The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development
Abstract
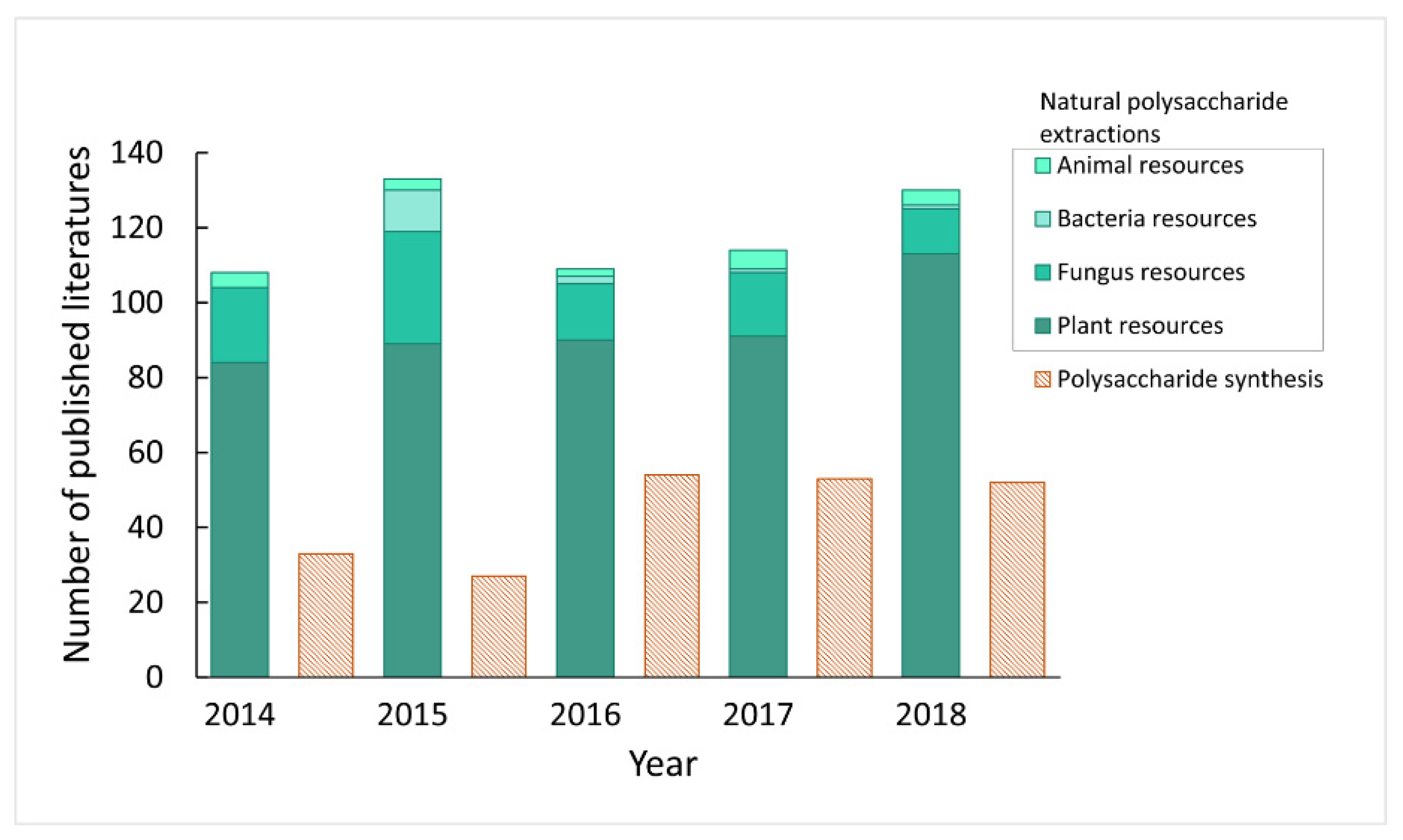
1. Introduction
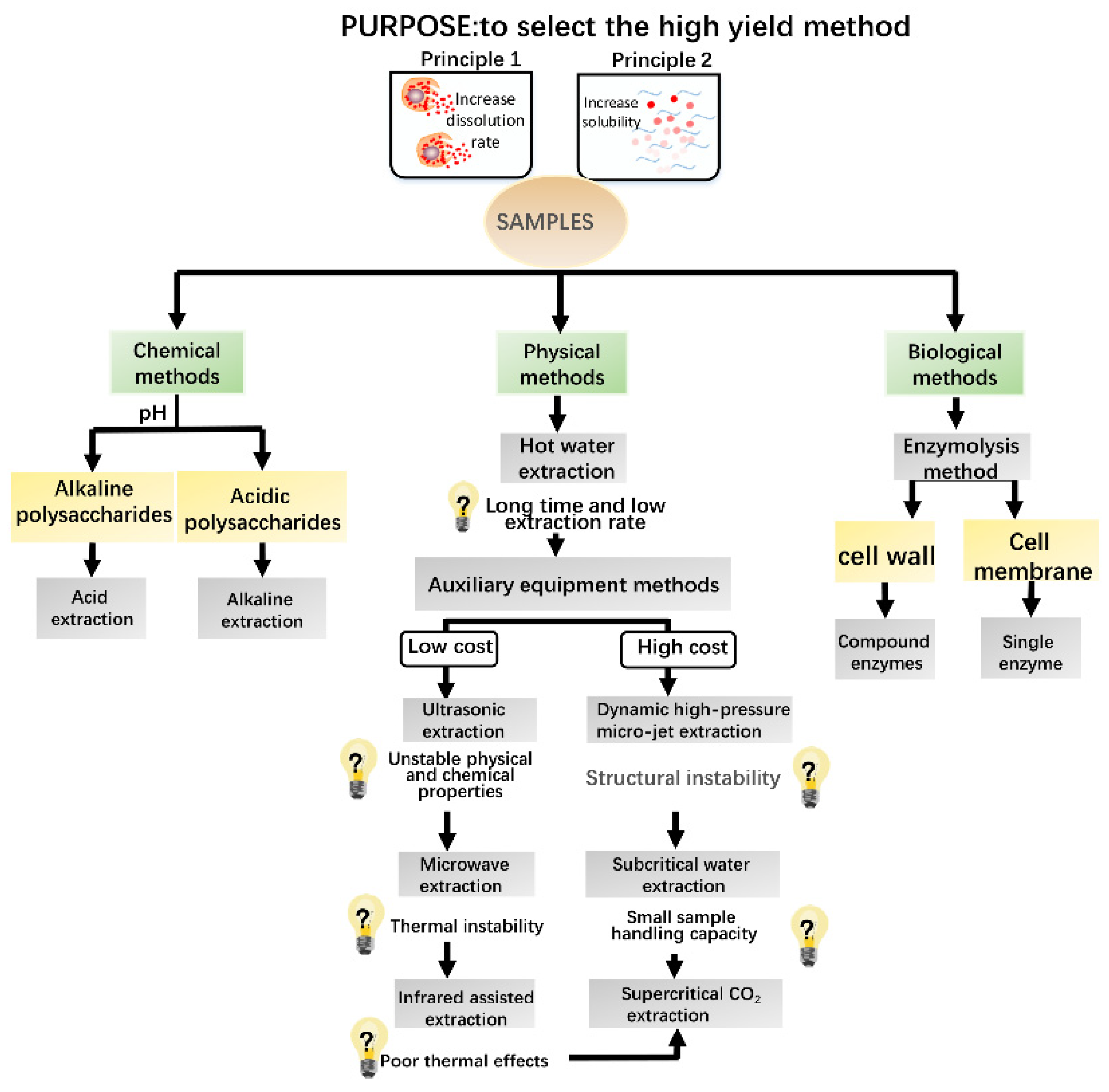
2. Extraction of Polysaccharides
2.1. Ultrasonic Extraction
2.2. Microwave Extraction
2.3. Supercritical CO2 Extraction
2.4. Subcritical Water Extraction
2.5. Other Extraction Methods
3. Separation and Purification of Polysaccharides
3.1. Separating Impurities from Polysaccharides
3.1.1. Protein Removal
3.1.2. Pigment Removal
3.2. Purification of Polysaccharides
3.2.1. Precipitation
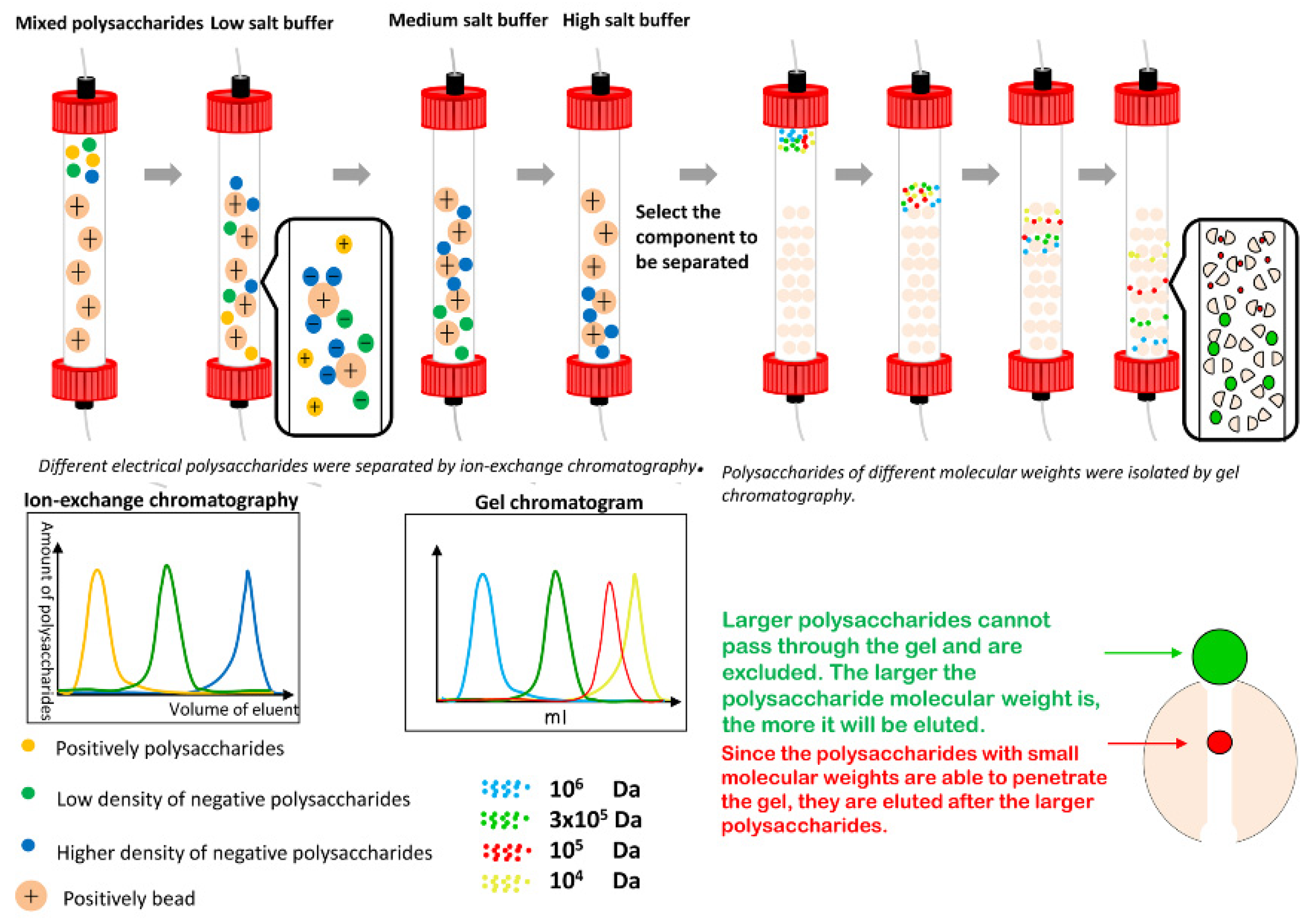
3.2.2. Chromatographic Separation
3.2.2.1. Anion-Exchange Column Chromatography
3.2.2.2. Gel Column Chromatography
3.2.3. Other Polysaccharide Purification Methods
4. Structural Analysis of Polysaccharides
4.1. Determination of Primary Structure
4.1.1. Detection of Polysaccharide Homogeneity
4.1.1.1. Chromatography
4.1.1.2. Other Methods
4.1.2. Determination of Molecular Weight of Polysaccharides
4.1.2.1. High Performance Liquid Chromatography
4.1.2.2. Gel Permeation Chromatography
4.1.2.3. Mass Spectrometry
4.1.2.4. Other Methods
4.1.3. Monosaccharide Composition
4.1.3.1. High-Performance Liquid Phase Chromatography
4.1.3.2. High-Performance Capillary Electrophoresis
4.1.3.3. Ion Chromatography
4.1.3.4. Formation of Methyl Glycosides
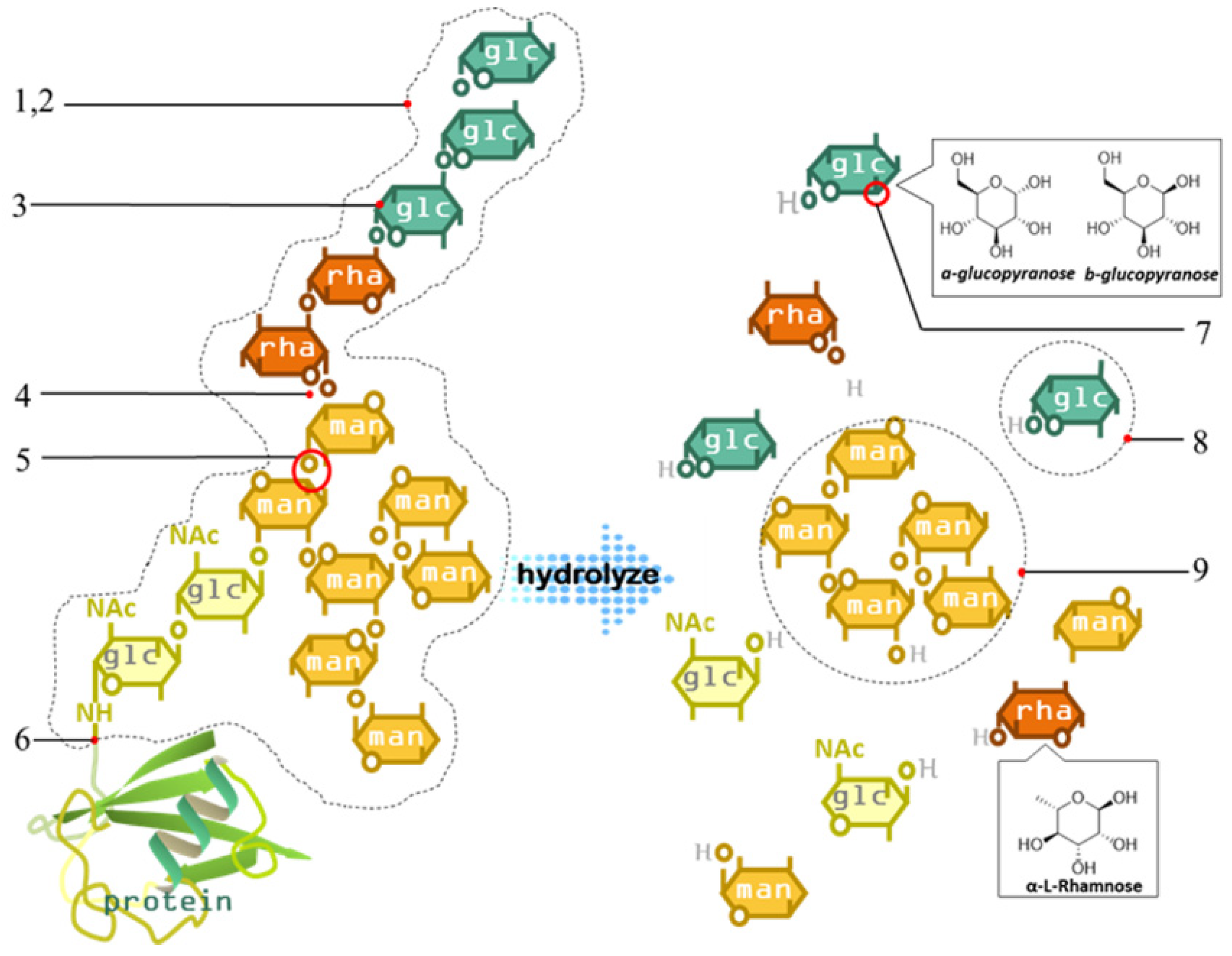
4.1.4. Analysis of Chain Structure of Polysaccharides
4.1.4.1. Methylation Analysis
4.1.4.2. Periodate Oxidation
4.1.4.3. Smith Degradation Reaction
4.1.4.4. Enzymatic Hydrolysis
4.1.4.5. Infrared Spectroscopy
4.1.4.6. Raman Spectroscopy
4.1.4.7. Nuclear Magnetic Resonance Spectroscopy (NMR)
4.2. Conformational Analysis of Polysaccharides
4.2.1. Scanning Electron Microscopy (SEM)
4.2.2. Atomic Force Microscopy (AFM)
4.2.3. X-ray Diffraction
4.2.4. Circular Dichroism Chromatography
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zong, A.Z.; Cao, H.Z.; Wang, F.S. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yao, F.K.; Ming, K.; Wang, D.Y.; Hu, Y.L.; Liu, J.G. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.Y.; Song, Q.Q.; Xie, J.H. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Oh, J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, M.; Zhang, Y. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019. [Google Scholar] [CrossRef]
- Wang, P.C.; Zhao, S.; Yang, B.Y.; Wang, Q.H.; Kuang, H.X. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr. Polym. 2016, 148, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Niu, Y.M.; Xing, P.F.; Wang, C.M. Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin. Med. 2019, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.; Li, J.; Yan, L.; Li, S.; Ye, X.; Liu, D.; Ding, T.; Linhardt, R.J.; Orfila, C.; et al. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocolloid. 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Song, Y.R.; Sung, S.K.; Jang, M.; Lim, T.G.; Cho, C.W.; Han, C.J.; Hong, H.D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer). Int. J. Biol. Macromol. 2018, 116, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Peng, C.C.; Kong, J.; You, L.J.; Ma, F.L. Optimization of ultrasonic-assisted extraction technology of lentinan polysaccharides by response surface methodology and its antioxidant activity. Mod. Food Sci. Technol. 2011, 27, 452–456. [Google Scholar]
- Zhu, W.L.; Xue, X.P.; Zhang, Z.J. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2016, 91, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.E.; de Magalhaes, W.T.; Moreira, L.M.; Rocha, M.V.P.; Bastos, A.K.P. Microwave-assisted extraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- Kumar, C.S.; Sivakumar, M.; Ruckmani, K. Microwave-assisted extraction of polysaccharides from Cyphomandra betacea and its biological activities. Int. J. Biol. Macromol. 2016, 92, 682–693. [Google Scholar]
- Chen, J.; Li, J.; Sun, A.D.; Zhang, B.L.; Qin, S.G.; Zhang, Y.Q. Supercritical CO2 extraction and pre-column derivatization of polysaccharides from Artemisia sphaerocephala Krasch. seeds via gas chromatography. Ind. Crops Prod. 2014, 60, 138–143. [Google Scholar] [CrossRef]
- Zou, X.; Liu, Y.; Tao, C.; Liu, Y.; Liu, M.; Wu, J.; Lv, Z. CO2 supercritical fluid extraction and characterization of polysaccharide from bamboo (Phyllostachys heterocycla) leaves. J. Food Meas. Charact. 2017, 12, 35–44. [Google Scholar] [CrossRef]
- Zhao, T.; Luo, Y.B.; Zhang, X.Y.; Zhang, W.J.; Qu, H.Y.; Mao, G.H.; Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; et al. Subcritical water extraction of bioactive compounds from Radix Puerariae and optimization study using response surface methodology. Chem. Eng. Commun. 2019, 206, 1218–1227. [Google Scholar] [CrossRef]
- Yang, R.F.; Zhao, C.; Chen, X.; Chan, S.W.; Wu, J.Y. Chemical properties and bioactivities of Goji (Lycium barbarum) polysaccharides extracted by different methods. J. Funct. Foods 2015, 17, 903–909. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Wang, H.; Kou, Y.; Wen, Q.H.; Fu, Z.F.; Chang, H.X. Response surface optimization and physicochemical properties of polysaccharides from Nelumbo nucifera leaves. Int. J. Biol. Macromol. 2015, 74, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, G.; Xie, M.; Chen, B.; Li, Y. Infrared-assisted extraction coupled with high-performance liquid chromatography for simultaneous determination of eight active compounds in Radix Salviae miltiorrhizae. J. Sep. Sci. 2010, 33, 2888–2897. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.H.; Liu, H.; Wu, C.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from Snow Chrysanthemum (Coreopsis Tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Zhang, D.N.; Qu, Z.; Yang, Q.H.; Han, Y.B. Purification, preliminary characterization and in vitro immunomodulatory activity of tiger lily polysaccharide. Carbohydr. Polym. 2014, 106, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.N.; Guo, X.Y.; Chen, Z.G. A novel and efficient method for the isolation and purification of polysaccharides from lily bulbs by Saccharomyces cerevisiae fermentation. Process Biochem. 2014, 49, 2299–2304. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Liu, T.T.; Han, Y.; Zhu, X.F.; Zhao, X.J.; Ma, X.J.; Jiang, D.Y.; Zhang, Q.H. An efficient method for decoloration of polysaccharides from the sprouts of Toona sinensis (A. Juss.) Roem by anion exchange macroporous resins. Food Chem. 2017, 217, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, W.S.; Chai, Y.; Liu, Y.M.; Yao, W.B.; Gao, X.D. Preliminary Structural Characterization and Hypoglycemic Effects of an acidic Polysaccharide SERP1 from the Residue of Sarcandra Glabra. Carbohydr. Polym. 2017, 176, 140–151. [Google Scholar] [CrossRef]
- Zhen, W.; Hong, L.; Tu, D.; Yong, Y.; Yong, Z. Extraction optimization, preliminary characterization, and in vitro antioxidant activities of crude polysaccharides from finger citron. Ind. Crops Prod. 2013, 44, 145–151. [Google Scholar]
- Wang, D.; Sun, S.Q.; Wu, W.Z.; Yang, S.L.; Tan, J.M. Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr. Polym. 2014, 105, 127–134. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Huang, W.W.; Zhao, H.T.; Wang, J.; Xu, R.B.; Hu, X.L.; Shen, S.Y.; Qin, D. Characterization and bioactivity of polysaccharides obtained from pine cones of Pinus koraiensis by graded ethanol precipitation. Molecules 2013, 18, 9933–9948. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ran, L.W.; Mi, J.; Yan, Y.M.; Lu, L.; Jin, B.; Li, X.Y.; Cao, Y.L. Isolation, characterization and antitumor effect on DU145 cells of a main polysaccharide in pollen of chinese wolfberry. Molecules 2018, 23, 2430. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qin, N.B.; Yan, C.Y.; Wang, S.M. Isolation, purification, characterization and bioactivities of a glucan from the root of Pueraria lobata. Food Funct. 2018, 9, 2644–2652. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.W.; Han, J.J.; Shu, H.M.; Liu, K.H. Isolation of polysaccharides from Dendrobium officinale leaves and anti-inflammatory activity in LPS-stimulated THP-1 cells. Chem. Cent. J. 2018, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hong, Y.; Yi, S.; Tu, Y.; Zeng, X. Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis). Food Chem. Toxicol. 2012, 50, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Shen, M.Y.; Nie, S.P.; Liu, X.; Zhang, H.; Xie, M.Y. Analysis of monosaccharide composition of cyclocarya paliurus polysaccharide with anion exchange chromatography. Carbohydr. Polym. 2013, 98, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, Z.; Yang, Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr. Polym. 2013, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, G.; Yu, Z.; Song, X.; Li, X.; Yang, Y.; Wang, L.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2016, 199, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Liu, X.C.; Fang, X.N.; Sun, H.Q.; Yang, X.Y.; Zhang, Y.M. Structural characterization and anti-tumor activity of polysaccharide produced by Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 82, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, H.; Wang, B.; Fu, L.; Yuan, M.; Liu, J.; Zhou, L.; Ding, C. Characterization and antioxidant activities of polysaccharides from the leaves of Lilium lancifolium Thunb. Int. J. Biol. Macromol. 2016, 92, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, S.; Ran, C.; Wang, L.; Kan, J. Extraction, characterization and antioxidant activity of water-soluble polysaccharides from Tuber huidongense. Int. J. Biol. Macromol. 2016, 91, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, C.; Cheng, Y.; Huang, L.; Wang, Z. Isolation and structural characterization of a polysaccharide LRP4-A from Lycium ruthenicum Murr. Carbohydr. Res. 2013, 365, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Meng, Y.; Liu, L.; Li, J.; Ren, D.; Guo, Y. Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple. Int. J. Biol. Macromol. 2015, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Miao, S.; Zhang, Y.; Lin, S.; Jian, Y.; Tian, Y.; Zheng, B. Isolation, preliminary structural characterization and hypolipidemic effect of polysaccharide fractions from Fortunella margarita (Lour.) Swingle. Food Hydrocolloids 2016, 52, 126–136. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, H.Y.; Li, W.J.; Ye, J.Z. Chemical constituents and structural characterization of polysaccharides from four typical bamboo species leaves. Molecules 2015, 20, 4162–4179. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Lu, X.; Xie, B. Isolation and chemical characterisation of a polysaccharide from green tea (Camellia sinensis L.). J. Sci. Food Agric. 2008, 88, 2523–2528. [Google Scholar] [CrossRef]
- Prashanth, M.R.S.; Muralikrishna, G. Arabinoxylan from finger millet (Eleusine coracana, v. Indaf 15) bran: Purification and characterization. Carbohydr. Polym. 2014, 99, 800–807. [Google Scholar] [CrossRef]
- Castro, L.; Pinheiro, T.D.; Castro, A.J.G.; Santos, M.D.N.; Soriano, E.M.; Leite, E.L. Potential anti-angiogenic, antiproliferative, antioxidant, and anticoagulant activity of anionic polysaccharides, fucans, extracted from brown algae Lobophora variegata. J. Appl. Phycol. 2015, 27, 1315–1325. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Feng, S.L.; Liu, J.; Zhou, L.J.; Yuan, M.; Ding, C.B. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016, 91, 1025–1032. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.Z.; Gao, Q.Y.; Zou, Y.X. Hypoglycemic effect of Chinese yam (Dioscorea opposita rhizoma) polysaccharide in different structure and molecular weight. J. Food Sci. 2017, 82, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Li, C.F.; Wang, S.S.; Mei, X.F.; Zhang, H.X.; Kan, J.Q. Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis): Effect of drying procedures. Food Chem. 2019, 292, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.W.; Ma, Q.Q.; Xue, Z.H.; Gao, X.D.; Chen, H.X. Studies on the binding characteristics of three polysaccharides with different molecular weight and flavonoids from corn silk (Maydis stigma). Carbohydr. Polym. 2018, 198, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, G.; Yang, B.; Onishi, A.; Li, L.; Sun, P.; Zhang, F.; Linhardt, R.J. Structural characterization of pharmaceutical heparins prepared from different animal tissues. J. Pharm. Sci. 2013, 102, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Lin, C. Isolation, structural characterization and antioxidant activity of a neutral polysaccharide from. Food Hydrocolloids 2014, 39, 10–18. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Amid, B.T.; Cheong, K.W. Effect of different drying methods on chemical and molecular structure of heteropolysaccharide–protein gum from durian seed. Food Hydrocolloids 2013, 31, 210–219. [Google Scholar] [CrossRef]
- Yin, J.Y.; Nie, S.P.; Guo, Q.B.; Wang, Q.; Cui, S.W.; Xie, M.Y. Effect of calcium on solution and conformational characteristics of polysaccharide from seeds of Plantago asiatica L. Carbohydr. Polym. 2015, 124, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, L.; Chen, C.; Teng, B.; Wang, C.; Xu, Z.; Hu, B.; Zhou, P. Structure characterization of a novel neutral polysaccharide isolated from Ganoderma lucidum fruiting bodies. Food Chem. 2012, 135, 1097–1103. [Google Scholar] [CrossRef]
- Nwokocha, L.M.; Williams, P.A. Rheological characterization of the galactomannan from Leucaena leucocephala seed. Carbohydr Polym. 2012, 90, 833–838. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Hu, X.; Kang, J.; Yada, R.Y. Structural characterization of a low-molecular-weight heteropolysaccharide (glucomannan) isolated from Artemisia sphaerocephala Krasch. Carbohydr. Res. 2012, 350, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Kar Mandal, E.; Maity, K.; Bhunia, S.K.; Behera, B.; Maiti, T.K.; Mallick, P.; Sikdar, S.R.; Islam, S.S. Structural study of an immunoenhancing polysaccharide isolated from an edible hybrid mushroom of Pleurotus florida and Lentinula edodes. Bioact. Carbohydr. Diet. Fibre 2013, 1, 72–80. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Liu, C.; Li, E.; Gao, Z.; Liu, C.; Gu, W.; Huang, Y.; Liu, J.; Wang, D.; et al. Structural characterization of an acidic Epimedium polysaccharide and its immune-enhancement activity. Carbohydr. Polym. 2016, 138, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.J.; Chang, J.M.; Ablise, M.; Li, G.R.; He, J.W. Isolation, purification, and structural elucidation of polysaccharides from Alhagi-honey. J. Asian Nat. Prod. Res. 2014, 16, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Song, F.F.; Zhang, H.; Huan, X.; Li, S.Y. Compositional monosaccharide analysis of morus nigra linn by hplc and hpce quantitative determination and comparison of polysaccharide from Morus nigra Linn by HPCE and HPLC. Curr. Pharm. Anal. 2017, 13, 433–437. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Z.; Zhao, J.; Chen, R.; Meng, F.; Zhang, M.; Ge, W. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011, 127, 434–440. [Google Scholar] [CrossRef]
- Yan, C.; Yin, Y.; Zhang, D.; Yang, W.; Yu, R. Structural characterization and in vitro antitumor activity of a novel polysaccharide from Taxus yunnanensis. Carbohydr. Polym. 2013, 96, 389–395. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrieres, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Mu, H.; Lv, Z.; Yang, Y.; Zhang, J. Structural elucidation and antioxidant activity of a novel polysaccharide (TAPB1) from Tremella aurantialba. Food Hydrocolloids 2015, 43, 459–464. [Google Scholar] [CrossRef]
- Miao, M.; Bai, A.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocolloids 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.R.; Zhang, Q.; Li, G.Y.; Shan, Y.; Ding, S.H. Morphological, structural, and physicochemical properties of starch isolated from different lily cultivars grown in China. Int. J. Food Prop. 2019, 22, 737–757. [Google Scholar] [CrossRef]
- Yang, W.F.; Wang, Y.; Li, X.P.; Yu, P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015, 117, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Sun, Y.; Yang, B.; Wang, Z.; Chai, G.; Guan, Y.; Zhu, W.; Shu, Z.; Lei, X.; et al. Purification, characterization and immunomodulatory effects of Plantago depressa polysaccharides. Carbohydr. Polym. 2014, 112, 63–72. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, X.; Zhang, Y.; Wang, J.; Sun, H.; Wang, J.; Cao, X.; Ye, Y. Isolation and prebiotic activity of water-soluble polysaccharides fractions from the bamboo shoots (Phyllostachys praecox). Carbohydr. Polym. 2016, 151, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; He, T.Z.; Zhang, Y.Q. The isolation and the characterization of two polysaccharides from the branch bark of mulberry (Morus alba L.). Arch. Pharmacal Res. 2016, 39, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.; Feng, W.; Mao, R.; Gu, X.; Li, T.; Li, Q.; Bao, Y.; Yang, L.; Wu, X. Isolation, characterization and antioxidant activity of polysaccharide from Schisandra sphenanthera. Carbohydr. Polym. 2014, 105, 26–33. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef]
- Yu, X.H.; Liu, Y.; Wu, X.L.; Liu, L.Z.; Fu, W.; Song, D.D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Tian, H.; Yin, X.; Zeng, Q.; Zhu, L.; Chen, J. Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Int. J. Biol. Macromol. 2015, 79, 577–582. [Google Scholar] [CrossRef]
- Liu, H.M.; Li, Y.R.; Wu, M.; Yin, H.S.; Wang, X.D. Two-step isolation of hemicelluloses from Chinese quince fruit: Effect of hydrothermal treatment on structural features. Ind. Crops Prod. 2018, 111, 615–624. [Google Scholar] [CrossRef]
- Kang, C.C.; Hao, L.M.; Zhang, L.M.; Zheng, Z.Q.; Yang, Y.W. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium Meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar]
- Carrero-Carralero, C.; Mansukhani, D.; Ruiz-Matute, A.I.; Martínez-Castro, I.; Ramos, L.; Sanz, M.L. Extraction and characterization of low molecular weight bioactive carbohydrates from mung bean (Vigna radiata). Food Chem. 2018, 266, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Jahanbin, K.; Abbasian, A.; Ahang, M. Isolation, purification and structural characterization of a new water-soluble polysaccharide from Eremurus stenophyllus (boiss. & buhse) baker roots. Carbohydr. Polym. 2017, 178, 386–393. [Google Scholar] [PubMed]
- Zhou, C.L.; Liu, W.; Kong, Q.; Song, Y.; Ni, Y.Y.; Li, Q.H.; O’Riordan, D. Isolation, characterisation and sulphation of soluble polysaccharides isolated from Cucurbita maxima. Int. J. Food Sci. Technol. 2014, 49, 508–514. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Zhang, J.; Kang, W. Isolation, purification, structural analysis and coagulatory activity of water-soluble polysaccharides from Ligustrum lucidum Ait flowers. Chem. Cent. J. 2017, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, J.E.; Nie, S.P.; Xie, M.Y.; Li, C. Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J. Funct. Foods 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Jahanbin, K. Structural characterization of a new water-soluble polysaccharide isolated from Acanthophyllum acerosum roots and its antioxidant activity. Int. J. Biol. Macromol. 2018, 107, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Jiang, H.; Zhou, H.; Li, P.; Wang, F. Isolation, structural characterization and neurotrophic activity of a polysaccharide from Phellinus ribis. Carbohydr. Polym. 2015, 127, 145–151. [Google Scholar] [CrossRef]
- Sun, M.; Li, Y.; Wang, T.; Sun, Y.; Xu, X.; Zhang, Z. Isolation, fine structure and morphology studies of galactomannan from endosperm of Gleditsia japonica var. delavayi. Carbohydr. Polym. 2018, 184, 127–134. [Google Scholar] [CrossRef]
- Mei, Y.X.; Yang, W.; Zhu, P.X.; Peng, N.; Zhu, H.; Liang, Y.X. Isolation, characterization, and antitumor activity of a novel heteroglycan from cultured mycelia of Cordyceps sinensis. Planta Med. 2014, 80, 1107–1112. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, F.; Ni, H.; Mo, Z.; Wan, J.B.; Hua, D.; Yan, C. Structural characterization, alpha-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 2016, 144, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, G.; Sun, Y.; Gu, X.; Huang, L.; Wang, Z. Isolation, structural characterization, and immunological activity of a polysaccharide LRLP4-A from the leaves of Lycium ruthenicum. J. Carbohydr. Chem. 2016, 35, 40–56. [Google Scholar] [CrossRef]
- Hua, D.; Zhang, D.; Huang, B.; Yi, P.; Yan, C. Structural characterization and DPPH. radical scavenging activity of a polysaccharide from Guara fruits. Carbohydr. Polym. 2014, 103, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Shan, X.; Zhao, X.; Cai, C.; Zhao, X.; Lang, Y.; Zhu, H.; Yu, G. Extraction, isolation, structural characterization and anti-tumor properties of an apigalacturonan-rich polysaccharide from the sea grass Zostera caespitosa Miki. Mar. Drugs 2015, 13, 3710–3731. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, F.; Yu, Z.; Lin, J.; Yang, L. Chemical characterization and in vitro antitumor activity of a single-component polysaccharide from Taxus chinensis var. mairei. Carbohydr. Polym. 2015, 133, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Cheng, D.; Han, L.; Chen, Y.; Wang, C. Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym. 2017, 157, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Si, H.Y.; Chen, N.F.; Chen, N.D.; Huang, C.; Li, J.; Wang, H. Structural characterisation of a water-soluble polysaccharide from tissue-cultured Dendrobium huoshanense C.Z. Tang et S.J. Cheng. Nat. Prod. Res. 2018, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.C.; Clerigo, M.M.; Paano, A.M. Extraction, isolation and MALDI-QTOF MS/MS analysis of beta-d-Glucan from the fruiting bodies of Daedalea quercina. Int. J. Biol. Macromol. 2016, 93, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Li, Y.; Xu, G.Y.; Li, Y.S. Isolation, purification and structural characterization of a water-soluble polysaccharide HM 41 from Halenia elliptica D. Don. Chin. Chem. Lett. 2016, 27, 979–983. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Shen, C.C.; Gao, F.L.; Wei, H.; Ren, D.F.; Lu, J. Isolation, purification and structural characterization of two novel water-soluble polysaccharides from Anredera cordifolia. Molecules 2017, 22, 1276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, L.; Yang, S.; Zhao, F.; Xu, L.; Yong, Q. Isolation, characterization and in vitro anticancer activity of an aqueous galactomannan from the seed of Sesbania cannabina. Int. J. Biol. Macromol. 2018, 113, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.P.; Jin, H.; Li, X.Z.; Yang, X.Y.; Cui, H.Y.; Xin, A.Y.; Zhao, R.M.; Qin, B. Purification, characterization and antioxidant activities of a polysaccharide from the roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol. 2019, 135, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.K.; Chatterjee, S.; Gauri, S.S.; Das, S.S.; Mishra, A.; Patra, M.; Ghosh, A.K.; Das, A.K.; Singh, S.M.; Dey, S. Isolation and characterization of extracellular polysaccharide Thelebolan produced by a newly isolated psychrophilic antarctic fungus Thelebolus. Carbohydr. Polym. 2014, 104, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, J.; Liu, J.; Gou, Y.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Kan, J.; Qian, C.; et al. Structural characterization and anti-inflammatory activity of alkali-soluble polysaccharides from purple sweet potato. Int. J. Biol. Macromol. 2019, 131, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Lv, Y.; Hu, M.; Fan, L.; Li, Q.; Cai, C.; Li, G.; Yu, G. Extraction, isolation and structural characterization of a novel polysaccharide from Cyclocarya paliurus. Int. J. Biol. Macromol. 2019, 132, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.L.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Dong, C.X.; Du, J. Structural characterization of three polysaccharides from the roots of Codonopsis pilosula and their immunomodulatory effects on RAW264.7 macrophages. Int. J. Biol. Macromol. 2019, 130, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, G.; Qin, C.; Chen, W.; Chen, G.; Wen, L. Structure Characterization and Otoprotective Effects of a New Endophytic Exopolysaccharide from Saffron. Molecules 2019, 24, 749. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 1964, 55, 205–208. [Google Scholar]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Ciucanu, I.; Costello, C.E. Elimination of oxidative degradation during the per-o-methylation of carbohydrates. J. Am. Chem. Soc. 2003, 125, 16213–16219. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Caprita, R. Per-o-methylation of neutral carbohydrates directly from aqueous samples for gas chromatography and mass spectrometry analysis. Anal. Chim. Acta. 2007, 585, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xiong, Q.; Li, S.; Zhao, X.; Zeng, X. Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis. Carbohydr. Polym. 2015, 115, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.; Gupta, P.K.; Soni, P.L. Structure of the oligosaccharides isolated from Prosopis juliflora (Sw.) DC. seed polysaccharide. Carbohydr. Polym. 2014, 101, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.B.; Liang, H.P.; Wu, Y. Isolation, purification and structural characterization of polysaccharide from Acanthopanax brachypus. Carbohydr. Polym. 2015, 127, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhou, C.; Li, W.; Hu, B.; Wang, X.; Zeng, X. Structural elucidation of polysaccharide fractions from brown seaweed Sargassum pallidum. Carbohydr. Polym. 2013, 97, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.X.; Sun, P.L. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Yin, Q.S.; Han, T.; Zhao, Y.X.; Su, J.J.; Li, M.Z.; Ling, J.Y. Purification and antioxidant effect of novel fungal polysaccharides from the stroma of Cordyceps kyushuensis. Ind. Crop. Prod. 2015, 69, 485–491. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Zhou, X.; Yang, X.; Shen, S.; Wang, J.; Wang, Z.; Geng, L. Isolation and characterization of antioxidant polysaccharides (PKCP-D70-2-a and PKCP-D70-2-b) from the Pinus koraiensis pinecone. RSC Adv. 2016, 6, 110706–110721. [Google Scholar] [CrossRef]
- Patel, B.K.; Campanella, O.H.; Janaswamy, S. Impact of urea on the three-dimensional structure, viscoelastic and thermal behavior of iota-carrageenan. Carbohydr. Polym. 2013, 92, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, R.; Wei, Y.; Liu, H.; Yang, H.; Zhao, Q. Carboxymethylated hyperbranched polysaccharide: Synthesis, solution properties, and fabrication of hydrogel. Carbohydr. Polym. 2015, 128, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Ma, X.; Ren, H.; Fan, W.; Leng, F.; Yang, M.; Wang, X. Extraction, purification, and bioactivities analyses of polysaccharides from Glycyrrhiza uralensis. Ind. Crop. Prod. 2018, 122, 596–608. [Google Scholar] [CrossRef]
- Kong, L.; Ling, Y.; Tao, F.; Yin, X.; Liu, T.; Lei, D. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Sun, R.G.; Zhang, J.; Chen, Y.Y.; Liu, N.N. Structure and antioxidant activity of polysaccharide POJ-U1a extracted by ultrasound from Ophiopogon japonicus. Fitoterapia 2012, 83, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, D.; Bertucci, C. Induced circular dichroism as a tool to investigate the binding of drugs to carrier proteins: Classic approaches and new trends. J. Pharm. Biomed. Anal. 2015, 113, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sanandiya, N.D.; Siddhanta, A.K. Chemical studies on the polysaccharides of Salicornia brachiata. Carbohydr. Polym. 2014, 112, 300–307. [Google Scholar] [CrossRef]
- Wang, J.; Niu, S.; Zhao, B.; Luo, T.; Liu, D.; Zhang, J. Catalytic synthesis of sulfated polysaccharides. II: Comparative studies of solution conformation and antioxidant activities. Carbohydr. Polym. 2014, 107, 221–231. [Google Scholar] [CrossRef] [PubMed]

| Method | Mechanism | Range of Application | Target Production Properties | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Fractional precipitation | The solubilities of polysaccharides are different in different solvents. | crude polysaccharides with different molecular weight distribution | obtain polysaccharides of different molecular weights | ① simplicity of process ② can obtain polysaccharides with different molecular weight distributions. | ① easy to produce co-precipitation ② low efficiency |
| Cetyltrimethylammonium ammonium bromide (CTAB) precipitation | Long chain quaternary ammonium salt can form complex with acidic polysaccharides or long chain polysaccharides and then precipitate. | most crude polysaccharides, especially the acidic polysaccharides. | obtain acidic and neutral crude polysaccharides | ① low cost ② simple equipment requirements | ① the great destruction of structures of polysaccharides ② low yield |
| Metal complexation | Polysaccharides can be complexed with specific ionic compounds to precipitate. | most crude polysaccharides | obtain free polysaccharides of different properties | ① simplicity of operator ② low cost | uneasy to control the degree of reaction leading to the irreversible changes of structures of polysaccharides |
| Anion-exchange chromatography | It is the same as ion-exchange with reversible adsorption and bond adsorption. | acidic, neutral, and viscous polysaccharides, especially complex polysaccharides that bind to proteins | obtain homogenous polysaccharides | having a large separation capacity and satisfactory effects | ① high cost ② The flow rate of eluent is easily affected by the changes of volumes, which is sensitive with the changes of eluent pH or the ion strength of solution |
| Gel column chromatography | molecular sieve principle, according to the size and shape of polysaccharides | most crude polysaccharides | obtain homogenous polysaccharides from different molecular weight ranges | quick, convenient, and effective separation process | ① strict conditions for separation ② unsuitable for the separation of mucopolysaccharides |
| Affinity column chromatography | molecular affinity | polysaccharides with affinity to groups on chromatographic columns | obtain homogenous polysaccharides with different properties | ① can separate polysaccharides with less content. ② one-time enrichment of polysaccharides is very high. | difficult to find suitable ligands |
| Cellulose column chromatography | molecular sieve principle | acidic and neutral polysaccharides | to obtain polysaccharides from different molecular weight ranges | polysaccharides with high purity | time-consuming, especially for the more viscous acid polysaccharides |
| Macroporous resin chromatography | molecular sieve and selective adsorption principle | most polysaccharides | to obtain polysaccharides from different molecular weight ranges | ① high adsorption capacity ② good selectivity ③ reproducibility | the ability to separate polysaccharides with different properties is weak |
| Order | Chromatography | Column Type | Elution Condition | References |
|---|---|---|---|---|
| Uniformity and molecular weight determination | ||||
| 1 | HPGPC | Shodex sugar KS-805+ guard column KS-G (300 × 7.8 mm) | unknown | [73] |
| 2 | HPGPC | TSK-gel column G4000PWXL | ultra-pure water containing 0.1% (w/w) NaN3, 0.5 mL/min | [74] |
| 3 | HPLC | TSK G4000PWXL (300 × 7.8 mm) | deionized water, 0.6 mL/min | [75] |
| 4 | HPLC | TSK-gel G4000PWXL (300 × 7.8 mm) | 0.003 mol/L CH3COONa solution, 0.8 mL/min | [76] |
| 5 | HPSEC | TSK-gel G4000 PWXL (300 × 7.8 mm) | distilled water, 0.6 mL/min | [77] |
| Monosaccharide composition analysis | ||||
| 1 | GC | HP-5 fused silica capillary column (30 m × 0.32 mm × 0.25 mm) | N2 carrier gas, 1.0 mL/min. | [78] |
| 2 | HPLC | Waters xbridge-C18 column | 0.1 mol/L potassium phosphate buffer, 1.0 mL/min | [79] |
| 3 | HPAEC | CarbopacTM PA-20 column (4 mm × 250 mm) | unknown | [80] |
| 4 | HPGPC | L-aquagel-OH 40 pre-column (300 × 7.5 mm) PL-aquagel-OH 30 separation column (300 × 7.5 mm) | NaAc solution (0.003 mol/L), 1.0 mL/min | [81] |
| 5 | GC-MS | polycarborane–siloxane capillary column (25 m × 0.22 mm i.d. ×0.1 µm film thickness) | Helium carrier gas, 1 mL/min | [82] |
| Polysaccharide separation and purification | ||||
| 1 | Anion exchange column chromatography and gel column chromatography | DEAE-Cellulose A52 (2.6 × 30 cm) Sephadex G-100 gel filtration column (1.6 × 70 cm) | NaCl aqueous solution (0–1 mol/L)/Deionized water, 9 mL/h | [83] |
| 2 | Anion exchange column chromatography and gel column chromatography | anion-exchange chromatography column (2.6 × 37 cm) DEAE-Sepharose chromatography column | NaCl (0–0.6 mol/L), 4 mL/min | [84] |
| 3 | Anion exchange column chromatography and gel column chromatography | DEAE-52 cellulose gel (2.5 × 60 cm) Sephadex G-100 column (1.5 × 100 cm) | NaCl (0–0.3 mol/L) | [85] |
| 4 | Gel column chromatography | DEAE-52 cellulose chromatography column (1.6 × 60 cm) | NaCl (0–0.3 mol/L), 0.64 mL/min | [86] |
| Name | Material Source | Monosaccharide Composition and Proportion | Molecular Weight | The Main Structure | Biological Activities | References |
|---|---|---|---|---|---|---|
| AAPS-1 | Acanthophyllum acerosum roots | Glc, Gal, Ara in a ratio of 1.6:5.1:1.0 | 23.2 kDa | →6)-α-d-Galp-(1→residues | anti-oxidation | [87] |
| PRG | Phellinus ribis | unknown | 5.16 kDa | β-d-glucan containing a (1→3) linked backbone | neuroprotection | [88] |
| EGSP | Gleditsia japonica var. delavayi seeds | unknown | 1913 kDa | β-1,4-d-mannopyranose | unknown | [89] |
| CSPS-1 | Cordyceps sinensis | Glc, Gal, Xyl, Man, Rha in the ratio of 30.67:13.37:5.40:2.39:1.0 | 11,700 kDa | (1→6)-linked α-d-Glc and α-d-Gal | antitumor | [90] |
| GP90-1B | Psidium guajava fruits | Glc, Ara in a molar ratio of 9.92:84.06 | unknown | (1→5)-linked-α-l-arabinose, (1→2,3,5)-linked-α-l-arabinose and (1→3)-linked-α-l-arabinose | anti-oxidation | [91] |
| LRLP4-A | Lycium ruthenicum leaves | unknown | 135 kDa | 1→6-linked β-galactopyranosyl residues substituted at O-3 by arabinosyl or galactosyl residues | immunomodulatory | [92] |
| GP70-2 | Psidium guajava fruits | D-Gla, L-Ara in a molar ratio of 1:1 | 74 kDa | (1→3) linked α-l-Ara and (1→3,6) linked β-d-Gal | anti-oxidation | [93] |
| ZCMP | Zostera caespitosa | GalA, Api, Gal, Rha, Ara, Xyl, Man in a molar ratio of 51.4:15.5:6.0:11.8:4.2:4.4:4.2 | 77.2 kDa | 70% AGA (α-1,4-d-galactopyranosyluronan), 30% RG-I, (→4GalAα1,2Rhaα1→, with a few α-l-arabinose) | anti-angiogenesis, immune regulation | [94] |
| IPSII | Isochrysis galbana | Glc, Gal, and Rha | 15.934 kDa | a β-type heteropolysaccharide with a pyran group | anti-oxidation | [95] |
| CPTC-2 | Taxus chinensis leaves | Glc, Man, Xyl, Ara, Rha, Gal in a molar ratio of 1.00:0.32:0.27:3.34:1.22:1.84 | 73.53 kDa | α-(1→3) Araf, α-(1→5) Araf and α-(1→4) Galp with branches composed of α-(1→3,5) Araf and β-(1→3,6) Manp | antitumor | [96] |
| GFP | Grifola frondosa fruits | Rha, Xyl, Man, Glc in a molar ratio of 1.00:1.04:1.11:6.21 | 155 kDa | every→3)-Glcp-(1→and one→3,4)-Glcp-(1→connected interval with a small amount of 1→, 1→4,1→6 glycosidic linkage | immunomodulatory | [97] |
| TC-DHPA4 | tissue-cultured Dendrobium huoshanense | Rha, Ara, Man, Glc, Gal, GlcA in a molar ratio of 1.28:1:1.67:4.71:10.43:1.42 | 800 kDa | →6)-β-Galp-(1→6)-β-Galp-(1→4)-β-GlcpA-(1→6)-β-Glcp-(1→6)-β-Glcp-(→ | unknown | [98] |
| DQW1Pa1 | Daedalea quercina | D-Mannose, D-Glccose, D-Galactose, D-Xylose, L-Fucose, L-Arabinose and L-Rhamnose | 16 kDa | 1-3 linked linear glucose backbone | unknown | [99] |
| HM41 | aerial part of Halenia elliptica | Rha, Ara, Xyl, Man, Gal, Glc in a molar ratio of 1.0:5.5:1.8:3.0:9.4:21 | 11.7 kDa | β-(1→4) Gal, β-(1→4) Glc and b-(1→6)Glc.β-(1→4)Gal | anti-oxidation | [100] |
| ACP1-1 | Anredera cordifolia seeds | Man, Glc, Gal in a molar ratio of 1.08:4.65:1.75 | 46.78 kDa | (1→3,6)-galacturonopyranosyl residues interspersed with (1→4)-residues and (1→3)-mannopyranosyl | unknown | [101] |
| GalM | Sesbania cannabina seeds | unknown | 1420 kDa | β-1,4-d-mannan | antitumor | [102] |
| LPR | Lilium davidii var. unicolor Cotton roots | Glc, Man in a molar ratio of 2.9:3.3 | 51.2 kDa | beta-(1→4)-linked D-glucopyranosyl and beta-(1→4)-linked D-mannopyanosyl | anti-oxidation | [103] |
| EPS | a newly isolated psychrophilic Antarcticfungus Thelebolus | unknown | unknown | (1→3)-linked β-d-Glccan backbone with (1→6)-linked branches of β-d-Glccopyranosyl units | antitumor | [104] |
| ASPP | purple sweet potato | Rha, Ara, Xyl, Man, Glc in the molar ratio of 2.8:1.9:1.0:7.6:53.3 | 18 kDa | 1,4-linked Glcp with side chains attached to the O-6 position | anti-inflammatory | [105] |
| CP-III | Cyclocarya paliurus leaves | Gal, Ara, GalA, Rha, Glc, Xyl and Man in a molar ratio of 31.1:27.5:22.0:6.7:5.8:3.8:3.1 | 72.7 kDa | →4)GalAp(α1→ and →2)Rhap(α1→4)GalAp(α1→ | pectin like polysaccharides | [106] |
| RCNP | Codonopsis pilosula roots | Ara, Gal in a molar ratio of nearly 3:1 | 11.4 kDa | arabinan region: (1→5)-linked Araf residues with side chains branched at the O-3 position, arabino galactan region: (1→4)-, (1→6)- or (1→3)-linked Galp along with small amounts of branches at the O-3 position of the (1→6)-linked Galp or O-6 position of the (1→3)-linked Galp residues | immunomodulatory activity | [107] |
| EPS-2 | Saffron | unkown | 40.4 kDa | (1→2)-linked -d-Manp, (1→2, 4)-linked-d-Manp, (1→4)-linked-d-Xylp, (1→2, 3, 5)-linked-d-Araf, (1→6)-linked-d-Glcp with-d-Glcp-(1→and-d-Galp-(1→as sidegroups | protection of cochlear hair cells from ototoxicity exposure | [108] |
| Raman Spectrum/cm−1 | Group/Atom |
|---|---|
| 350–600 | Pyranose ring |
| 600–950 | Heterocarbon model |
| 950–1200 | Glycosidic bond type |
| 1200–1500 | CH2 and C-OH deformation |
| Number | Polysaccharide Structure Analysis and Determination Project | Common Method |
|---|---|---|
| 1 | Overall structural analysis Homogeneity and molecular weight determination | High performance gel permeation chromatography (HPGPC), osmotic pressure, light scattering, viscosity, polypropylene gel electrophoresis, etc. |
| 2 | Overall structural analysis Monosaccharide composition and proportion | Complete acid hydrolysis, HPLC, GC, GC-MS, high performance ion chromatography, etc. |
| 3 | Glycoside ring form | Raman spectroscopy such as infrared spectroscopy. |
| 4 | Glycosidic linkage sequence | Selective acid hydrolysis, sequential hydrolysis of glycosidase, nuclear magnetic resonance, etc. |
| 5 | Hydroxyl substitution | Methylation, periodate oxidation, Smith degradation, GC-MS, nuclear magnetic resonance, etc. |
| 6 | Polysaccharides chain-peptide bond linkage | Dilute alkali hydrolysis, hydrazine reaction, amino acid composition reaction, etc. |
| 7 | Amorphic form substituted by glycosides | Glycosidase hydrolysis, nuclear magnetic resonance, infrared chromatography, laser, etc. |
| 8 | Monosaccharide residue type and glycosidic linkage site | Methylation analysis, GC-MS, etc. |
| 9 | Oligosaccharide determination | Partial acid hydrolysis, GC-MS, MS, etc. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. https://doi.org/10.3390/molecules24173122
Ren Y, Bai Y, Zhang Z, Cai W, Del Rio Flores A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules. 2019; 24(17):3122. https://doi.org/10.3390/molecules24173122
Chicago/Turabian StyleRen, Yan, Yueping Bai, Zhidan Zhang, Wenlong Cai, and Antonio Del Rio Flores. 2019. "The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development" Molecules 24, no. 17: 3122. https://doi.org/10.3390/molecules24173122
APA StyleRen, Y., Bai, Y., Zhang, Z., Cai, W., & Del Rio Flores, A. (2019). The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules, 24(17), 3122. https://doi.org/10.3390/molecules24173122





