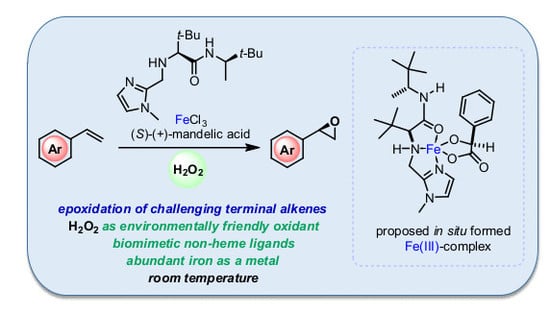
Biomimetic Non-Heme Iron-Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant
Abstract
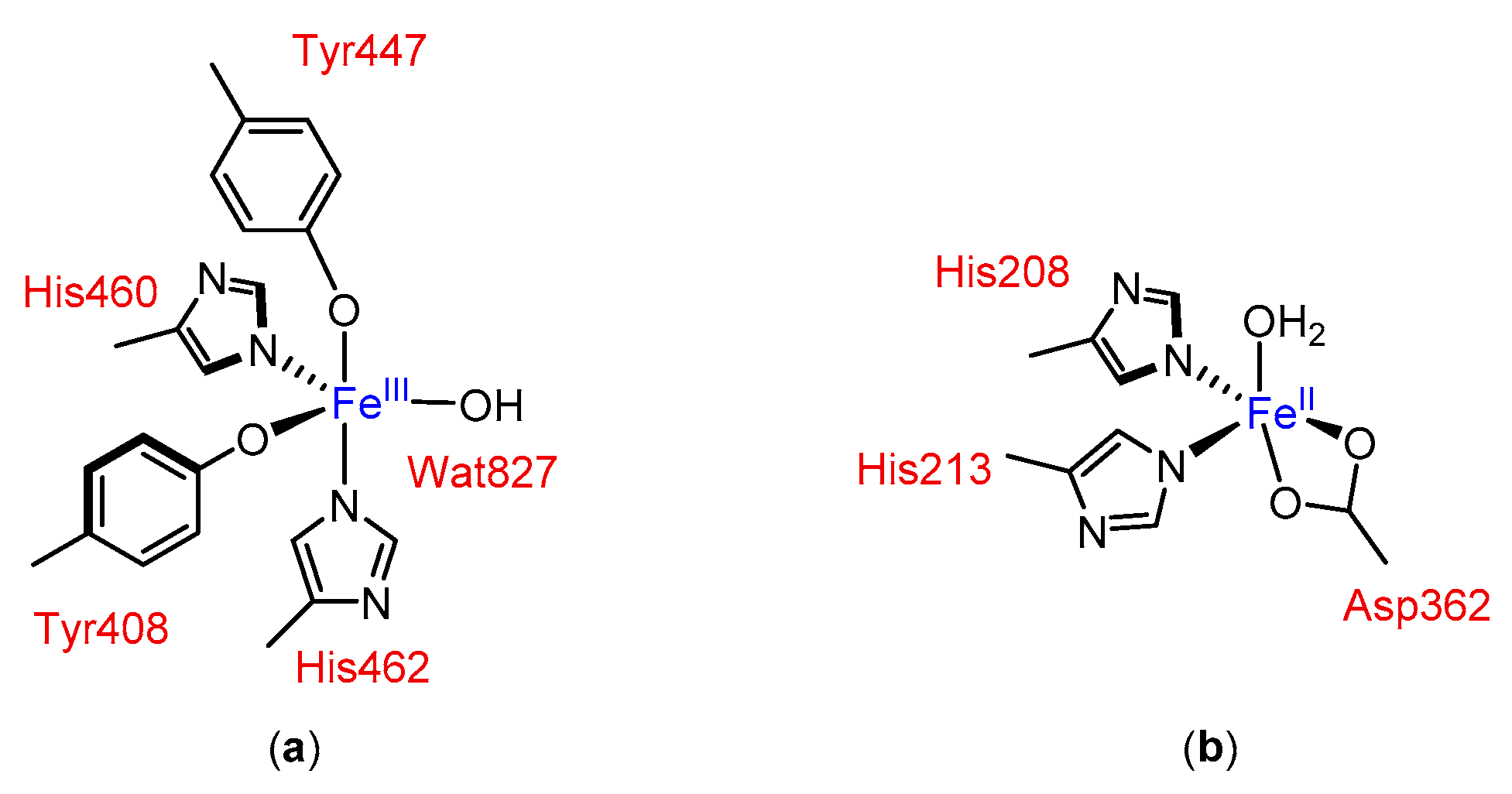
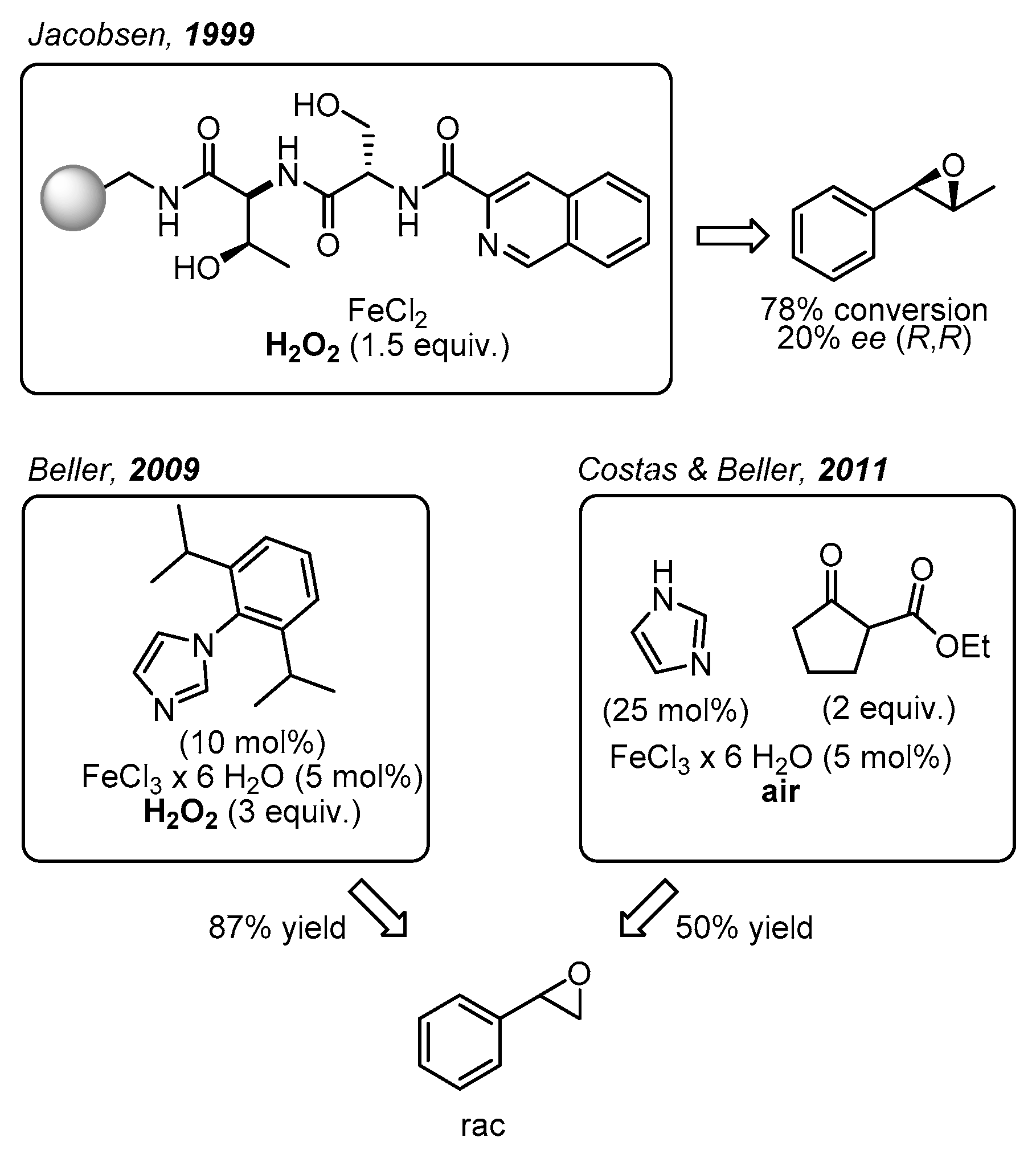
1. Introduction
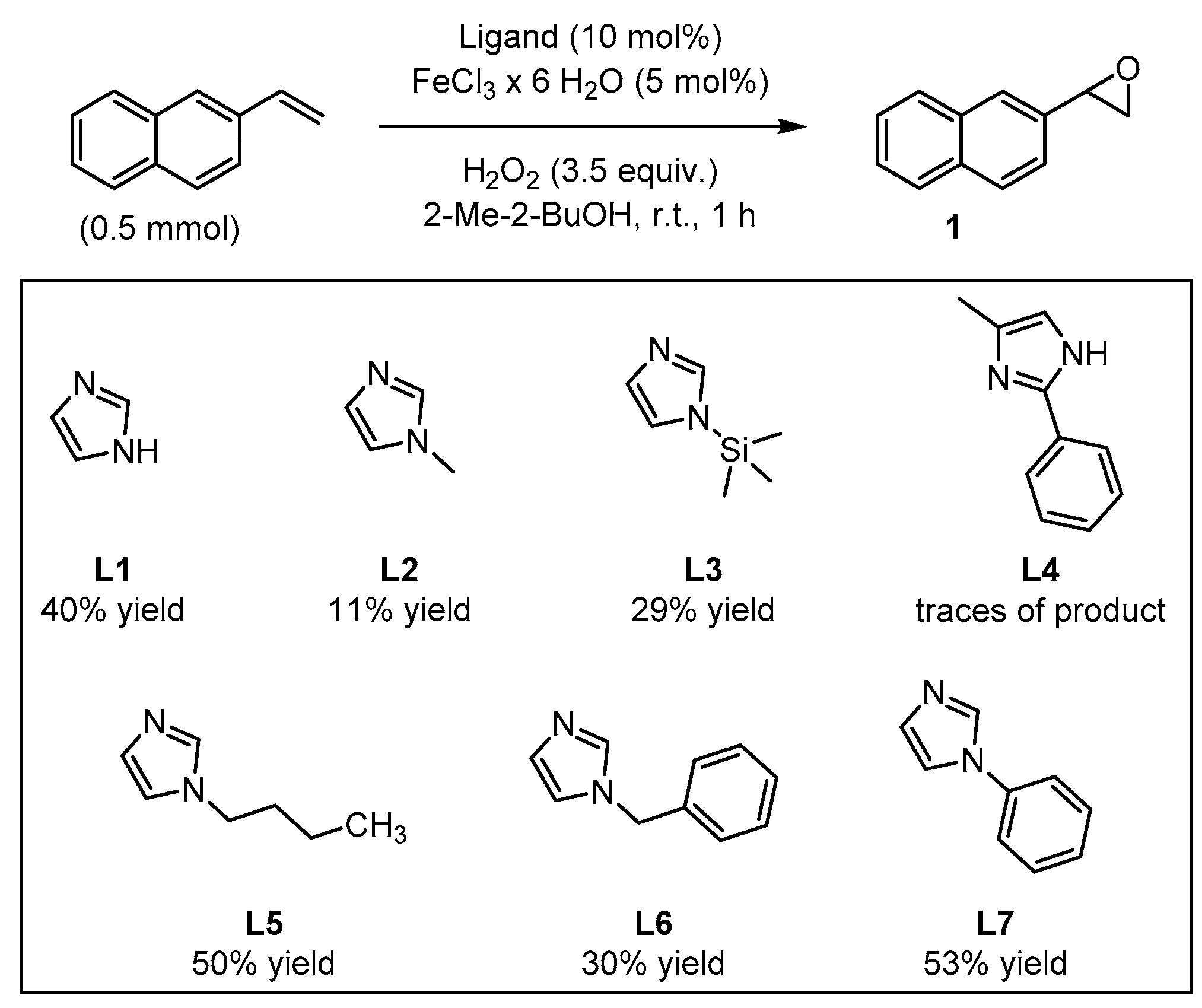
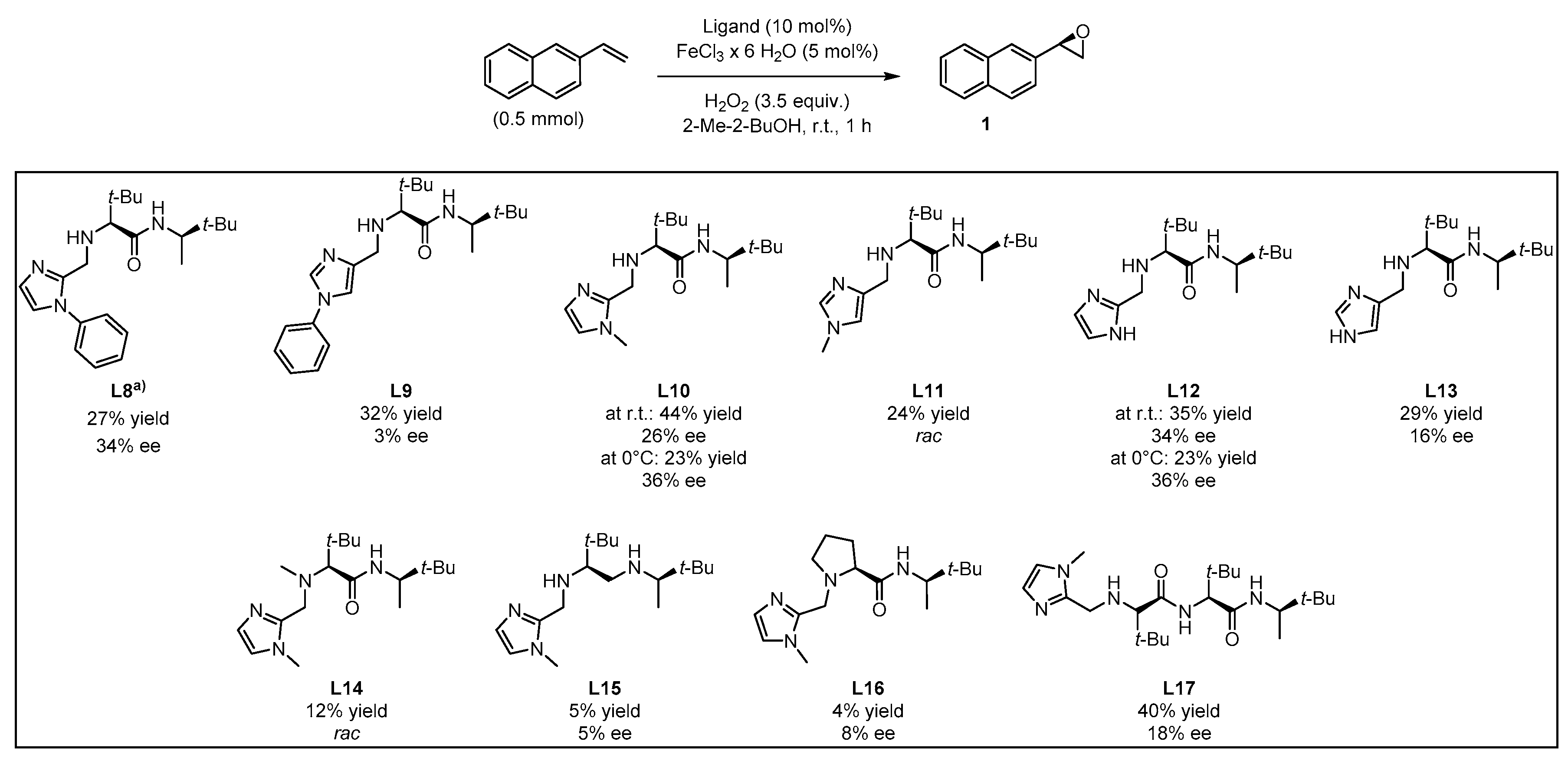
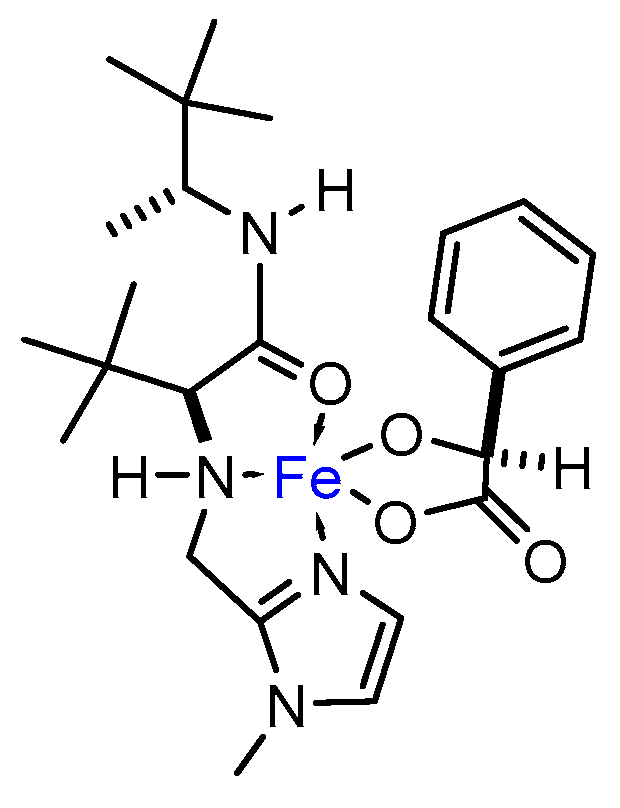
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Catalytic Procedure
3.2.1. Catalysis without Additive
3.2.2. Catalysis with (S)-(+)-Mandelic Acid
3.2.3. Substrate and Racemic Product References
3.3. General Procedure of Olefin Epoxidation with m-CPBA
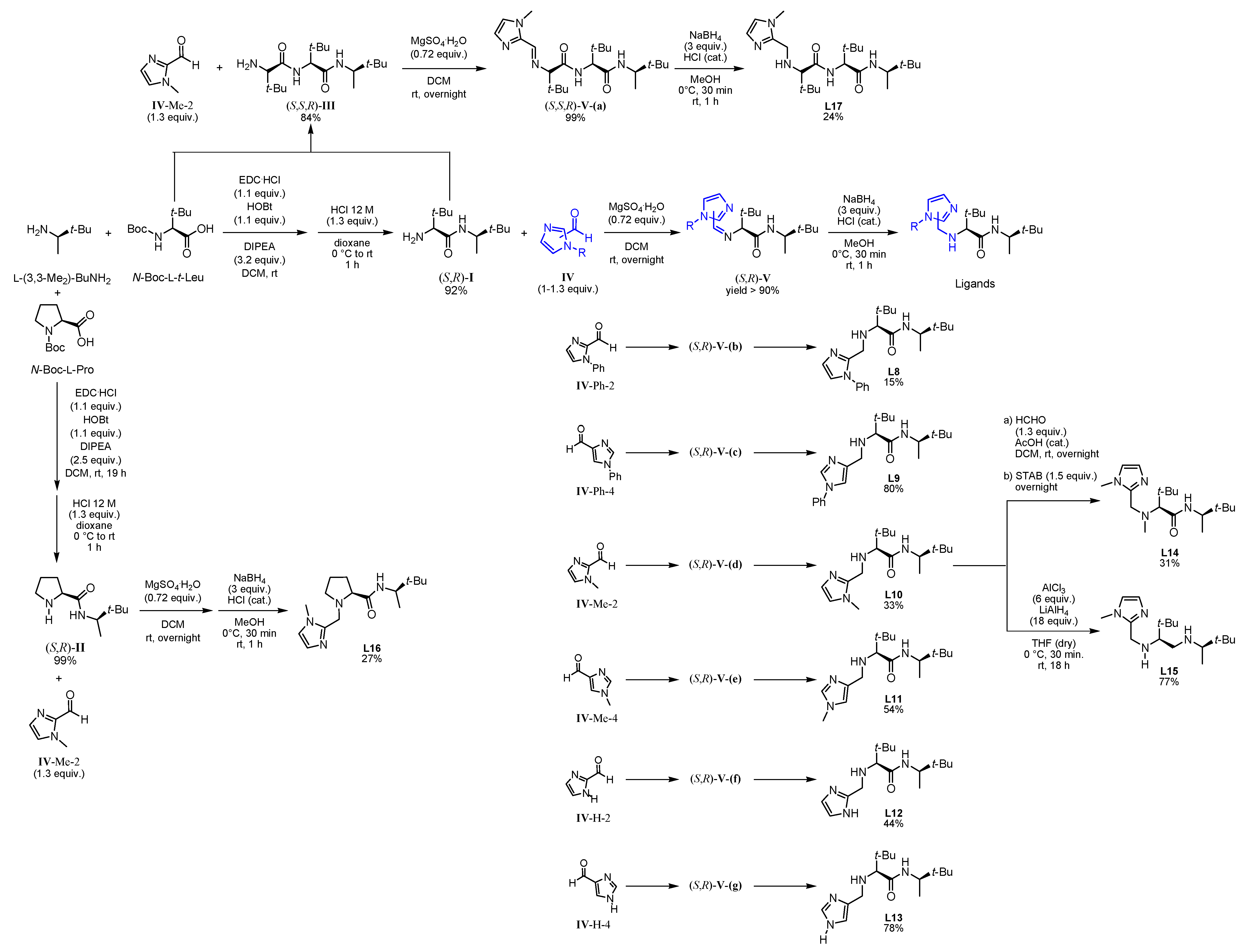
3.3.1. Synthesis of Ligands
3.3.2. Synthesis of l-Proline or l-tert-Leucine Based Amines
3.3.3. Synthesis of Imidazole Based Aldehyde
3.3.4. Synthesis of Imidazole Based Peptide Like Ligand
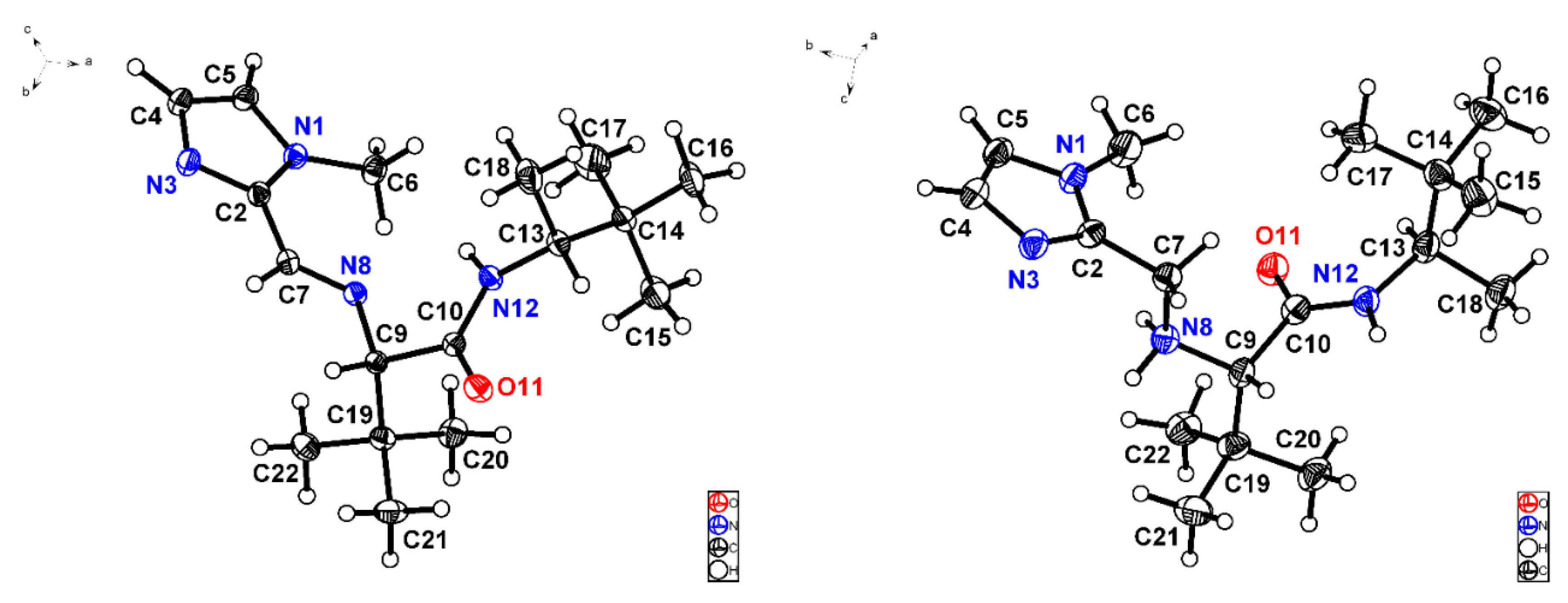
3.4. X-ray Crystallographic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yudin, A.K. (Ed.) Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Beauvais, B.; Sarfati, C.; Challier, S.; Derouin, F. In vitro model to assess effect of antimicrobial agents on Encephalitozoon cuniculi. Antimicrob. Agents Chemother. 1994, 38, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.L.; Kevin, N.J.; Kirk, B.A.; Chapman, K.T.; Schleif, W.A.; Olsen, D.B.; Stahlhut, M.; Rutkowski, C.A.; Kuo, L.C.; Jin, L.; et al. Synthesis and activity of novel HIV protease inhibitors with improved potency against multiple PI-resistant viral strains. Bioorg. Med. Chem. 2002, 12, 2423–2426. [Google Scholar] [CrossRef]
- Nachbauer, L.; Brückner, R. Synthesis of a Cn–Cn+6 Building Block Common to Important Polyol, Polyene Antibiotics from a Divinylcarbinol by a Desymmetrizing Sharpless Epoxidation. Eur. J. Org. Chem. 2013, 2013, 6545–6562. [Google Scholar] [CrossRef]
- Nakazawa, M.; Uehara, T.; Nomura, Y. Koningic Acid (a Potent Glyceraldehyde-3-Phosphate Dehydrogenase Inhibitor)-Induced Fragmentation and Condensation of DNA in NG108-15 Cells. J. Neurochem. 1997, 68, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Sunohara, K.; Mitsuhashi, S.; Shigetomi, K.; Ubukata, M. Discovery of N-(2,3,5-triazoyl)mycophenolic amide and mycophenolic epoxyketone as novel inhibitors of human IMPDH. Bioorg. Med. Chem. Lett. 2013, 23, 5140–5144. [Google Scholar] [CrossRef] [PubMed]
- Piontek, A.; Bisz, E.; Szostak, M. Iron-catalyzed cross-couplings in the synthesis of pharmaceuticals: In pursuit of sustainability. Angew. Chem. Int. Ed. 2018, 57, 11116–11128. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; Junge, K.; Beller, M. Sustainable Metal Catalysis with Iron: From Rust to a Rising Star? Angew. Chem. Int. Ed. 2008, 47, 3317–3321. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.T.; Myers, R.S. Catalytic asymmetric epoxidations with chiral iron porphyrins. J. Am. Chem. Soc. 1983, 105, 5791–5796. [Google Scholar] [CrossRef]
- Groves, J.T.; Viski, P. Asymmetric hydroxylation by a chiral iron porphyrin. J. Am. Chem. Soc. 1989, 111, 8537–8538. [Google Scholar] [CrossRef]
- Collman, J.P.; Wang, Z.; Straumanis, A.; Quelquejeu, M.; Rose, E. An Efficient Catalyst for Asymmetric Epoxidation of Terminal Olefins. J. Am. Chem. Soc. 1999, 121, 460–461. [Google Scholar] [CrossRef]
- Darwish, M.; Wills, M. Asymmetric catalysis using iron complexes—‘Ruthenium Lite’? Catal. Sci. Technol. 2012, 2, 243–255. [Google Scholar] [CrossRef]
- Schomburg, D.; Schomburg, I.; Chang, A. (Eds.) Class 1 Oxidoreductases X: EC 1.9–1.13; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Karlsson, A.; Parales, J.V.; Parales, R.E.; Gibson, D.T.; Eklund, H.; Ramaswamy, S. Crystal Structure of Naphthalene Dioxygenase: Side-on Binding of Dioxygen to Iron. Science 2003, 299, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.; Buurmans, I.L.; Gosiewska, S.; Moelands, M.A.; Lutz, M.; Spek, A.L.; van Koten, G.; Klein Gebbink, R.J. Iron(II) Complexes with Bio-Inspired N,N,O Ligands as Oxidation Catalysts: Olefin Epoxidation and cis-Dihydroxylation. Chem. Eur. J. 2008, 14, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Join, B.; Amali, A.J.; Junge, K.; Ribas, X.; Costas, M.; Beller, M. A Biomimetic Iron Catalyst for the Epoxidation of Olefins with Molecular Oxygen at Room Temperature. Angew. Chem. Int. Ed. 2011, 50, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Enthaler, S.; Bitterlich, B.; Schulz, T.; Spannenberg, A.; Tse, M.K.; Junge, K.; Beller, M. Design of and Mechanistic Studies on a Biomimetic Iron–Imidazole Catalyst System for Epoxidation of Olefins with Hydrogen Peroxide. Chem. Eur. J. 2009, 15, 5471–5481. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.B.; Jacobsen, E.N. Discovery of Novel Catalysts for Alkene Epoxidation from Metal-Binding Combinatorial Libraries. Angew. Chem. Int. Ed. 1999, 38, 937–941. [Google Scholar] [CrossRef]
- Hasan, K.; Brown, N.; Kozak, C.M. Iron-catalyzed epoxidation of olefins using hydrogen peroxide. Green Chem. 2011, 13, 1230–1237. [Google Scholar] [CrossRef]
- Jiao, M.; Matsunaga, H.; Ishizuka, T. A simple, Iron-Catalyzed, Pyridine-Assisted Hydrogen Peroxide Epoxidation System. Chem. Pharm. Bull. 2011, 59, 799–801. [Google Scholar] [CrossRef]
- Gelalcha, F.G.; Bitterlich, B.; Anilkumar, G.; Tse, M.K.; Beller, M. Iron-Catalyzed Asymmetric Epoxidation of Aromatic Alkenes Using Hydrogen Peroxide. Angew. Chem. Int. Ed. 2007, 46, 7293–7296. [Google Scholar] [CrossRef]
- Gelalcha, F.G.; Anilkumar, G.; Tse, M.K.; Bruckner, A.; Beller, M. Biomimetic Iron-Catalyzed Asymmetric Epoxidation of Aromatic Alkenes by Using Hydrogen Peroxide. Chem. Eur. J. 2008, 14, 7687–7698. [Google Scholar] [CrossRef]
- Bitterlich, B.; Schröder, K.; Tse, M.K.; Beller, M. An Improved Iron-Catalyzed Epoxidation of Aromatic and Aliphatic Olefins with Hydrogen Peroxide as Oxidant. Eur. J. Org. Chem. 2008, 4867–4870. [Google Scholar] [CrossRef]
- Schröder, K.; Tong, X.; Bitterlich, B.; Tse, M.K.; Gelalcha, F.G.; Brückner, A.; Beller, M. Novel biomimetic iron-catalysts for environmentally benign epoxidations of olefins. Tetrahedron Lett. 2007, 48, 6339–6342. [Google Scholar] [CrossRef]
- Schröder, K.; Junge, K.; Spannenberg, A.; Beller, M. Design of a bio-inspired imidazole-based iron catalyst for epoxidation of olefins: Mechanistic insights. Catal. Today 2010, 157, 364–370. [Google Scholar] [CrossRef]
- Costas, M.; Que, J.L. Ligand Topology Tuning of Iron-Catalyzed Hydrocarbon Oxidations. Angew. Chem. Int. Ed. 2002, 41, 2179–2181. [Google Scholar] [CrossRef]
- Mas-Balleste, R.; Costas, M.; van den Berg, T.; Que, L., Jr. Ligand Topology Effects on Olefin Oxidations by Bio-Inspired [FeII(N2Py2)] Catalysts. Chem. Eur. J. 2006, 12, 7489–7500. [Google Scholar] [CrossRef] [PubMed]
- Cusso, O.; Garcia-Bosch, I.; Ribas, X.; Lloret-Fillol, J.; Costas, M. Asymmetric Epoxidation with H2O2 by Manipulating the Electronic Properties of Non-heme Iron Catalysts. J. Am. Chem. Soc. 2013, 135, 14871–14878. [Google Scholar] [CrossRef] [PubMed]
- Cusso, O.; Ribas, X.; Lloret-Fillol, J.; Costas, M. Synergistic Interplay of a Non-Heme Iron Catalyst and Amino Acid Coligands in H2O2 Activation for Asymmetric Epoxidation of α-Alkyl-Substituted Styrenes. Angew. Chem. Int. Ed. 2015, 54, 2729–2733. [Google Scholar] [CrossRef]
- Cusso, O.; Giuliano, M.W.; Ribas, X.; Miller, S.J.; Costas, M. A bottom up approach towards artificial oxygenases by combining iron coordination complexes and peptides. Chem. Sci. 2017, 8, 3660–3667. [Google Scholar] [CrossRef]
- Wu, M.; Miao, C.-X.; Wang, S.; Hu, X.; Xia, C.; Kühn, F.E.; Sun, W. Chiral Bioinspired Non-Heme Iron Complexes for Enantioselective Epoxidation of α,β-Unsaturated Ketones. Adv. Synth. Catal. 2011, 353, 3014–3022. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Xia, C.; Sun, W. Highly Enantioselective Epoxidation of Multisubstituted Enones Catalyzed by Non-Heme Iron Catalysts. Chem. Eur. J. 2012, 18, 7332–7335. [Google Scholar] [CrossRef]
- Lyakin, O.Y.; Ottenbacher, R.V.; Bryliakov, K.P.; Talsi, E.P. Asymmetric Epoxidations with H2O2 on Fe and Mn Aminopyridine Catalysts: Probing the Nature of Active Species by Combined Electron Paramagnetic Resonance and Enantioselectivity Study. ACS Catal. 2012, 2, 1196–1202. [Google Scholar] [CrossRef]
- Lancaster, M. Green Chemistry: An Introductory Text, 3rd ed.; RSC Internet Services: London, UK, 2016. [Google Scholar]
- Lane, B.S.; Burgess, K. Metal-Catalyzed Epoxidations of Alkenes with Hydrogen Peroxide. Chem. Rev. 2003, 103, 2457–2473. [Google Scholar] [CrossRef] [PubMed]
- Held, F.E.; Wei, S.; Eder, K.; Tsogoeva, S.B. One-pot route to β-adrenergic blockers via enantioselective organocatalysed epoxidation of terminal alkenes as a key step. RSC Adv. 2014, 4, 32796–32801. [Google Scholar] [CrossRef]
- Fingerhut, A.; Serdyuk, O.V.; Tsogoeva, S.B. Non-heme iron catalysts for epoxidation and aziridination reactions of challenging terminal alkenes: Towards sustainability. Green Chem. 2015, 17, 2042–2058. [Google Scholar] [CrossRef]
- Yeung, H.L.; Sham, K.C.; Tsang, C.S.; Lau, T.C.; Kwong, H.L. A chiral iron-sexipyridine complex as a catalyst for alkene epoxidation with hydrogen peroxide. Chem. Commun. 2008, 3801–3803. [Google Scholar] [CrossRef] [PubMed]
- Hieke, M.; Greiner, C.; Thieme, T.M.; Schubert-Zsilavecz, M.; Werz, O.; Zettl, H. A novel class of dual mPGES-1/5-LO inhibitors based on the α-naphthyl pirinixic acid scaffold. Bioorg. Med. Chem. Lett. 2011, 21, 1329–1333. [Google Scholar] [CrossRef]
- Shukla, M.R.; Chaudhari, V.D.; Sayyed, M.B.; Phadtare, R.D.; Walke, N.B.; Kulkarni, S.A.; Palle, V.P.; Kamboj, R.K. Substituted Morpholines as Modulators for the Calcium Sensing Receptor. Patent Number WO2012120476 A1, 13 Septemper 2012. [Google Scholar]
- Bitterlich, B.; Anilkumar, G.; Gelalcha, F.G.; Spilker, B.; Grotevendt, A.; Jackstell, R.; Tse, M.K.; Beller, M. Development of a General and Efficient Iron-Catalyzed Epoxidation with Hydrogen Peroxide as Oxidant. Chem. Asian J. 2007, 2, 521–529. [Google Scholar] [CrossRef]
- Stingl, K.A.; Weiß, K.M.; Tsogoeva, S.B. Asymmetric vanadium- and iron-catalyzed oxidations: New mild (R)-modafinil synthesis and formation of epoxides using aqueous H2O2 as a terminal oxidant. Tetrahedron 2012, 68, 8493–8501. [Google Scholar] [CrossRef]
- Muñiz, K. Asymmetrische Katalyse mit Metall-Komplexen. Bild oder Spiegelbild? Chem. Unserer Zeit 2006, 40, 112–124. [Google Scholar] [CrossRef]
- White, M.C.; Doyle, A.G.; Jacobsen, E.N. A Synthetically Useful, Self-Assembling MMO Mimic System for Catalytic Alkene Epoxidation with Aqueous H2O2. J. Am. Chem. Soc. 2001, 123, 7194–7195. [Google Scholar] [CrossRef]
- Dubois, G.; Murphy, A.; Stack, T.D. Simple Iron Catalyst for Terminal Alkene Epoxidation. Org. Lett. 2003, 5, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Que, L., Jr. In situ Formation of Peracetic Acid in Iron-Catalyzed Epoxidations by Hydrogen Peroxide in the Presence of Acetic Acid. Adv. Synth. Catal. 2004, 346, 190–194. [Google Scholar] [CrossRef]
- Yeori, A.; Gendler, S.; Groysman, S.; Goldberg, I.; Kol, M. Salalen: A hybrid Salan/Salen tetradentate [ONNO]-type ligand and its coordination behavior with group IV metals. Inorg. Chem. Commun. 2004, 7, 280–282. [Google Scholar] [CrossRef]
- Matsumoto, K.; Saito, B.; Katsuki, T. Asymmetric catalysis of metal complexes with non-planar ONNO ligands: Salen, salalen and salan. Chem. Commun. 2007, 3619–3627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mitra, A.W.; Hoveyda, A.H.; Snapper, M.L. Kinetic Resolution of 1,2-Diols through Highly Site- and Enantioselective Catalytic Silylation. Angew. Chem. Int. Ed. 2007, 46, 8471–8474. [Google Scholar] [CrossRef]
- Rodrigo, J.M.; Zhao, Y.; Hoveyda, A.H.; Snapper, M.L. Regiodivergent Reactions through Catalytic Enantioselective Silylation of Chiral Diols. Synthesis of Sapinofuranone, A. Org. Lett. 2011, 13, 3778–3781. [Google Scholar] [CrossRef] [PubMed]
- Manville, N.; Alite, H.; Haeffner, F.; Hoveyda, A.H.; Snapper, M.L. Enantioselective silyl protection of alcohols promoted by a combination of chiral and achiral Lewis basic catalysts. Nat. Chem. 2013, 5, 768–774. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Hoveyda, A.H.; Snapper, M.L. Catalytic Enantioselective Silylation of Acyclic and Cyclic Triols: Application to Total Syntheses of Cleroindicins D., F., and C. Angew. Chem. Int. Ed. 2009, 48, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Hong, L.; Sun, W.; Yang, D.; Li, G.; Wang, R. Additive Effects on Asymmetric Catalysis. Chem. Rev. 2016, 116, 4006–4123. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; López-Ortiz, M.; Vega-Peñaloza, A.; Regla, I.; Juaristi, E. Use of (R)-Mandelic Acid as Chiral Co-Catalyst in the Michael Addition Reaction Organocatalyzed by (1S,4S)-2-Tosyl-2,5-diazabicyclo [2.2.1]heptane under Solvent-Free Conditions. Asymmetric Catal. 2015, 2, 37–44. [Google Scholar] [CrossRef]
- Dicks, A.P.; Hent, A. Atom Economy and Reaction Mass Efficiency. In Green Chemistry Metrics: A Guide to Determining and Evaluating Process Grenness; Springer: Heidelberg, Germany, 2015; pp. 17–44. [Google Scholar]
- Zhang, C.P.; Wang, Z.L.; Chen, Q.Y.; Zhang, C.T.; Gu, Y.C.; Xiao, J.C. Generation of the CF3 radical from trifluoromethylsulfonium triflate and its trifluoromethylation of styrenes. Chem. Commun. 2011, 47, 6632–6634. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.Z.; Li, Q.Q.; Wang, M.M.; Ning, X.S.; Kang, Y.B. (E)-Specific direct Julia-olefination of aryl alcohols without extra reducing agents promoted by bases. Chem. Commun. 2015, 51, 7729–7732. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Inukai, S.; Asai, N.; Oyamada, M.; Ikegawa, S.; Sugiyama, Y.; Hamamoto, H.; Shioiri, T.; Matsugi, M. A comparative study of the asymmetric epoxidation of aromatic olefins using the first generation manganese salen epoxidation catalysts and their light fluorous variants: An interesting discovery on the use of benzotrifluoride as a cosolvent. Tetrahedron Asymmetry 2014, 25, 1209–1214. [Google Scholar] [CrossRef]
- Yadav, V.K.; Kapoor, K.K. 1,8-diazabicyclo [5.4.0] undec-7-ene: A remarkable base in the epoxidation of α,β-unsaturated-δ-lactones and other enones with anhydrous t-BuOOH. Tetrahedron 1995, 51, 8573–8584. [Google Scholar] [CrossRef]
- Zandbergen, P.; van den Niewendijk, A.M.C.H.; Brussee, J.; van der Gen, A.; Kruse, C.G. A one-pot reduction-transimition-reduction synthesis of N-substituted β-ethanolamines from cyanohydrins. Tetrahedron 1992, 48, 3977–3982. [Google Scholar] [CrossRef]
- Robinson, M.W.; Davies, A.M.; Buckle, R.; Mabbett, I.; Taylor, S.H.; Graham, A.E. Epoxide ring-opening and Meinwald rearrangement reactions of epoxides catalyzed by mesoporous aluminosilicates. Org. Biomol. Chem. 2009, 7, 2559–2564. [Google Scholar] [CrossRef]
- Pedragosa-Moreau, S.; Morisseau, C.; Zylber, J.; Archelas, A.; Baratti, J.; Furstoss, R. Microbiological Transformations. 33. Fungal Epoxide Hydrolases Applied to the Synthesis of Enantiopure Para-Substituted Styrene Oxides. A Mechanistic Approach. J. Org. Chem. 1996, 61, 7402–7407. [Google Scholar] [CrossRef]
- Grigg, R.D.; Rigoli, J.W.; Pearce, S.D.; Schomaker, J.M. Synthesis of Propargylic and Allenic Carbamates via the C–H Amination of Alkynes. Org. Lett. 2012, 14, 280–283. [Google Scholar] [CrossRef]
- Fristrup, P.; Dideriksen, B.B.; Tanner, D.; Norrby, P.O. Probing Competitive Enantioselective Approach Vectors Operating in the Jacobsen−Katsuki Epoxidation: A Kinetic Study of Methyl-Substituted Styrenes. J. Am. Chem. Soc. 2005, 127, 13672–13679. [Google Scholar] [CrossRef]
- Alvaro, G.; Décor, A.; Fontana, S.; Hamprecht, D.; Large, C.; Marasco, A. Imidazolidinedione Derivatives. Patent Number WO2011069951 A1, 16 June 2011. [Google Scholar]
- Huy, P.; Neudorfl, J.M.; Schmalz, H.G. A Practical Synthesis of Trans-3-Substituted Proline Derivatives through 1,4-Addition. Org. Lett. 2011, 13, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Gebert, U.; Kerékjártó, B.V. Modellreaktionen für die enzymatische Katalyse, IV1) Untersuchungen zur Struktur-Wirkung-Beziehung neuer Transaminatoren mit Imidazol-, Thiazol- und Benzimidazolgerüst. Justus Liebigs Ann. Chem. 1974, 1974, 644–654. [Google Scholar] [CrossRef]
- Kodera, M.; Terasako, N.; Kita, T.; Tachi, Y.; Kano, K.; Yamazaki, M.; Koikawa, M.; Tokii, T. Synthesis, Crystal Structures, and Catalytic Activity of Dicopper(II) Complexes with Dinucleating Tetraimidazole Ligands [Cu2(RO)(mbipl)](ClO4)2 (R = Me, Et, 2-Pr; Hmbipl = 1,5-Bis[bis[(1-methyl-4-imidazolyl)methyl]amino]-3-pentanol) and [Cu2(MeO)(pbipl)](ClO4)2 (Hpbipl = 1,5-Bis[bis[(1-isopropyl-4-imidazolyl)methyl]amino]-3-pentanol). Inorg. Chem. 1997, 36, 3861–3868. [Google Scholar]
- Zhao, Y.; Rodrigo, J.; Hoveyda, A.H.; Snapper, M.L. Enantioselective silyl protection of alcohols catalysed by an amino-acid-based small molecule. Nature 2006, 443, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the ligands (S,R)-I, L10 and L17 are available from the authors. |
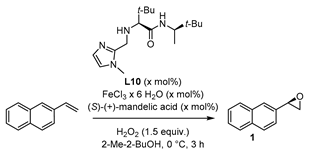 | |||||
|---|---|---|---|---|---|
| Entry | Ligand L10 (mol%) | FeCl3∙ 6H2O (mol%) | (S)-(+)-mandelic acid (mol%) | Yield 1 (%) | ee 2 (%) |
| 1 | 10 | 5 | 5 | 27 | 42 |
| 2 | 5 | 5 | 15 | 20 | 37 |
| 3 | 20 | 10 | 10 | 43 | 38 |
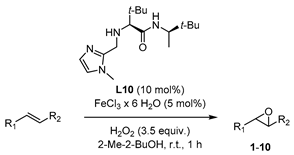 | |||
|---|---|---|---|
| Entry | Product | Yield 1,2 | ee 3 |
| 1 | 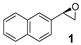 | 44 | 26 (R) |
| 2 | 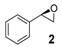 | 30 | 27 (R) |
| 3 |  | 14 | 29 (S) |
| 4 |  | 25 | 25 (R) 4 |
| 5 | 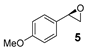 | 5 4 | 14 (R) 4 |
| 6 | 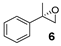 | 22 | 16 (S) |
| 7 | 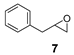 | <5 5 | n.d. 6 |
| 8 |  | 32 | 50 |
| 9 | 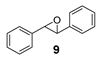 | 27 | 16 (R) |
| 10 | 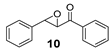 | <5 | 27 (2R,3S) |
| 11 7 | 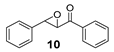 | 7 | 51 (2R,3S) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fingerhut, A.; Vargas-Caporali, J.; Leyva-Ramírez, M.A.; Juaristi, E.; Tsogoeva, S.B. Biomimetic Non-Heme Iron-Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant. Molecules 2019, 24, 3182. https://doi.org/10.3390/molecules24173182
Fingerhut A, Vargas-Caporali J, Leyva-Ramírez MA, Juaristi E, Tsogoeva SB. Biomimetic Non-Heme Iron-Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant. Molecules. 2019; 24(17):3182. https://doi.org/10.3390/molecules24173182
Chicago/Turabian StyleFingerhut, Anja, Jorge Vargas-Caporali, Marco Antonio Leyva-Ramírez, Eusebio Juaristi, and Svetlana B. Tsogoeva. 2019. "Biomimetic Non-Heme Iron-Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant" Molecules 24, no. 17: 3182. https://doi.org/10.3390/molecules24173182
APA StyleFingerhut, A., Vargas-Caporali, J., Leyva-Ramírez, M. A., Juaristi, E., & Tsogoeva, S. B. (2019). Biomimetic Non-Heme Iron-Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant. Molecules, 24(17), 3182. https://doi.org/10.3390/molecules24173182