Recognition of Thiols in Living Cells and Zebrafish Using an Imidazo[1,5-α]pyridine-Derivative Indicator
Abstract
1. Introduction
2. Results and Discussion
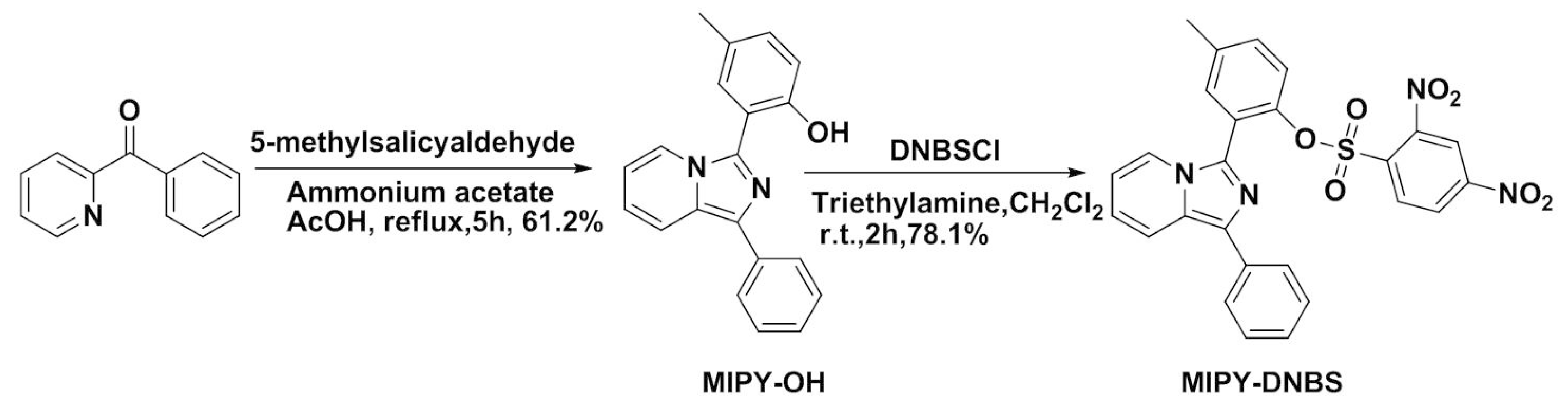
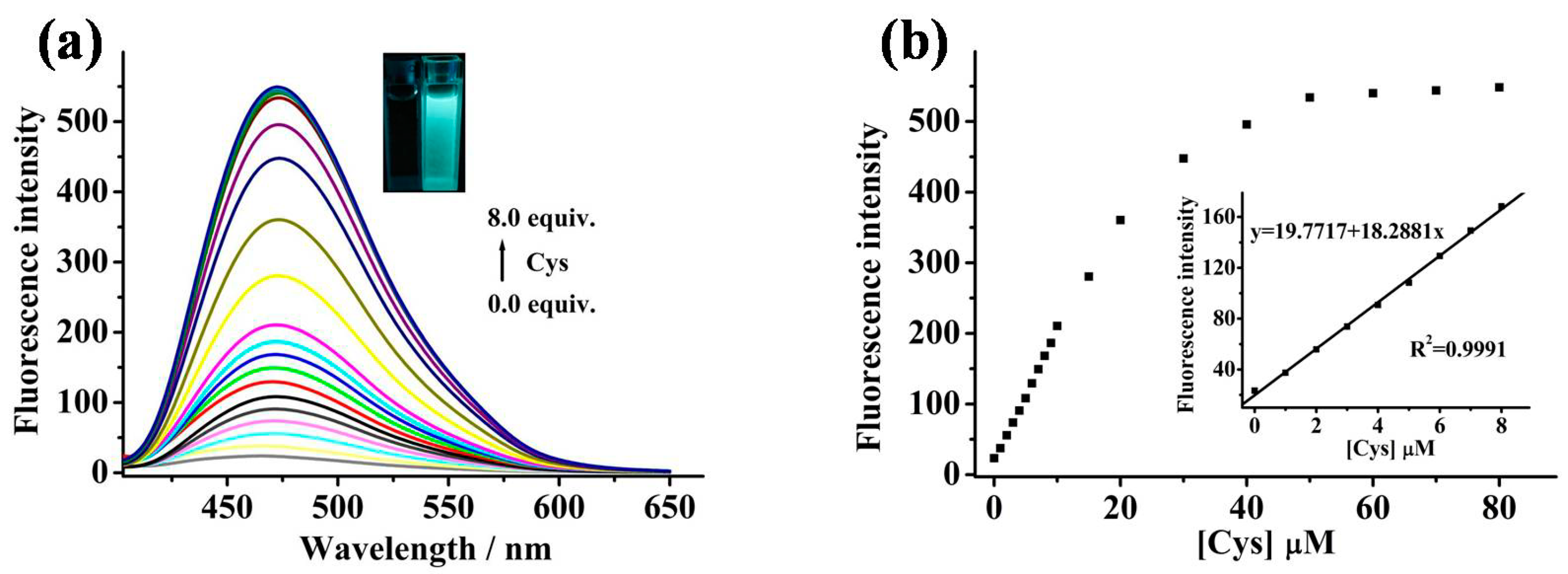
2.1. Design and Spectroscopic Studies
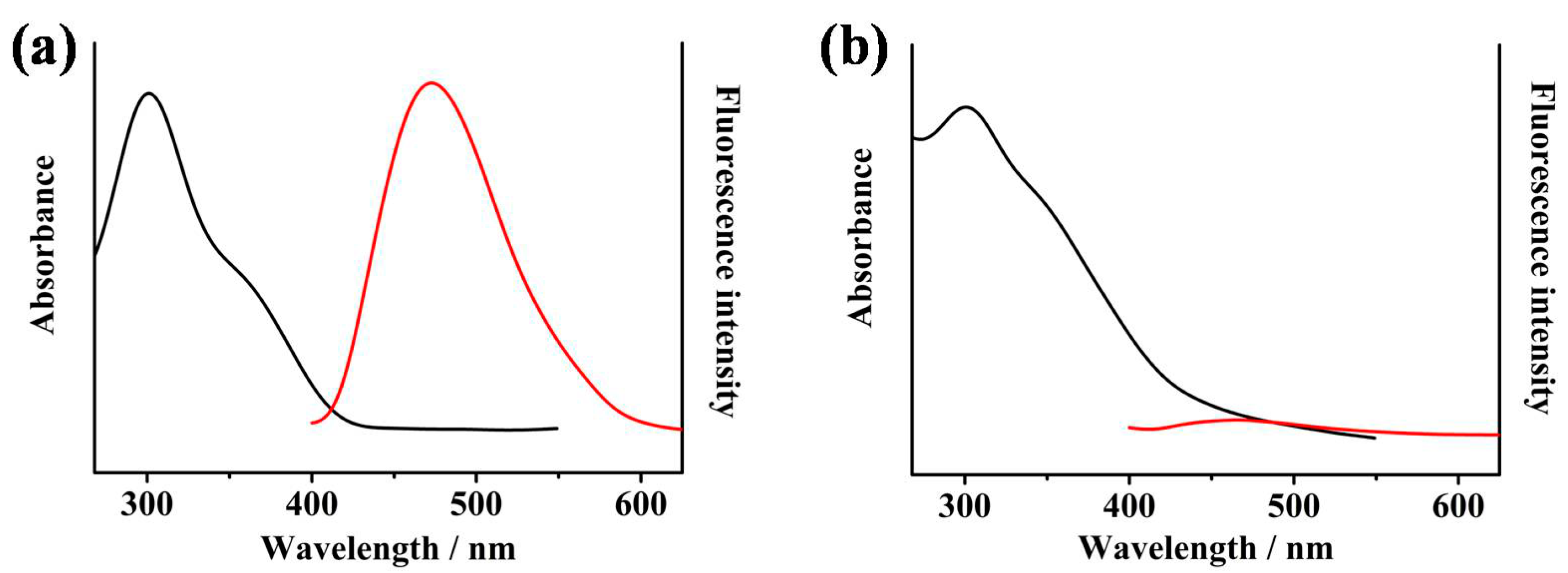
2.2. Sensing Properties of MIPY-DNBS to Thiols
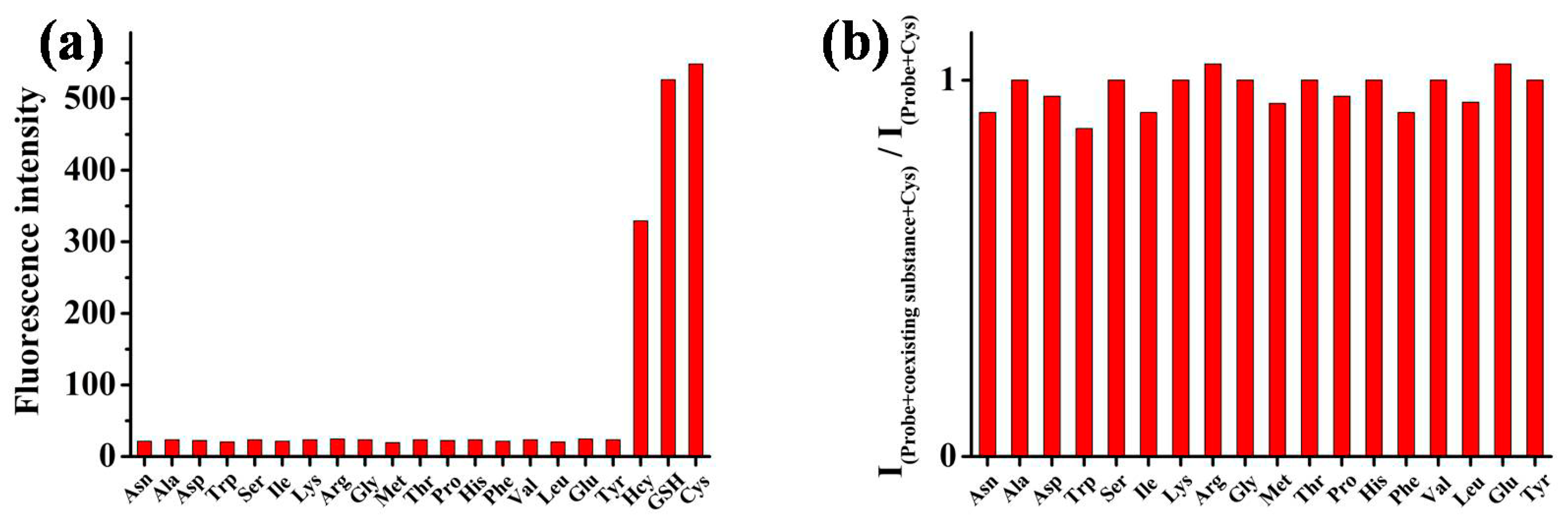
2.3. Specificity Evaluation
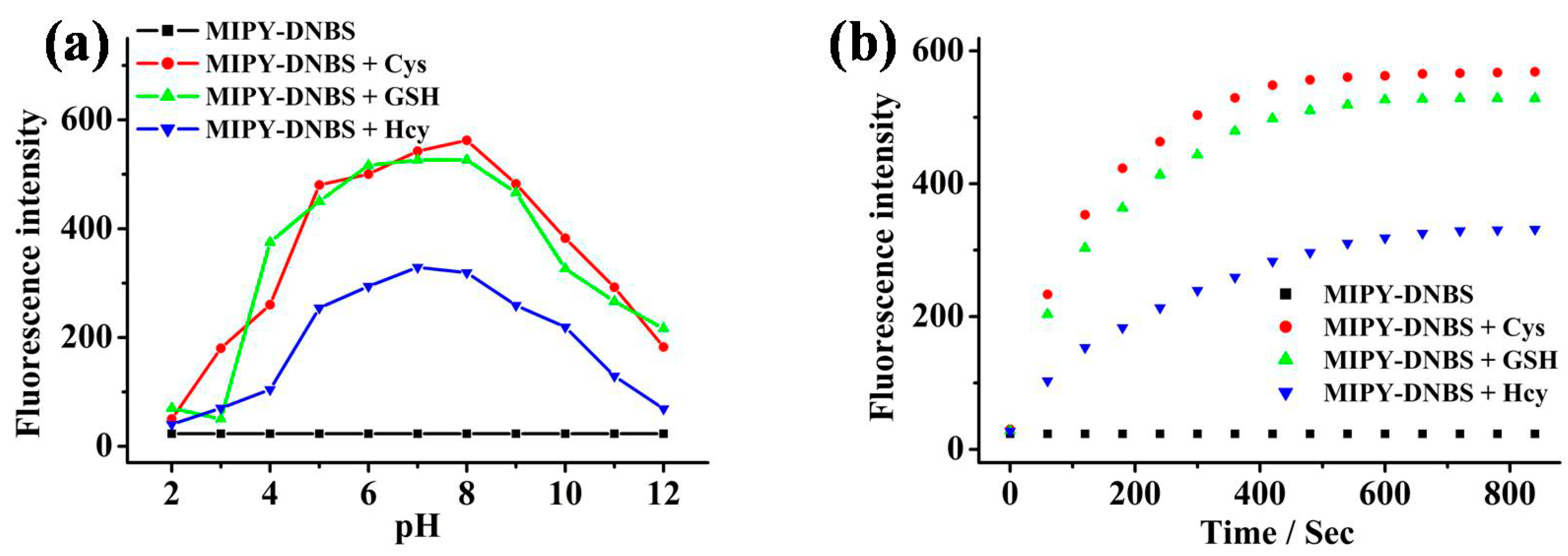
2.4. pH Stability on Thiols Studies
2.5. Reaction Time on Sensing Thiols
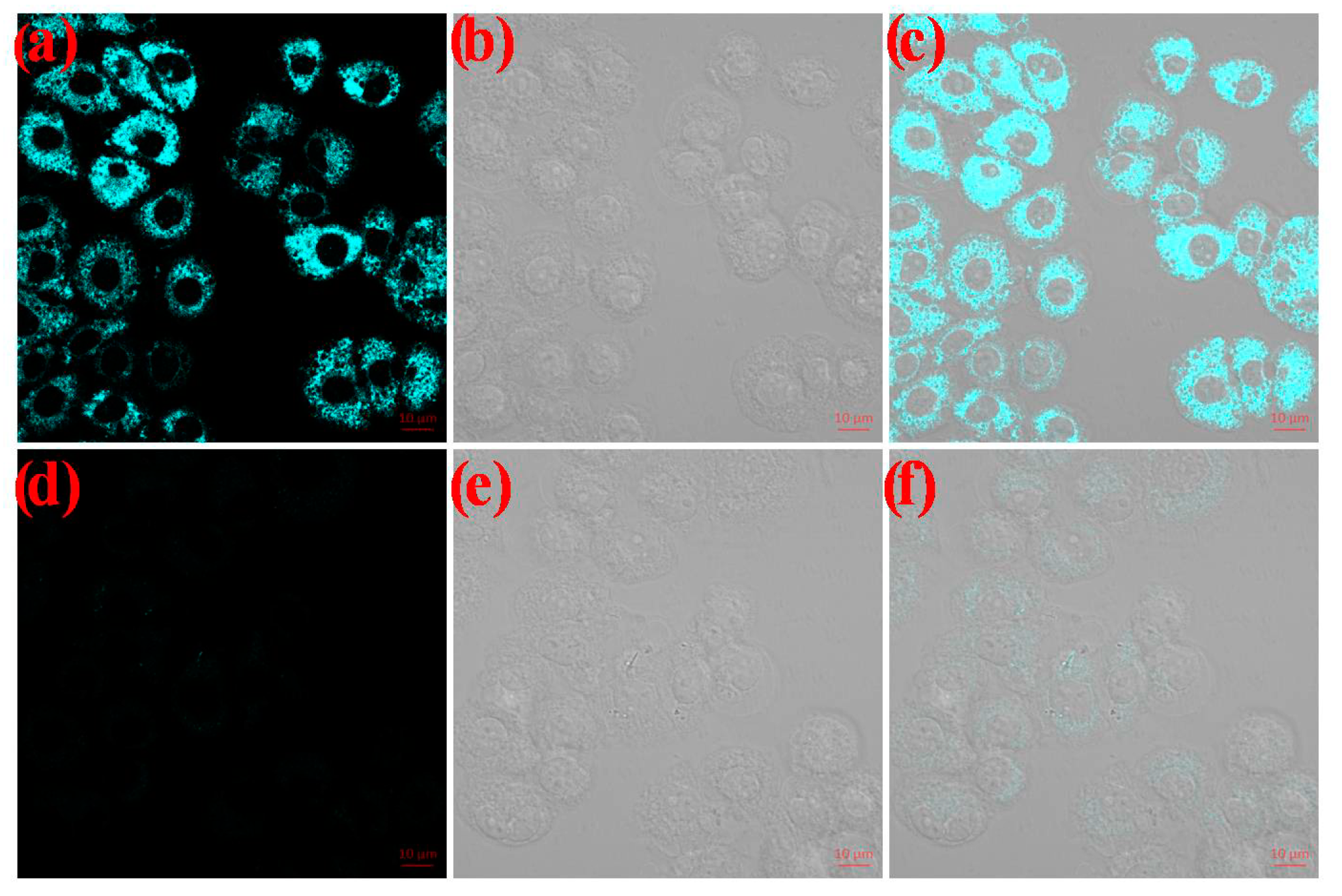
2.6. Fluorescence Imaging in Living MCF-7 Cells
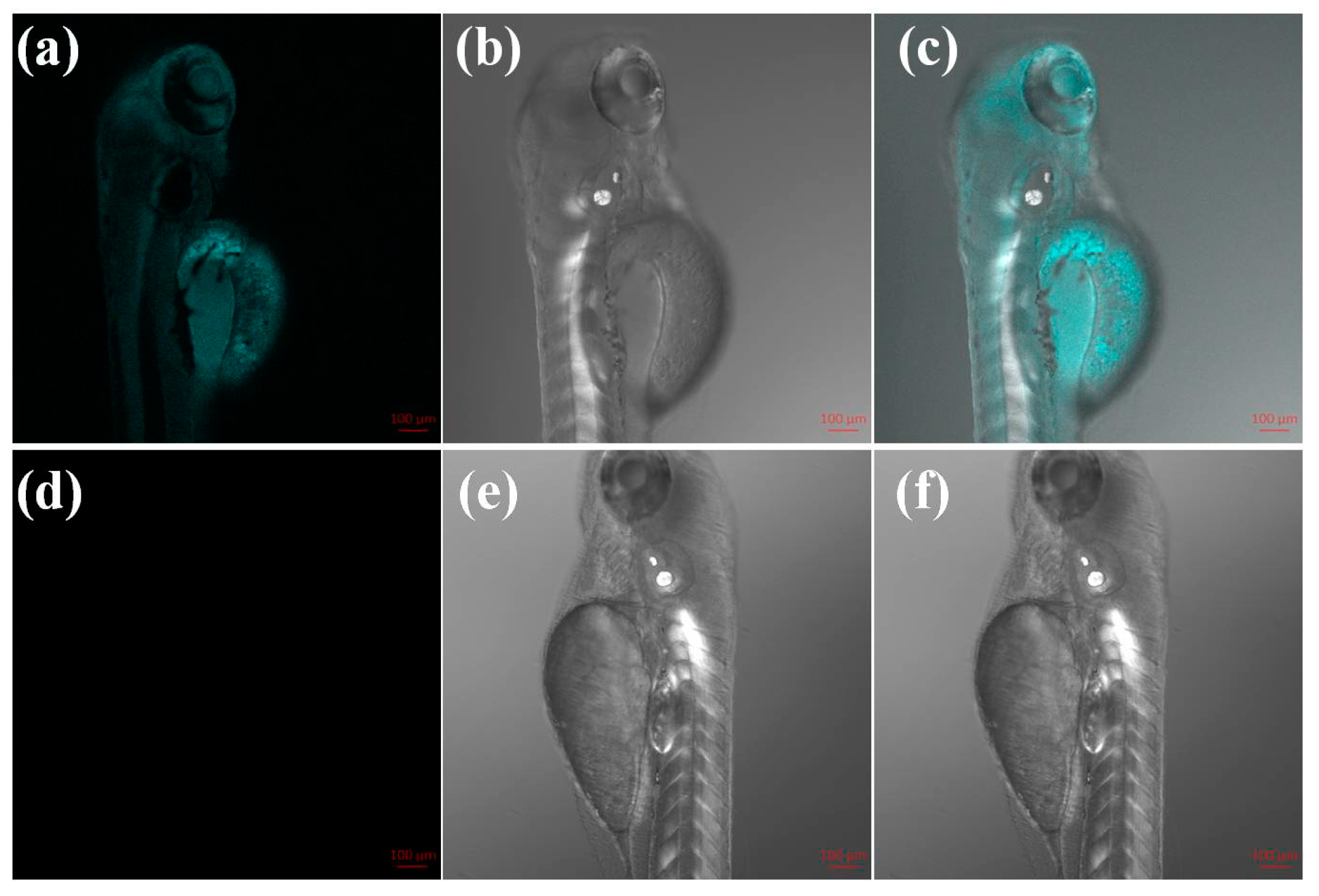
2.7. Fluorescence Imaging in Zebrafish
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Spectroscopic Methods
3.3. Synthesis of Probe MIPY-DNBS
3.4. Cells Culture and Fluorescence Imaging
3.5. Fluorescence Imaging in Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, T.; Ding, H.; Xu, H.J.; Lv, Y.L.; Liu, H.; Wang, H.D.; Tian, Z.Y. Dual-functional probes for sequential thiol and redox homeostasis sensing in live cells. Analyst 2015, 140, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ong, C.N.; Shen, H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yu, Y.W.; Wang, B.X.; Jiang, Y.L. A sensitive fluorescent probe based on coumarin for detection of cysteine in living cells. J. Photochem. Photobiol. A 2017, 338, 178–182. [Google Scholar] [CrossRef]
- Voehringer, D.W.; McConkey, D.J.; McDonnell, T.J.; Brisbay, S.; Meyn, R.E. Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc. Natl. Acad. Sci. USA 1998, 95, 2956–2960. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Liu, M.Y. Amino acid cystine induces senescence and decelerates cell growth in melanoma. J. Funct. Foods 2015, 18, 455–462. [Google Scholar] [CrossRef]
- Wilmer, M.J.; Kluijtmans, L.A.J.; Van der Velden, T.J.; Willems, P.H.; Scheffer, P.G.; Masereeuw, R.; Monnens, L.A.; Van den Heuvel, L.P.; Levtchenko, E.N. Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells. Bba-Mol. Basis Dis. 2011, 1812, 643–651. [Google Scholar] [CrossRef]
- Yin, C.X.; Huo, F.J.; Zhang, J.J.; Martinez-Manez, R.; Yang, Y.T.; Lv, H.G.; Li, S.D. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharm. 2007, 7, 355–359. [Google Scholar] [CrossRef]
- Niu, L.Y.; Guan, Y.S.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef]
- Nekrassova, O.; Lawrence, N.S.; Compton, R.G. Analytical determination of homocysteine: A review. Talanta 2003, 6, 1085–1095. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.Q.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharm. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Butterfield, D.A. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim. Biophys. Acta. 2012, 1822, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.J.; Yin, P.; Shen, Y.M.; Zhang, L.L.; Deng, J.H.; Xue, S.Y.; Li, H.T.; Guo, B.; Zhang, Y.Y.; Yao, S.Z. A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells. Chem. Common. 2013, 49, 4640–4642. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, H.R.; Sun, H.B.; Liu, S.J.; Wang, J.X.; Zhao, Q.; Liu, X.M.; Xu, W.J.; Li, S.B.; Huang, W. Rational design of an “off-on” phosphorescent chemodosimeter based on an iridium(III) complex and its application for time-resolved luminescent detection and bioimaging of cysteine and homocysteine. Chem. Eur. J. 2013, 19, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Y.; Seefeldt, T.; Guan, X. Determination of thiols and disulfidesvia HPLC quantification of 5-thio-2-nitrobenzoic acid. J. Pharm. Biomed. Anal. 2008, 48, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Yu, J.J.; Xu, X.H.; Jiang, Y.L.; Wang, B.X. A novel fluorescent probe for highly sensitive and selective detection of cysteine and its application in cell imaging. Sens. Actuat. B-Chem. 2017, 251, 902–908. [Google Scholar] [CrossRef]
- Li, W.X.; Zhou, S.M.; Zhang, L.L.; Yang, Z.M.; Chen, H.; Chen, W.Q.; Qin, J.K.; Shen, X.C.; Zhao, S.L. A red emitting fluorescent probe for sensitively monitoring hydrogen polysulfides in living cells and zebrafish. Sens. Actuat. B-Chem. 2018, 258, 125–132. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.L.; Xiong, H.Q.; Zhang, Y.; Chen, W.Q.; Sheng, J.R.; Song, X.Z. An endoplasmic reticulum-targetable fluorescent probe for highly selective detection of hydrogen sulfide. Org. Biomol. Chem. 2019, 17, 1436–1441. [Google Scholar] [CrossRef]
- Lv, Y.; Cheng, D.; Su, D.; Chen, M.; Yin, B.C.; Yuan, L.; Zhang, X. Visualization of Oxidative Injury in the Mouse Kidney by Selective Superoxide Anion Fluorescent Probes. Chem. Sci. 2018, 9, 7606–7613. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Wang, J.; Fu, S.; Liu, L. A highly sensitive fluorescent probe based on the Michael addition mechanism with a large Stokes shift for cellular thiols imaging. Anal. Bioanal. Chem. 2018, 410, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, P.; Wang, J.; Fu, S.; Liu, L. A simple but effective fluorescent probe with large stokes shift for specific detection of cysteine in living cells. J. Photochem. Photobiol. A 2018, 363, 7–12. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Zhao, W.; Zhang, X.; Luo, X.; Corkins, M.E.; Cole, S.L.; Wang, C.; Xiao, Y.; Bi, X.; et al. Two-Photon Near Infrared Fluorescent Turn-On Probe Toward Cysteine and Its Imaging Applications. ACS Sens. 2016, 1, 882–887. [Google Scholar] [CrossRef]
- Liu, X.J.; Gao, L.; Yang, L.; Zou, L.F.; Chen, W.Q.; Song, X.Z. A phthalimide-based fluorescent probe for thiol detection with a large Stokes shift. RSC Adv. 2015, 5, 18177–18182. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, N.; Fang, Z. A Novel Dicyanoisophorone-Based Ratiometric Fluorescent Probe for Selective Detection of Cysteine and Its Bioimaging Application in Living Cells. Molecules 2018, 23, 475. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Hao, Y.Q.; Liu, J.X.; Wu, G.G.; Liu, L. A green-emitting fluorescent probe based on a benzothiazole derivative for imaging biothiols in living cells. Molecules 2019, 24, 411. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.F.; Lan, J.S.; Li, X.-D.; Liang, H.-F.; Liao, Y.; Lu, Y.-J.; Zhang, T.; Ding, Y. A Fluorescent Coumarin-Based Probe for the Fast Detection of Cysteine with Live Cell Application. Molecules 2017, 22, 1618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.N.; Yang, S.L.; Lu, W.; Gao, B.H.; Xu, L.; Sun, X.; Jiang, D.; Xu, H.J.; Ma, M.T.; Cao, F.L. A novel fluorescence “turn off-on” nano-sensor for detecting Cu2+ and Cysteine in living cells. J. Photochem. Photobiol. A 2018, 362, 14–20. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Zhang, Y.; Zhang, C.; Jin, J.; Li, H.; Yao, S. A novel colorimetric/fluorescence dual-channel sensor based on NBD for the rapid and highly sensitive detection of cysteine and homocysteine in living cells. Anal. Methods 2016, 8, 2420–2426. [Google Scholar] [CrossRef]
- Fang, M.X.; Xia, S.; Bi, J.H.; Wigstrom, T.P.; Valenzano, L.; Wang, J.B.; Tanasova, M.; Luck, R.L.; Liu, H.Y. Detecting Zn(II) ions in live cells with near-infrared fluorescent probes. Molecules 2019, 24, 1592. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, T.B.; Fang, M.X.; Mikesell, L.; Steenwinkel, T.E.; Wan, S.L.; Phillips, T.; Luck, R.L.; Werner, T.; Liu, H.Y. A FRET-Based Near-Infrared Fluorescent Probe for Ratiometric Detection of Cysteine in Mitochondria. Chem. Biol. Chem. 2019, 20, 1986–1994. [Google Scholar]
- Chen, S.; Li, H.M.; Hou, P. A large stokes shift fluorescent probe for sensing of thiophenols based on imidazo 1,5-alpha pyridine in both aqueous medium and living cells. Anal. Chim. Acta 2017, 993, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, H.M.; Hou, P. A novel imidazo 1,5-alpha pyridine-based fluorescent probe with a large Stokes shift for imaging hydrogen sulfide. Sens. Actuat. B-Chem. 2018, 256, 1086–1092. [Google Scholar] [CrossRef]
- Hou, P.; Wang, J.; Fu, S.; Liu, L.; Chen, S. A new turn-on fluorescent probe with ultra-large fluorescence enhancement for detection of hydrogen polysulfides based on dual quenching strategy. Spectrochim. Acta A 2019, 213, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, P.; Wang, J.; Fu, S.; Liu, L. A rapid and selective fluorescent probe with a large Stokes shift for the detection of hydrogen sulfide. Spectrochim. Acta A 2018, 203, 258–262. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Hou, P.; Sun, J.; Wang, H.; Liu, L. Recognition of Thiols in Living Cells and Zebrafish Using an Imidazo[1,5-α]pyridine-Derivative Indicator. Molecules 2019, 24, 3328. https://doi.org/10.3390/molecules24183328
Chen S, Hou P, Sun J, Wang H, Liu L. Recognition of Thiols in Living Cells and Zebrafish Using an Imidazo[1,5-α]pyridine-Derivative Indicator. Molecules. 2019; 24(18):3328. https://doi.org/10.3390/molecules24183328
Chicago/Turabian StyleChen, Song, Peng Hou, Jingwen Sun, Haijun Wang, and Lei Liu. 2019. "Recognition of Thiols in Living Cells and Zebrafish Using an Imidazo[1,5-α]pyridine-Derivative Indicator" Molecules 24, no. 18: 3328. https://doi.org/10.3390/molecules24183328
APA StyleChen, S., Hou, P., Sun, J., Wang, H., & Liu, L. (2019). Recognition of Thiols in Living Cells and Zebrafish Using an Imidazo[1,5-α]pyridine-Derivative Indicator. Molecules, 24(18), 3328. https://doi.org/10.3390/molecules24183328




