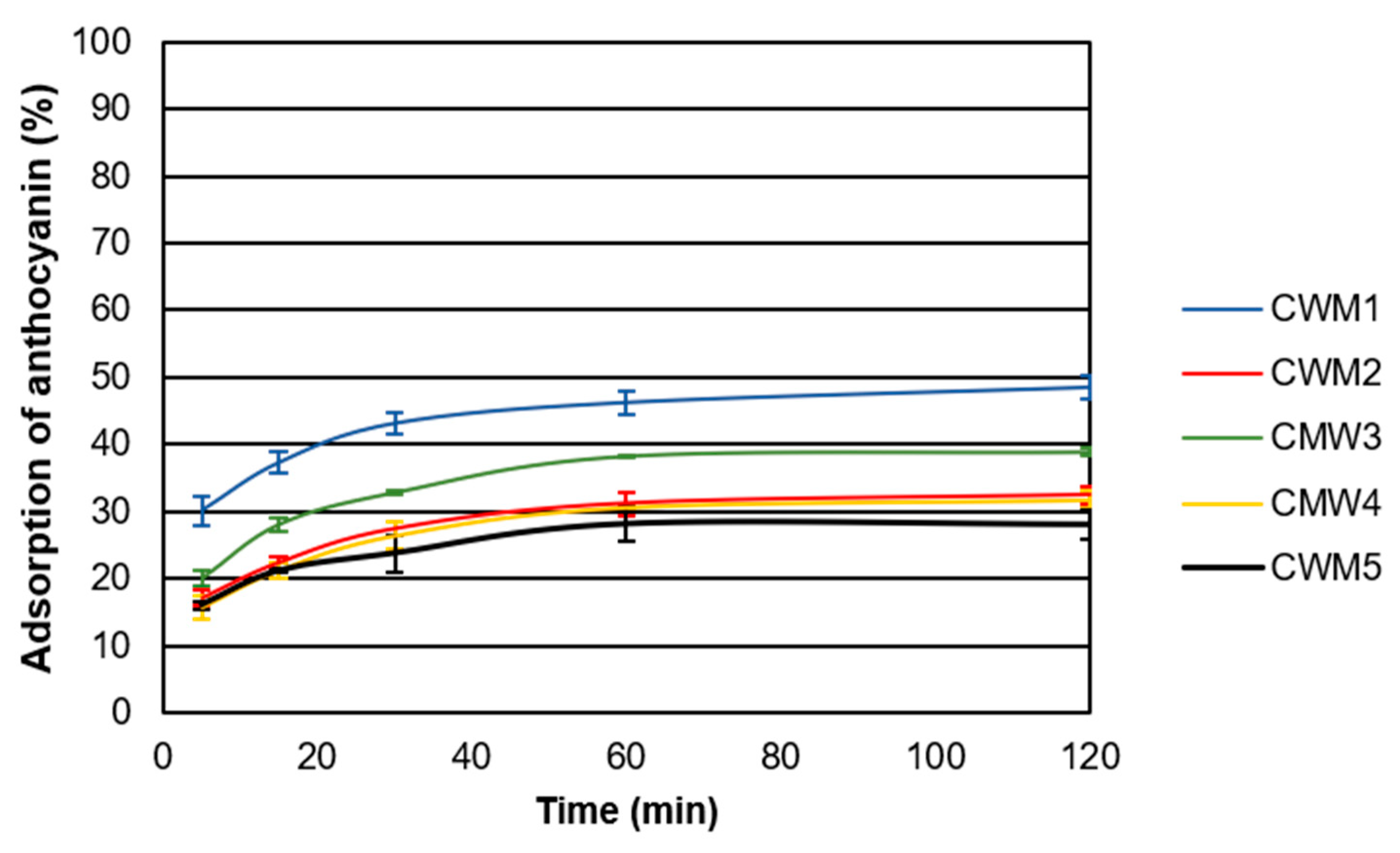
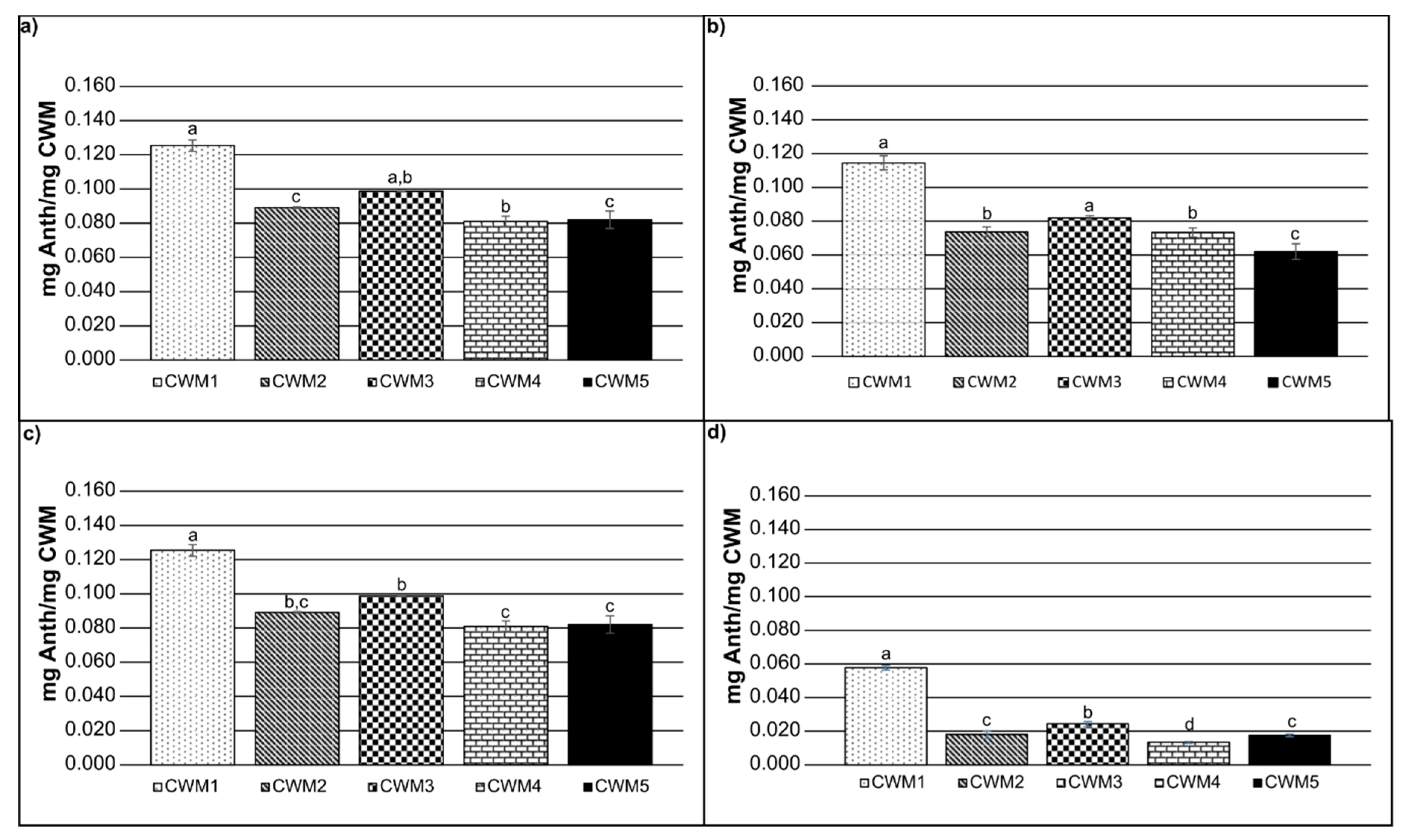
2.2. Adsorption Kinetics
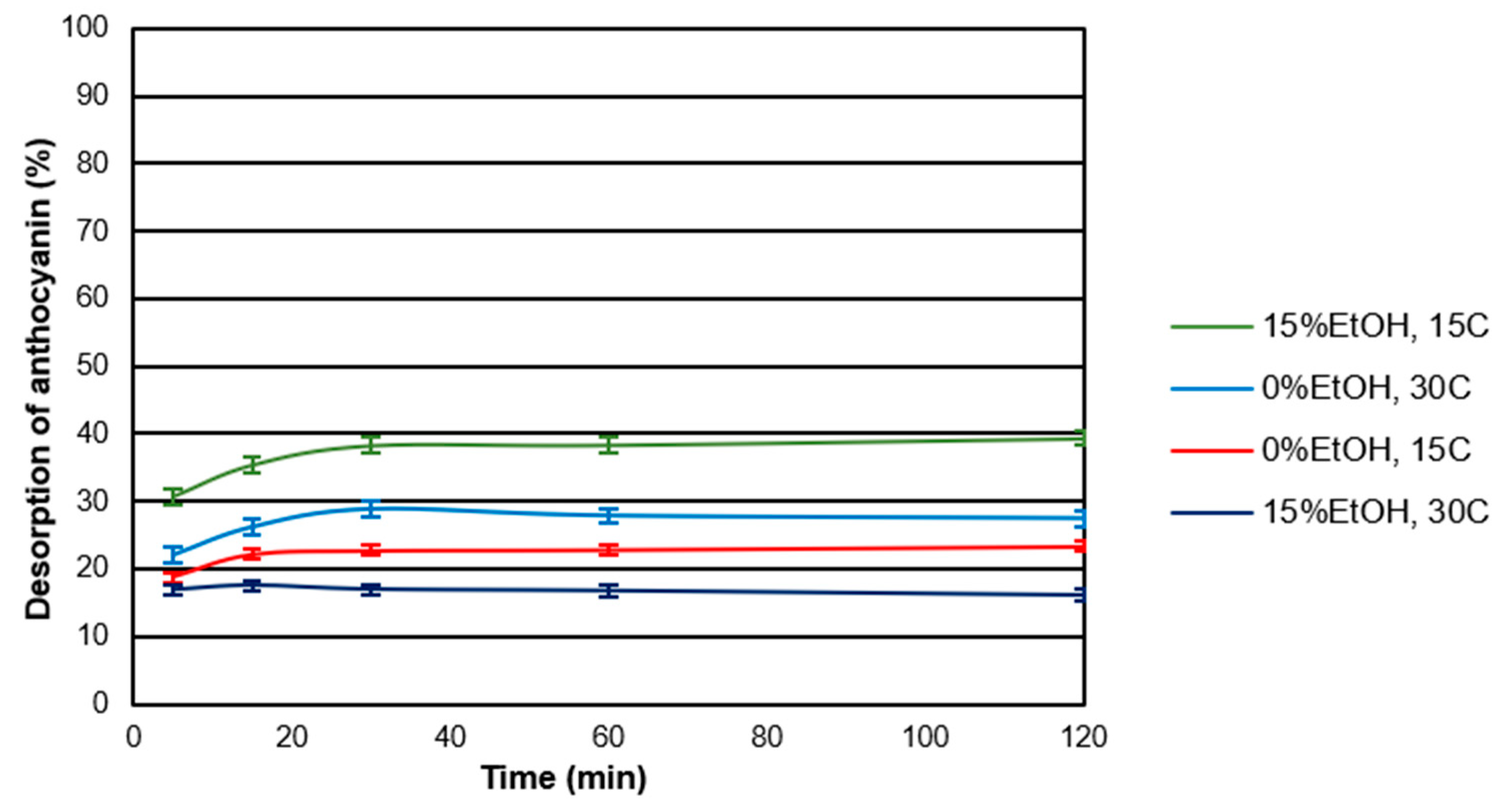
2.3. Desorption Kinetics
3. Materials and Methods
3.1. Reagents
3.2. Instrumentation
3.3. Isolation of Cell Wall Material
3.4. Isolation of Anthocyanins
3.5. Cell Wall Material Characterization
3.5.1. Carbohydrate Composition and Ash Content
3.5.2. Lipid Analysis
3.5.3. Protein Analysis
3.5.4. Uronic Acid Analysis
3.5.5. Phenolic Content
3.6. Adsorption Kinetics of Isolated Anthocyanin onto Cell Wall Material
3.7. Desorption Kinetics Experiments
3.8. Determination of Anthocyanin and Break Down Products by LC-DAD-MS
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iwashina, T. Contribution to flower colors of flavonoids including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Guidoni, S.; Hunter, J.J. Anthocyanin profile in berry skins and fermenting must/wine, as affected by grape ripeness level of Vitis vinifera cv. Shiraz/R99. Eur. Food Res. Technol. 2012, 235, 397–408. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Vasserot, Y.; Caillet, S.; Maujean, A. Study of Anthocyanin Adsorption by Yeast Lees. Effect of Some Physicochemical Parameters. Am. J. Enol. Vitic. 1997, 48, 433–437. [Google Scholar]
- Sparrow, A.M.; Dambergs, R.G.; Bindon, K.A.; Smith, P.A.; Close, D.C. Interactions of grape skin, seed, and pulp on tannin and anthocyanin extraction in pinot noir wines. Am. J. Enol. Vitic. 2015, 66, 472–481. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Cano-Lechuga, M.; Ruiz-García, Y.; Gómez-Plaza, E. Interactions between grape skin cell wall material and commercial enological tannins. Practical implications. Food Chem. 2014, 152, 558–565. [Google Scholar] [CrossRef]
- Bindon, K.A.; Bacic, A.; Kennedy, J.A. Tissue-Speci fi c and Developmental Modi fi cations of Grape Cell Walls In fl uence the Adsorption of Proanthocyanidins. J. Agric. Food Chem. 2012, 60, 9249–9260. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues - Part 2: Phenolic acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bouchet, B.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. Biochim. Biophys. Acta Gen. Subj. 2005, 1725, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A.G. Changes in Anthocyanins and Color Characteristics of Pinot Noir Wines during Different Vinification Processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Froix, M.; Goedde, A. The effect of temperature on the cellulose/water interaction from NMR relaxation times. Macromolecules 1976, 9, 428–430. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material: Part I. Effect of some environmental parameters. Biochim. Biophys. Acta Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.; Bisson, L.; Adams, D. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic 2005, 56, 197–206. [Google Scholar]
- DuBois, M.; Gilles, K.; Hamilton, J.; Rebers, P. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation ans Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Ruiz-Garcia, Y.; Smith, P.A.; Bindon, K.A. Selective extraction of polysaccharide affects the adsorption of proanthocyanidin by grape cell walls. Carbohydr. Polym. 2014, 114, 102–114. [Google Scholar] [CrossRef]
- Vicens, A.; Fournand, D.; Williams, P.; Sidhoum, L.; Moutounet, M.; Doco, T. Changes in Polysaccharide and Protein Composition of Cell Walls in Grape Berry Skin (Cv. Shiraz during Ripening and Over-ripening. Phytochemistry 2009, 57, 2955–2960. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.A.; Peng, A.C. Lipid Composition of “Concord” grapes. Am. J. Enol. Vitic. 1976, 27, 32–35. [Google Scholar]
- Bautista-Ortín, A.B.; Martínez-Hernández, A.; Ruiz-García, Y.; Gil-Muñoz, R.; Gómez-Plaza, E. Anthocyanins influence tannin-cell wall interactions. Food Chem. 2016, 206, 239–248. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; López-Roca, J.M.; Gómez-Plaza, E.; Ros-García, J.M. Application and comparison of four selected procedures for the isolation of cell-wall material from the skin of grapes cv. Monastrell. Anal. Chim. Acta 2010, 660, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.-F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Gennis, R.B. Biomembranes: Molecular Structure and Function; Springer: New York, NT, USA, 1989; ISBN 9781475720679. [Google Scholar]
- Lerno, L.; Reichwager, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of cap and overall fermentation temperature on phenolic extraction in cabernet sauvignon fermentations. Am. J. Enol. Vitic. 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Piffaut, B.; Kader, F.; Girardin, M.; Metche, M. Comparative degradation pathways of malvidin 3,5-diglucoside after enzymatic and thermal treatments. Food Chem. 1994, 50, 115–120. [Google Scholar] [CrossRef]
- Lopes, P.; Richard, T.; Saucier, D.; Teissedre, P.-L.; Monti, J.-P.; Glories, Y. Anthocyanone A: A Quinone Methide Derivative Resulting from Malvidin 3-O-Glucoside Degradation. J. Agric. Food Chem. 2007, 55, 2698–2704. [Google Scholar] [CrossRef]
- Ben-Naim, A. Hydrophobic Interactions; Springer: Berlin, Germany, 1980; ISBN 9781468435450. [Google Scholar]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues - Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Vidal, S.; Williams, P.; O’Neill, M.A.; Pellerin, P. Polysaccharides from grape berry cell walls. Part I: Tissue distribution and structural characterization of the pectic polysaccharides. Carbohydr. Polym. 2001, 45, 315–323. [Google Scholar] [CrossRef]
- Olle, D.; Lozano, Y.F.; Brillouet, J.M. Isolation and characterization of soluble polysaccharides and insoluble cell wall material of the pulp from four mango (Mangifera indica L) cultivars. J. Agric. Food Chem. 1996, 44, 2658–2662. [Google Scholar] [CrossRef]
- Peng, Z.; Iland, P.G.; Oberholster, A.; Sefton, M.A.; Waters, E.J. Analysis of pigmented polymers in red wine by reverse phase HPLC. Aust. J. Grape Wine Res. 2002, 8, 70–75. [Google Scholar] [CrossRef]
- Lurie, S.; Levin, A.; Greve, L.C.; Labavitch, J.M. Pectic polymer changes in nectarines during normal and abnormal ripening. Phytochemistry 1994, 36, 11–17. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar]
- AOAC International Official method of analysis of AOAC International, 19th ed.; George, W.; Latimer, J. (Eds.) AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B. Handbook of Enology; John Wiley: Hoboken, NJ, USA, 2006; ISBN 0470010371. [Google Scholar]
- Lerno, L.; Reichwage, M.; Panprivech, S.; Ponangi, R.; Hearne, L.; Oberholster, A.; Block, D.E. Chemical Gradients in Pilot-Scale Cabernet Sauvignon Fermentations and Their Effect on Phenolic Extraction. Am. J. Enol. Vitic. 2017, 68, 401–411. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| CWM | Protein (mg BSA/g CWM) | Lipids (mg/g CWM) | Soluble Polysaccharides (mg/g CWM) | Non-Cellulosic Glucose (mg/g CWM) | Cellulosic Glucose (mg/g CWM) | Lignin (mg/g CWM) | Uronic Acid (mg/g CWM) | Total Polyphenolic Content (mg Gallic Acid/g CWM) | Ash (mg/g CWM) |
|---|---|---|---|---|---|---|---|---|---|
| CWM1 | 51.29 ± 1.14 c | 18.51 ± 2.38 bc | 3.00 ± 0.04 c | 84.17 ± 0.59 b | 51.23 ± 1.82 c | 407.23 ± 10.51 ab | 0.22 ± 0.00 a | 0.035 ± 0.000 c | 15.43 ± 2.08 e |
| CWM2 | 54.91 ± 1.74 b | 13.62 ± 3.46 c | 5.49 ± 0.35 b | 94.23 ± 5.42 b | 55.06 ± 0.80 b | 418.42 ± 19.70 ab | 0.21 ± 0.01 a | 0.043 ± 0.003 b | 19.34 ± 3.27 c |
| CWM3 | 48.86 ± 0.87 c | 33.61 ± 5.04 a | 3.04 ± 0.14 b | 87.44 ± 2.30 b | 54.10 ± 2.03 d | 408.52 ± 40.68 ab | 0.22 ± 0.01 a | 0.049 ± 0.000 a | 34.24 ± 5.01 b |
| CWM4 | 57.96 ± 2.36 a | 15.08 ± 2.34 bc | 2.33 ± 0.20 bc | 111.29 ± 2.86 a | 57.04 ± 1.12 a | 371.96 ± 43.47 b | 0.20 ± 0.01 a | 0.043 ± 0.002 b | 15.96 ± 2.33 d |
| CWM5 | 56.46 ± 1.73 ab | 24.23 ± 5.18 ab | 10.17 ± 0.88 a | 111.13 ± 7.06 a | 55.90 ± 0.29 ab | 475.54 ± 27.83 a | 0.22 ± 0.01 a | 0.021 ± 0.002 d | 49.41 ± 6.36 a |
| Condition | Delph (%) | Pet (%) | Peo (%) | Malv (%) | Malv-Acet (%) |
|---|---|---|---|---|---|
| 0%EtOH; 15 °C | 78.57 | 75.94 | 32.60 | 60.55 | 54.79 |
| 15%EtOH; 15 °C | 68.89 | 65.10 | 42.04 | 38.34 | 29.08 |
| 0%EtOH; 30 °C | 69.27 | 63.28 | 47.55 | 29.68 | 47.43 |
| 15%EtOH; 30 °C | 23.58 | 20.73 | 16.81 | 15.06 | 16.16 |
| EtOH (%) | T (°C) | CWM | Adsorbed (mg Anth/mg CWM) | Desorbed (mg Anth/mg CWM) | Percentage Desorbed (%) |
|---|---|---|---|---|---|
| 0 | 15 | CWM1 | 0.160 | 0.029 | 18.12 |
| 0 | 15 | CWM2 | 0.155 | 0.028 | 18.06 |
| 0 | 15 | CWM3 | 0.172 | 0.025 | 14.53 |
| 0 | 15 | CWM4 | 0.159 | 0.025 | 15.72 |
| 0 | 15 | CWM5 | 0.148 | 0.025 | 16.89 |
| 15 | 15 | CWM1 | 0.128 | 0.037 | 28.90 |
| 15 | 15 | CWM2 | 0.108 | 0.043 | 39.81 |
| 15 | 15 | CWM3 | 0.114 | 0.035 | 30.70 |
| 15 | 15 | CWM4 | 0.103 | 0.035 | 33.98 |
| 15 | 15 | CWM5 | 0.102 | 0.030 | 29.41 |
| 0 | 30 | CWM1 | 0.137 | 0.024 | 17.51 |
| 0 | 30 | CWM2 | 0.128 | 0.037 | 28.90 |
| 0 | 30 | CWM3 | 0.132 | 0.025 | 18.94 |
| 0 | 30 | CWM4 | 0.127 | 0.030 | 23.62 |
| 0 | 30 | CWM5 | 0.125 | 0.029 | 23.20 |
| 15 | 30 | CWM1 | 0.112 | 0.014 | 12.50 |
| 15 | 30 | CWM2 | 0.100 | 0.013 | 13.00 |
| 15 | 30 | CWM3 | 0.104 | 0.016 | 15.38 |
| 15 | 30 | CWM4 | 0.093 | 0.013 | 13.97 |
| 15 | 30 | CWM5 | 0.092 | 0.012 | 13.04 |
| Type of CWM | 70% Acetone | Buffer HEPES | Phenol Solution | MeOH/Chloroform (1:1 v/v) | Composition |
|---|---|---|---|---|---|
| CWM1 | √ | √ | √ | √ | Clean cell wall material |
| CWM2 | √ | × | √ | √ | CWM1 + Soluble Polysaccharides |
| CWM3 | √ | √ | √ | × | CWM1 + Lipids |
| CWM4 | √ | √ | × | √ | CWM1 + Cytoplasmic proteins |
| CWM5 | √ | × | × | × | “Crude” cell wall material |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Plaza, C.; Beaver, J.W.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions. Molecules 2019, 24, 3350. https://doi.org/10.3390/molecules24183350
Medina-Plaza C, Beaver JW, Lerno L, Dokoozlian N, Ponangi R, Blair T, Block DE, Oberholster A. Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions. Molecules. 2019; 24(18):3350. https://doi.org/10.3390/molecules24183350
Chicago/Turabian StyleMedina-Plaza, Cristina, Jordan W. Beaver, Larry Lerno, Nick Dokoozlian, Ravi Ponangi, Tom Blair, David E. Block, and Anita Oberholster. 2019. "Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions" Molecules 24, no. 18: 3350. https://doi.org/10.3390/molecules24183350
APA StyleMedina-Plaza, C., Beaver, J. W., Lerno, L., Dokoozlian, N., Ponangi, R., Blair, T., Block, D. E., & Oberholster, A. (2019). Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions. Molecules, 24(18), 3350. https://doi.org/10.3390/molecules24183350