Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of C8-OG on S. mutans
2.2. Suppressive Activity of C8-OG on S. mutans Biofilm Formation on Polystyrene Surfaces
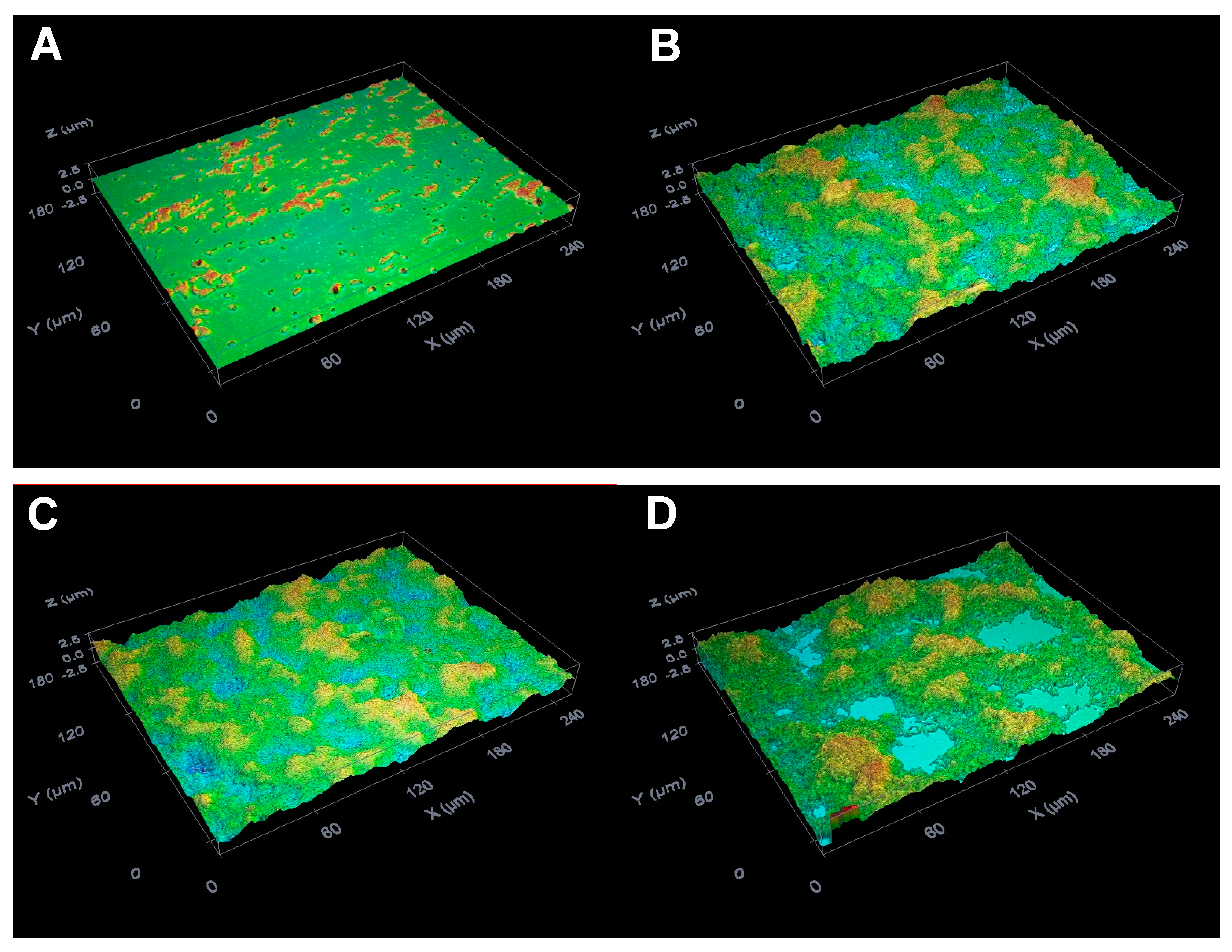
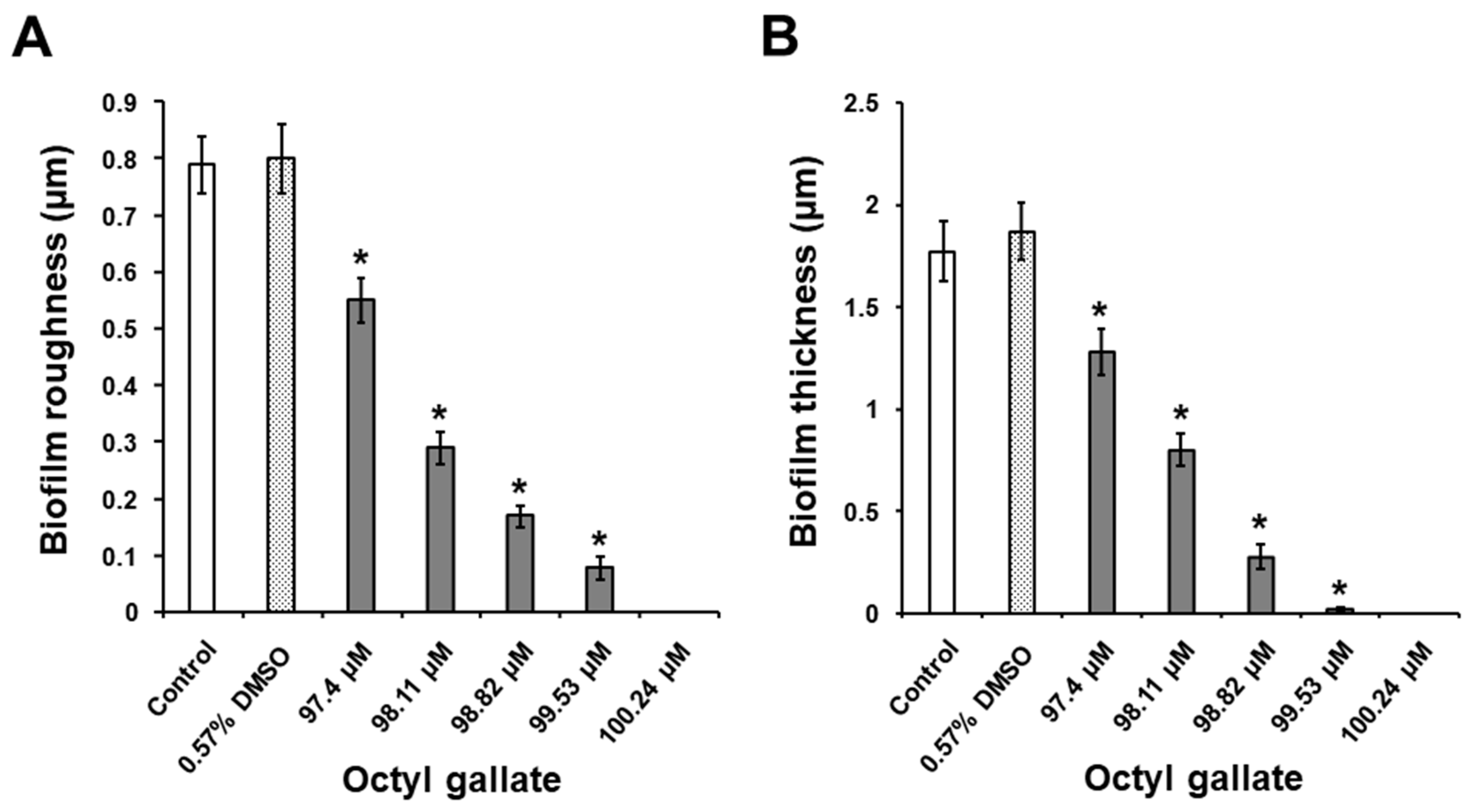
2.3. Suppressive Activity of C8-OG on S. mutans Biofilm Formation on the Glass Surfaces
2.4. Suppressive Activity of C8-OG on S. mutans Biofilm Acidogenicity
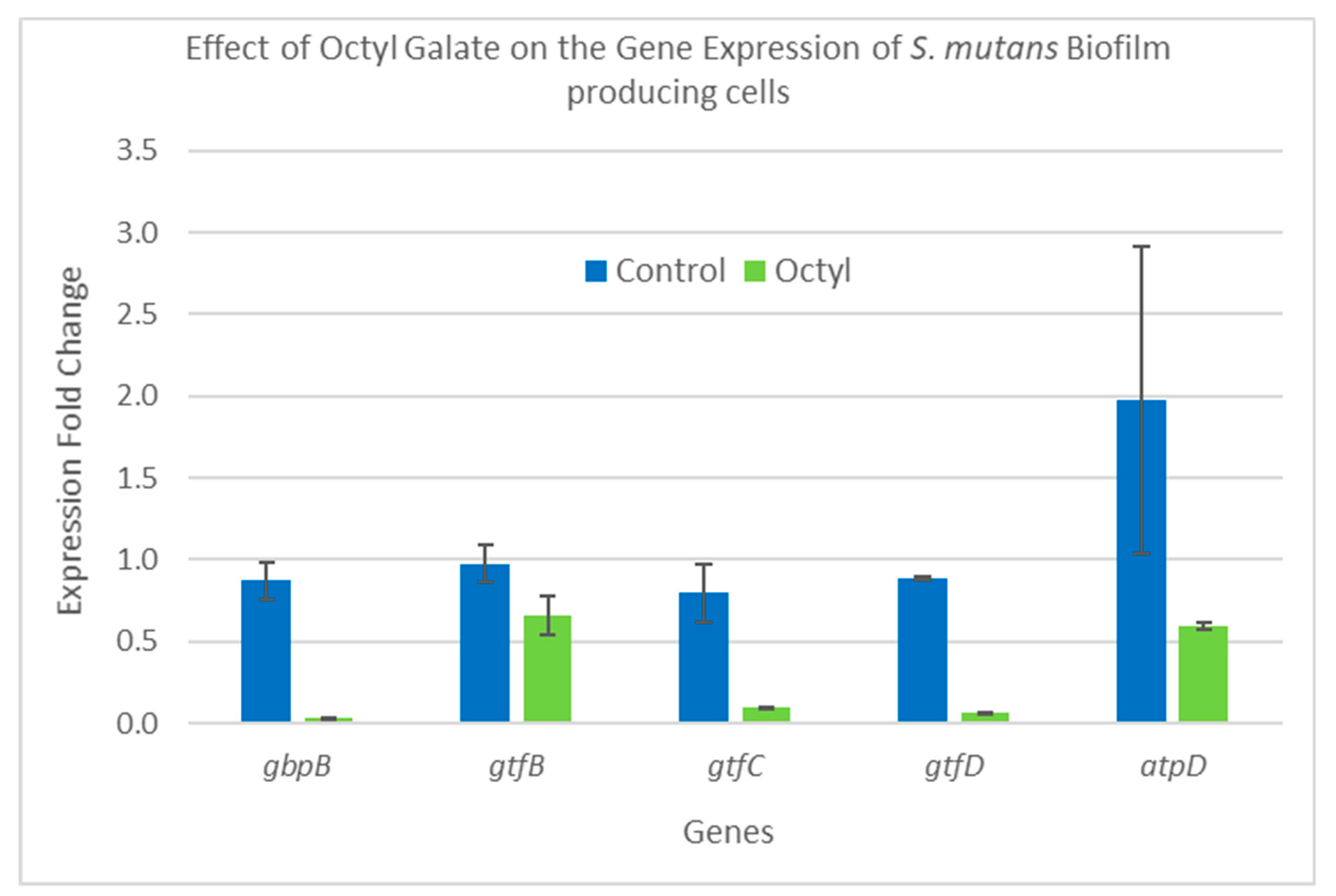
2.5. Gene Expression Analysis
3. Materials and Methods
3.1. The Source of Chemicals
3.2. Terminalia Bellerica Plant Extraction
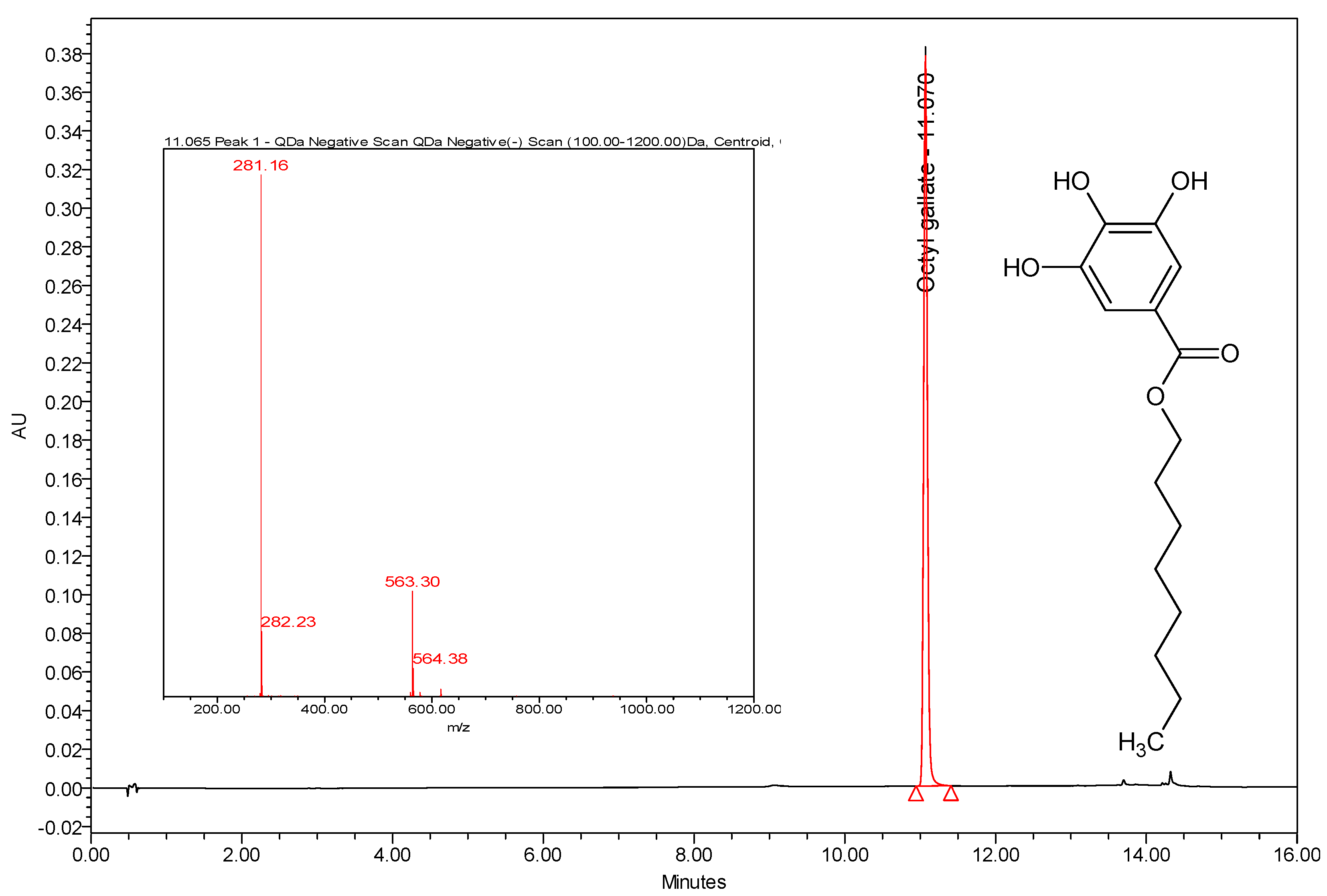
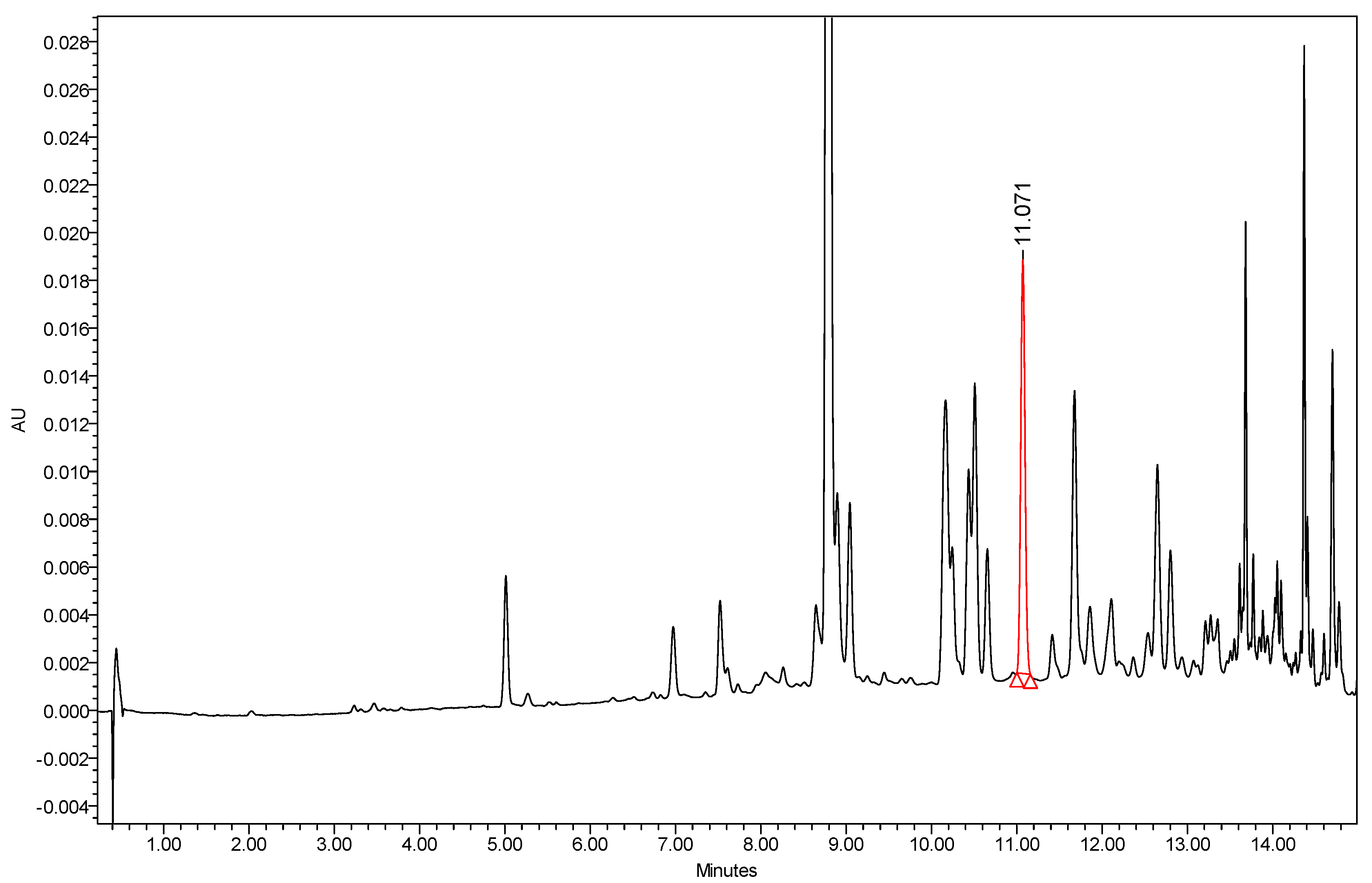
3.3. Instrumentation and Chromatographic Conditions
3.4. Calibration Curve of Octyl Gallate
3.5. Bacterial Culture
3.6. Microdilution Test for Determining the Minimum Inhibitory Concentration (MIC)
3.7. Protocol for the Biofilm Development and Treatment with C8-OG
3.8. Biofilm Analysis by the Colorimetric Method
3.9. Biofilm Analysis by the Optical Profilometry Method
3.10. Evaluation of the Biofilm Acidogenicity
3.11. Analysis of Gene Expression
3.12. RNA Isolation
3.13. Relative RT-qPCR for the Estimation of Biofilm-Associated Gene Expression Following EG Exposure
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Ooshima, T.; Matsumura, M.; Hoshino, T.; Kawabata, S.; Sobue, S.; Fujiwara, T. Contributions of three glycosyltransferases to sucrose-dependent adherence of Streptococcus mutans. J. Dent. Res. 2001, 80, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tian, X.L.; Sutherland, T.; Sisson, G.; Mai, J.; Ling, J.; Li, Y.H. Global transcriptional analysis of acid-inducible genes in Streptococcus mutans: Multiple two-component systems involved in acid adaptation. Microbiology 2009, 155, 3322–3332. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Jepsen, S.; Blanco, J.; Buchalla, W.; Carvalho, J.C.; Dietrich, T.; Dorfer, C.; Eaton, K.A.; Figuero, E.; Frencken, J.E.; Graziani, F.; et al. Prevention and control of dental caries and periodontal diseases at individual and population level: Consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S85–S93. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Abu-Lafi, S.; Rayan, M.; Masalha, M.; Abu-Farich, B.; Al-Jaas, H.; Abu-Lafi, M.; Rayan, A. Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L. Medicines 2019, 6, 53. [Google Scholar] [CrossRef]
- Rayan, M.; Abdallah, Z.; Abu-Lafi, S.; Masalha, M.; Rayan, A. Indexing Natural Products for their Antifungal Activity by Filters-based Approach: Disclosure of Discriminative Properties. Curr. Comput. Aided Drug Des. 2019, 15, 235–242. [Google Scholar] [CrossRef]
- Masalha, M.; Abu-Lafi, S.; Abu-Farich, B.; Rayan, M.; Issa, N.; Zeidan, M.; Rayan, A. A New Approach for Indexing Honey for Its Heath/Medicinal Benefits: Visualization of the Concept by Indexing Based on Antioxidant and Antibacterial Activities. Medicines 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Masalha, M.; Rayan, M.; Adawi, A.; Abdallah, Z.; Rayan, A. Capturing antibacterial natural products with in silico techniques. Mol. Med. Rep. 2018, 18, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory Effects of Ethyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry and Gene Expression Analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef] [PubMed]
- Kacergius, T.; Abu-Lafi, S.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Qutob, M.; Stukas, R.; Utkus, A.; Zeidan, M.; et al. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Latha, R.C.; Daisy, P. Therapeutic potential of octyl gallate isolated from fruits of Terminalia bellerica in streptozotocin-induced diabetic rats. Pharm. Biol. 2013, 51, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.S.; Roy, A.; Antony, L.; Banu, S.K.; Jeyaraman, S.; Manikkam, R. Octyl gallate and gallic acid isolated from Terminalia bellarica regulates normal cell cycle in human breast cancer cell lines. Biomed. Pharm. 2018, 103, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, M.P.J.; Manoraj, A.; Thevanesam, V.; Ekanayake, A.; Kumar, N.S.; Liyanapathirana, V.; Abeyratne, E.; Bandara, B.M.R. Terminalia bellirica fruit extracts: In-vitro antibacterial activity against selected multidrug-resistant bacteria, radical scavenging activity and cytotoxicity study on BHK-21 cells. BMC Complementary Altern. Med. 2018, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Abdelfattah, M.A.O.; Osman, S.; Rashid, H.O.; El-Shazly, A.M.; Wink, M. Chemical composition, antioxidant and hepatoprotective activities of methanol extracts from leaves of Terminalia bellirica and Terminalia sericea (Combretaceae). PeerJ 2019, 7, e6322. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Synergistic antibiofilm efficacy of a gallotannin 1,2,6-tri-O-galloyl-beta-D-glucopyranose from Terminalia chebula fruit in combination with gentamicin and trimethoprim against multidrug resistant uropathogenic Escherichia coli biofilms. PLoS ONE 2017, 12, e0178712. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.K.; Roy, N.; Acharyya, S.; Saha, D.R.; Koley, H.; Sarkar, P.; Bhowmik, P. In vivo fluid accumulation-inhibitory, anticolonization and anti-inflammatory and in vitro biofilm-inhibitory activities of methyl gallate isolated from Terminalia chebula against fluoroquinolones resistant Vibrio cholerae. Microb. Pathog. 2019, 128, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Uozaki, M.; Yamasaki, H.; Katsuyama, Y.; Higuchi, M.; Higuti, T.; Koyama, A.H. Antiviral effect of octyl gallate against DNA and RNA viruses. Antivir. Res. 2007, 73, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubo, I. Antifungal activity of octyl gallate. Int. J. Food Microbiol. 2002, 79, 193–201. [Google Scholar] [CrossRef]
- Krol, E.; de Sousa Borges, A.; da Silva, I.; Polaquini, C.R.; Regasini, L.O.; Ferreira, H.; Scheffers, D.J. Antibacterial activity of alkyl gallates is a combination of direct targeting of FtsZ and permeabilization of bacterial membranes. Front. Microbiol. 2015, 6, 390. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of octyl gallate (E 311) as a food additive. EFSA J. 2015, 13, 4248. [Google Scholar] [CrossRef]
- Buckley, H.L.; Hart-Cooper, W.M.; Kim, J.H.; Faulkner, D.M.; Cheng, L.W.; Chan, K.L.; Vulpe, C.D.; Orts, W.J.; Amrose, S.E.; Mulvihill, M.J. Design and Testing of Safer, More Effective Preservatives for Consumer Products. ACS Sustain. Chem. Eng. 2017, 5, 4320–4331. [Google Scholar] [CrossRef]
- Oh, E.; Bae, J.; Kumar, A.; Choi, H.J.; Jeon, B. Antioxidant-based synergistic eradication of methicillin-resistant Staphylococcus aureus (MRSA) biofilms with bacitracin. Int. J. Antimicrob. Agents 2018, 52, 96–99. [Google Scholar] [CrossRef]
- Jordon-Thaden, I.E.; Chanderbali, A.S.; Gitzendanner, M.A.; Soltis, D.E. Modified CTAB and TRIzol protocols improve RNA extraction from chemically complex Embryophyta. Appl. Plant Sci. 2015, 3. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound Octyl Gallate is available from Prof. Anwar Rayan. |



| Compound | MIC |
|---|---|
| Octyl gallate | 97 µg/mL (343.5 µM) |
| Erythromycin | 4.8 µg/mL (6.54 µM) |
| DMSO | 25% (v/v) |
| Experimental Group | pH |
|---|---|
| Blank | 7.37 ± 0.02 * |
| Control | 4.23 ± 0.01 |
| DMSO (0.57%) | 4.22 ± 0.01 |
| C8-OG (97.4 µM) | 6.27 ± 0.38 * |
| C8-OG (98.11 µM) | 6.83 ± 0.23 * |
| C8-OG (98.82 µM) | 7.19 ± 0.04 * |
| C8-OG (99.53 µM) | 7.23 ± 0.03 * |
| C8-OG (100.24 µM) | 7.28 ± 0.03 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Zeidan, M.; Abu-Farich, B.; Austys, D.; Masalha, M.; Rayan, A. Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression. Molecules 2019, 24, 3170. https://doi.org/10.3390/molecules24173170
Gabe V, Kacergius T, Abu-Lafi S, Zeidan M, Abu-Farich B, Austys D, Masalha M, Rayan A. Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression. Molecules. 2019; 24(17):3170. https://doi.org/10.3390/molecules24173170
Chicago/Turabian StyleGabe, Vika, Tomas Kacergius, Saleh Abu-Lafi, Mouhammad Zeidan, Basheer Abu-Farich, Donatas Austys, Mahmud Masalha, and Anwar Rayan. 2019. "Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression" Molecules 24, no. 17: 3170. https://doi.org/10.3390/molecules24173170
APA StyleGabe, V., Kacergius, T., Abu-Lafi, S., Zeidan, M., Abu-Farich, B., Austys, D., Masalha, M., & Rayan, A. (2019). Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression. Molecules, 24(17), 3170. https://doi.org/10.3390/molecules24173170








