Zwitterionic Acetylated Cellulose Nanofibrils
Abstract
1. Introduction
2. Results
2.1. Acetylated CNF (ACNF)
2.2. Oxidized Acetylated CNF
2.3. Aminated Acetylated CNF
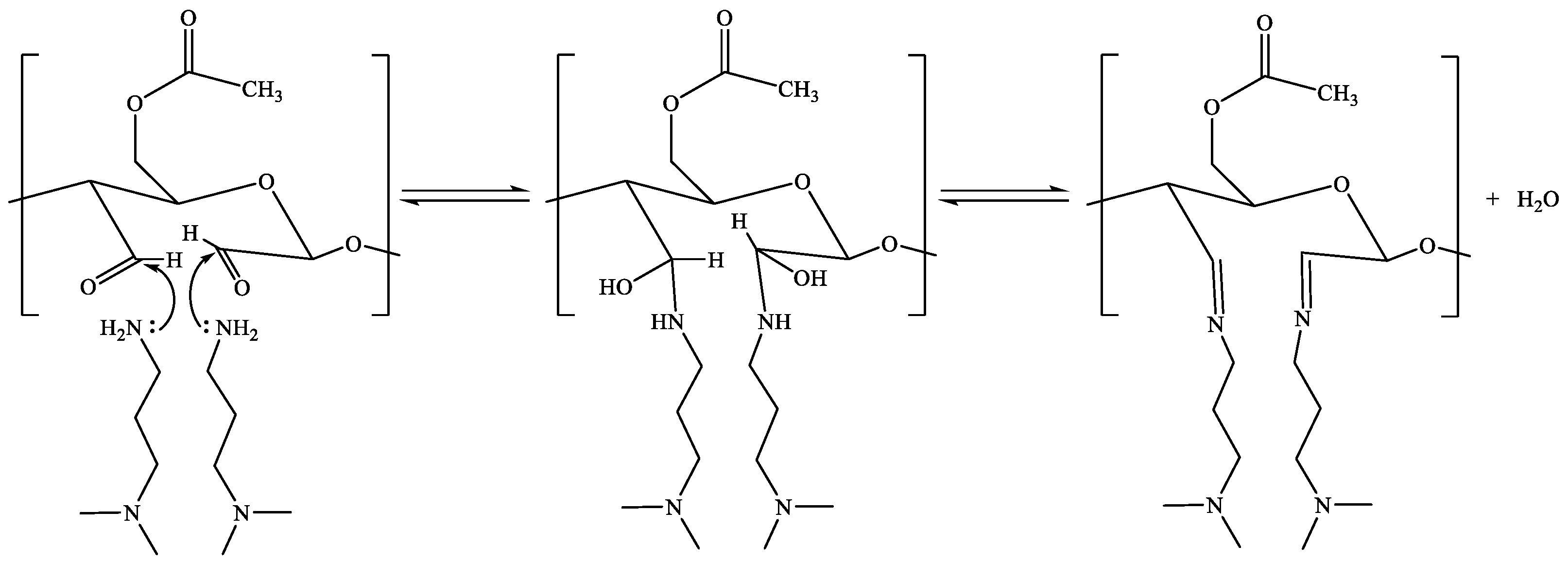
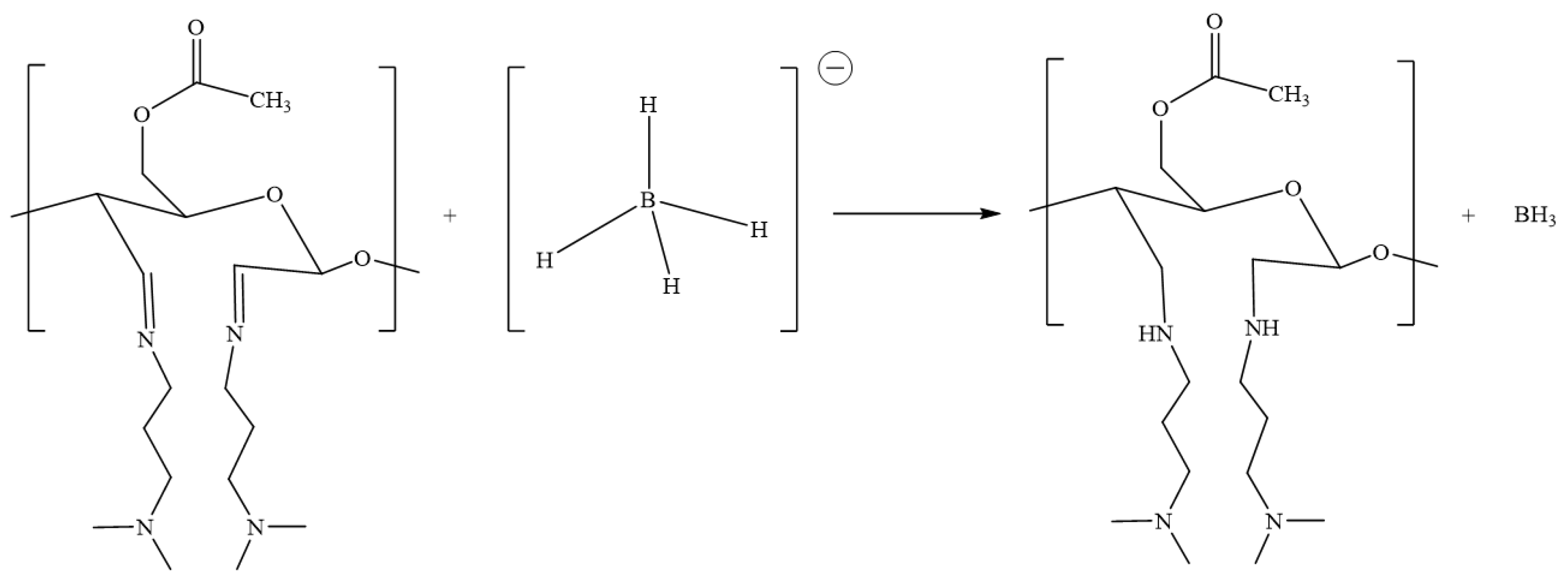
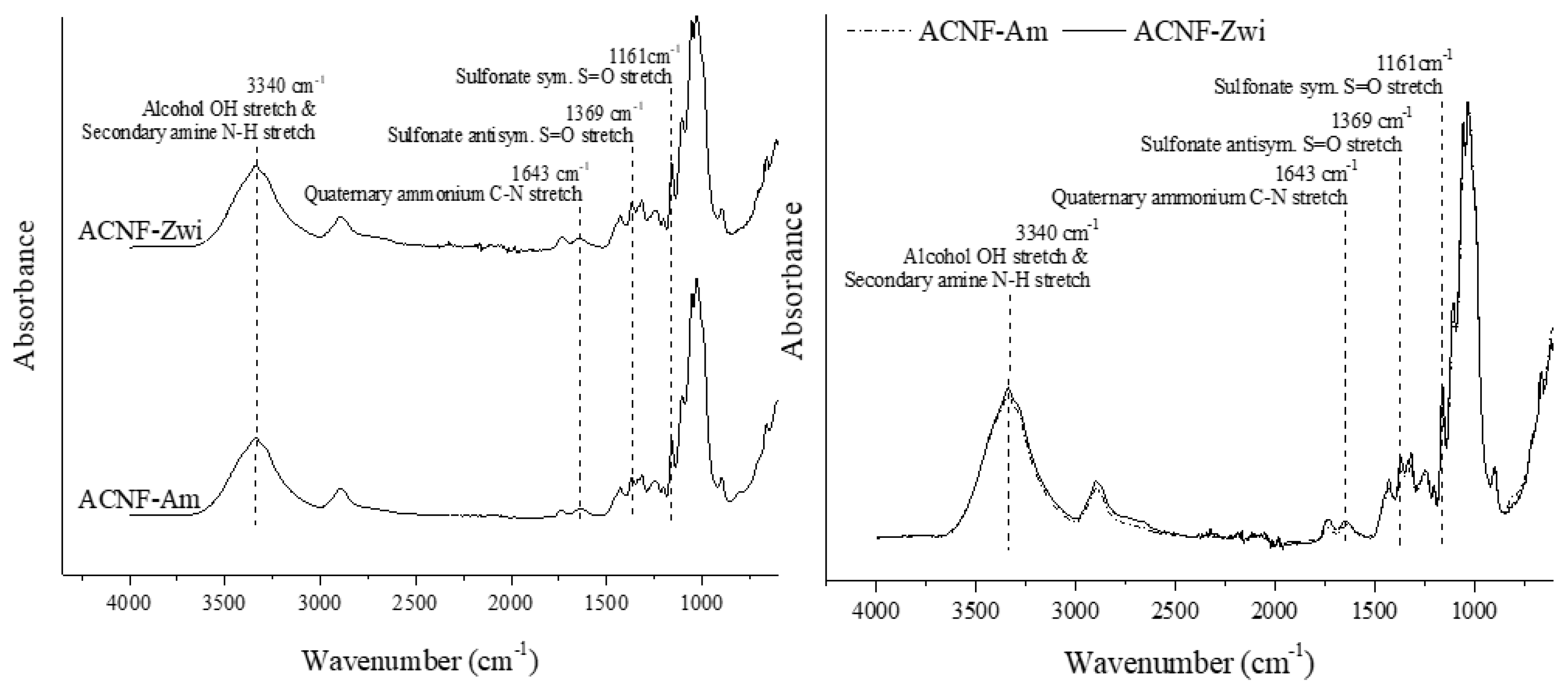
2.4. Zwitterionic Acetylated CNF
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Lyophilization
4.3. Acetylation of CNF (ACNF)
4.4. Oxidation of Acetylated CNF (ACNF-Ox)
4.4.1. Oxidation in DMSO
4.4.2. Oxidation in Water
4.5. CNF Schiff Base Formation and Reduction (ACNF-Am)
4.6. Zwitterionic CNF Formation by Quaternary Ammonium Formation (ACNF-Zwi)
4.7. Characterization
4.7.1. Moisture Content Determination
4.7.2. Fourier Transform Infrared (FTIR) Spectroscopy
4.7.3. Zeta (ζ) Potential Measurements
4.7.4. Scanning Electron Microscopy (SEM) and Elemental Analysis via Energy-Dispersive Spectroscopy (EDS)
4.7.5. Acetyl Content Determination
4.7.6. Carbonyl Content Determination
4.7.7. Atomic Force Microscopy (AFM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A. Nanofibrillated cellulose. Properties reinvestigated. Cellulose 2017, 24, 1933–1945. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose Nanomaterials—Binding Properties and Applications: A Review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials, 2nd ed.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018. [Google Scholar]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the polymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Rustemeyer, P. History of CA and evolution of the markets. Macromol. Symp. 2004, 208, 1–6. [Google Scholar] [CrossRef]
- Akim, E. On the mechanism of cellulose acetylation. Pure Appl. Chem. 1967, 14, 475–480. [Google Scholar] [CrossRef][Green Version]
- Heinze, T.; Liebert, T. Chemical characteristics of cellulose acetate. Macromol. Symp. 2004, 208, 167–238. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Edgar, K.J.; Wilson, A.K. Preparation and characterization of cellulose monoacetates: The relationship between structure and water solubility. Macromolecules 1991, 24, 3060–3064. [Google Scholar] [CrossRef]
- Edgar, K.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Syverud, K. Pretreatment-dependent surface chemistry of wood nanocellulose for pH-sensitive hydrogels. J. Biomater. Appl. 2014, 29, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Lindh, J.; Hong, J.; Strømme, M.; Mihranyan, A.; Ferraz, N. Blood Compatibility of Sulfonated Cladophora Nanocellulose Beads. Molecules 2018, 23, 601. [Google Scholar] [CrossRef] [PubMed]
- Littunen, K.; Snoei de Castro, J.; Samoylenko, A.; Xu, Q.; Quaggin, S.; Vainio, S.; Seppälä, J. Synthesis of cationized nanofibrillated cellulose and its antimicrobial properties. Eur. Polym. J. 2016, 75, 116–124. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; Mautner, A.; Perez de Larraya, U.; Pfenninger, N.; Tingaut, P.; Zimmermann, T. Cationic cellulose nanofibers from waste pulp residues and their nitrate, fluoride, sulphate and phosphate adsorption properties. Carbohydr. Polym. 2016, 135, 334–340. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4117–4189. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Wang, S.F.; Guo, H.X.; Zhang, B.H.; Zhang, L.; Gu, K.; Gu, J. Fabrication and performance study of a zwitterionic polyimide antifouling ultrafiltration membrane. RSC Adv. 2015, 5, 21316–21325. [Google Scholar] [CrossRef]
- Calabrese, V.; da Silva, M.A.; Schmitt, J.; Muñoz-Garcia, J.C.; Gabrielli, V.; Scott, J.L.; Angulo, J.; Khimyak, Y.Z.; Edler, K.J. Surfactant controlled zwitterionic cellulose nanofibril dispersions. Soft Matter 2018, 14, 7793–7800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Huo, P.; Gu, J. Exploration of zwitterionic cellulose acetate antifouling ultrafiltration membrane for bovine serum albumin (BSA) separation. Carbohydr. Polym. 2017, 165, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A. The role of acid catalysts in the manufacture of cellulose acetate. Pure Appl. Chem. 1967, 14, 535–546. [Google Scholar] [CrossRef]
- Steinmeier, H. Acetate manufacturing, process and technology. Macromol. Symp. 2004, 208, 49–60. [Google Scholar] [CrossRef]
- Dupont, A.-L.; Tétreault, J. Cellulose degradation in an acetic acid environment. Stud. Conserv. 2000, 45, 201–210. [Google Scholar]
- Olaru, N.; Olaru, L.; Vasile, C.; Ander, P. Surface modified cellulose obtained by acetylation without solvents of bleached and unbleached kraft pulps. Polimery 2011, 56, 834–840. [Google Scholar] [CrossRef]
- Kulkarni, V.S. Handbook of Non-invasive Drug Delivery Systems: Science and Technology, 1st ed.; Elsevier: New York, NY, USA, 2009. [Google Scholar]
- Maekawa, E.; Koshijima, T. Properties of 2, 3-dicarboxy cellulose combined with various metallic ions. J. Appl. Polym. Sci. 1984, 29, 2289–2297. [Google Scholar] [CrossRef]
- Höglund, E. Production of Dialdehyde Cellulose and Periodate Regeneration: Towards feasible oxidation processes. Ph.D. Thesis, Karlstad University, Karlstad, Sweden, 2015. [Google Scholar]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Cordes, E.H.; Jencks, W.P. On the Mechanism of Schiff Base Formation and Hydrolysis. J. Am. Chem. Soc. 1962, 84, 832–837. [Google Scholar] [CrossRef]
- Martin, R.B. Reactions of carbonyl compounds with amines and derivatives. J. Phys. Chem. 1964, 68, 1369–1377. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Pittman, C.U. Reductions of Carboxylic Acids and Esters with NaBH4 in Diglyme at 162 °C. Synt. Commun. 2003, 33, 1733–1750. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Critical Review on Sustainable Homogeneous Cellulose Modification: Why Renewability Is Not Enough. ACS Sustainable Chem. Eng. 2019, 7, 1826–1840. [Google Scholar] [CrossRef]
- Valencia, L.; Kumar, S.; Jalvo, B.; Mautner, A.; Salazar-Alvarez, G.; Mathew, A.P. Fully bio-based zwitterionic membranes with superior antifouling and antibacterial properties prepared via surface-initiated free-radical polymerization of poly(cysteine methacrylate). J. Mater. Chem. A 2018, 6, 16361–16370. [Google Scholar] [CrossRef]
- Navarro, J.; Wennmalm, S.; Godfrey, J.; Breitholtz, M.; Edlund, U. A Luminescent Nanocellulose Platform: From Controlled Graft Block Copolymerization to Bio-marker Sensing. Biomacromolecules 2016, 17, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- López Durán, V.; Hellwig, J.; Larsson, P.T.; Wågberg, L.; Larsson, P.A. Effect of chemical functionality on the mechanical and barrier performance of all-cellulose composites. ACS Appl. Nano Mater. 2018, 1, 1959–1967. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2001; Chapter 23. [Google Scholar]
- Kim, D.-Y.; Nishiyama, Y.; Kuga, S. Surface acetylation of bacterial cellulose. Cellulose 2002, 9, 361–367. [Google Scholar] [CrossRef]
- López Durán, V.; Larsson, P.A.; Wågberg, L. On the relationship between fibre composition and material properties following periodate oxidation and borohydride reduction of lignocellulosic fibres. Cellulose 2016, 23, 3495–3510. [Google Scholar] [CrossRef]
Sample Availability: Not available. |






| Sample | Average ζ-Potential (mV) |
|---|---|
| CNF | −23.5 ± 1.21 |
| ACNF | −29.8 ± 0.66 |
| ACNF-Ox | −33.7 ± 0.23 |
| ACNF-Am | 1.5 ± 1.21 |
| ACNF-Zwi | −6.5 ± 0.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami, J.; Mathew, A.P.; Edlund, U. Zwitterionic Acetylated Cellulose Nanofibrils. Molecules 2019, 24, 3147. https://doi.org/10.3390/molecules24173147
Rostami J, Mathew AP, Edlund U. Zwitterionic Acetylated Cellulose Nanofibrils. Molecules. 2019; 24(17):3147. https://doi.org/10.3390/molecules24173147
Chicago/Turabian StyleRostami, Jowan, Aji P. Mathew, and Ulrica Edlund. 2019. "Zwitterionic Acetylated Cellulose Nanofibrils" Molecules 24, no. 17: 3147. https://doi.org/10.3390/molecules24173147
APA StyleRostami, J., Mathew, A. P., & Edlund, U. (2019). Zwitterionic Acetylated Cellulose Nanofibrils. Molecules, 24(17), 3147. https://doi.org/10.3390/molecules24173147






