Identification of Constituents Affecting the Secretion of Pro-Inflammatory Cytokines in LPS-Induced U937 Cells by UHPLC-HRMS-Based Metabolic Profiling of the Traditional Chinese Medicine Formulation Huangqi Jianzhong Tang
Abstract
1. Introduction
2. Results
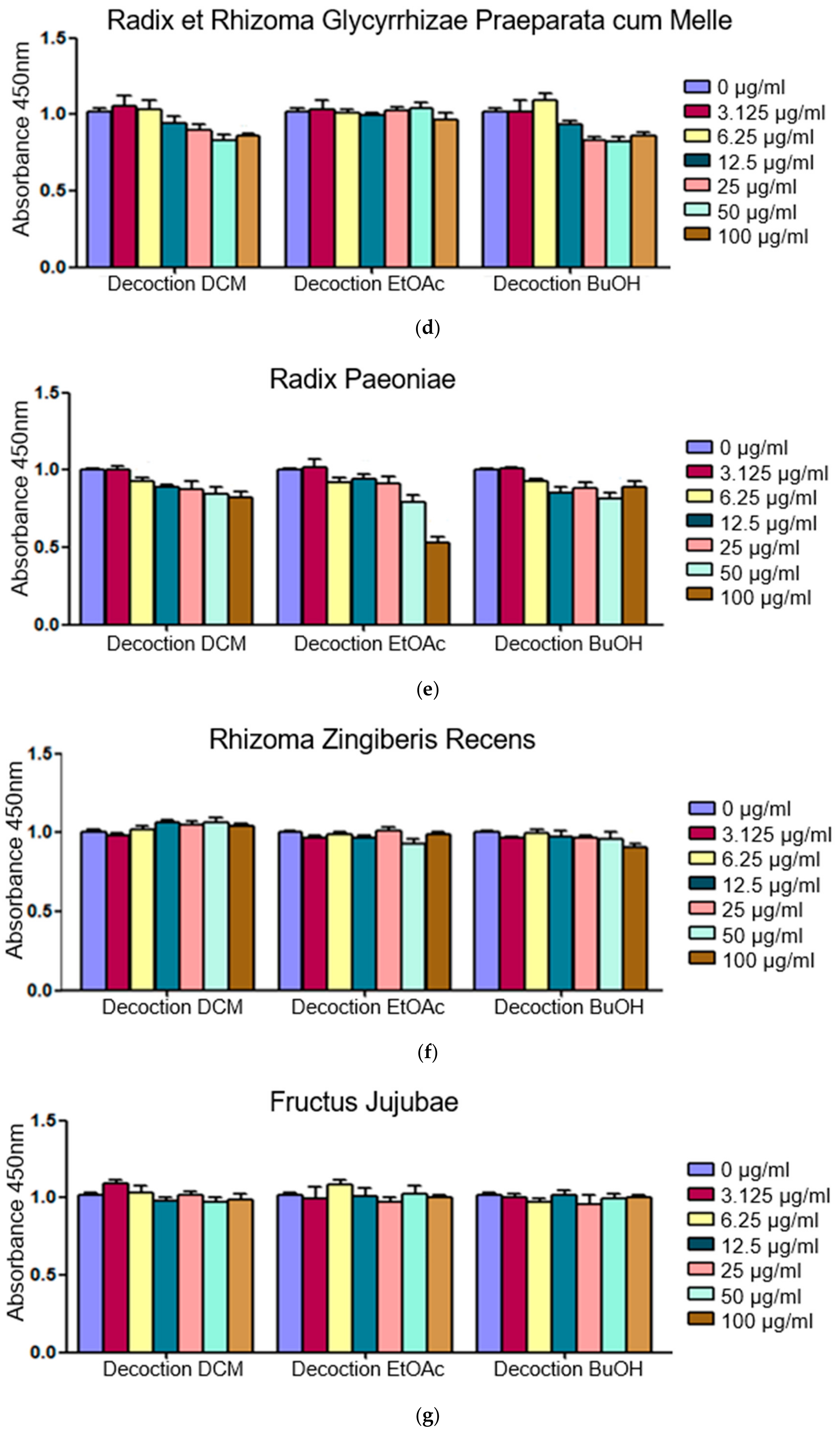
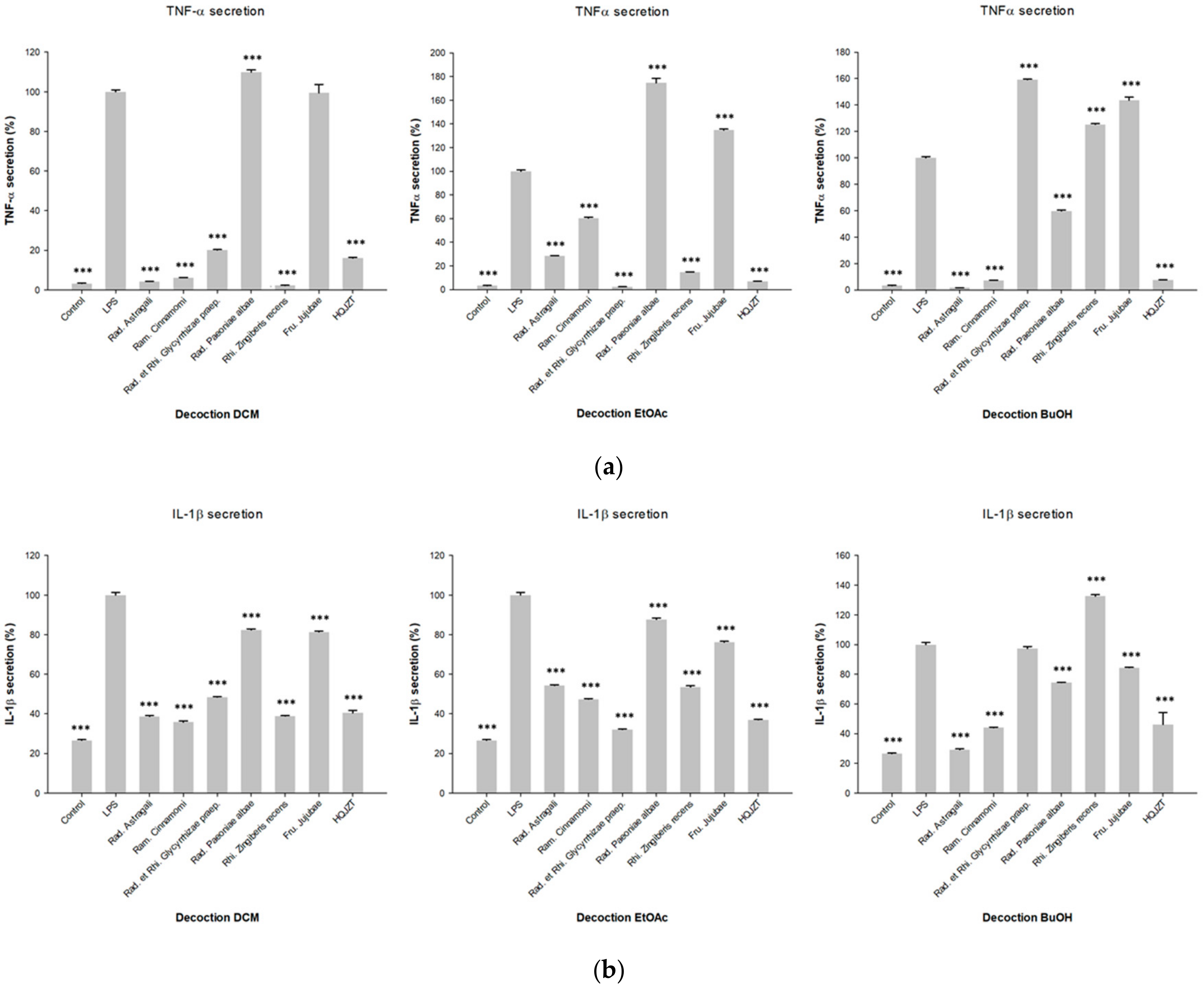
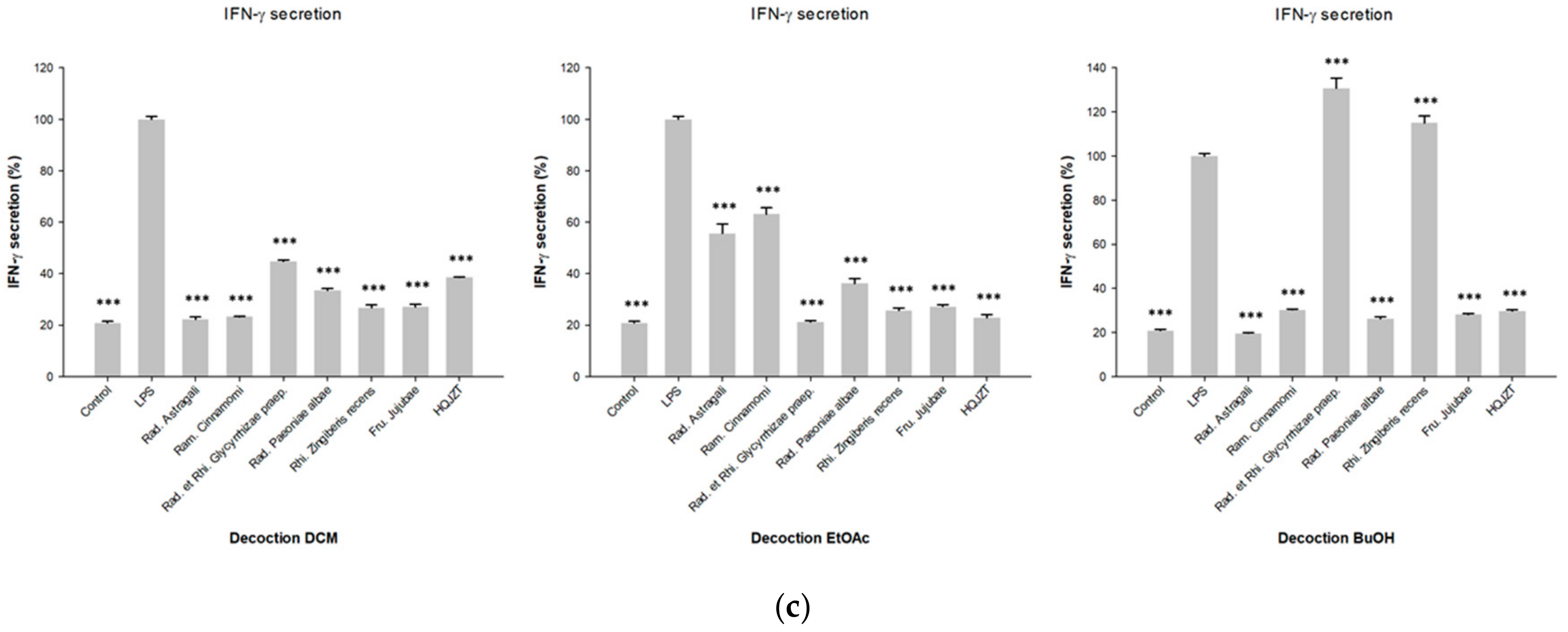
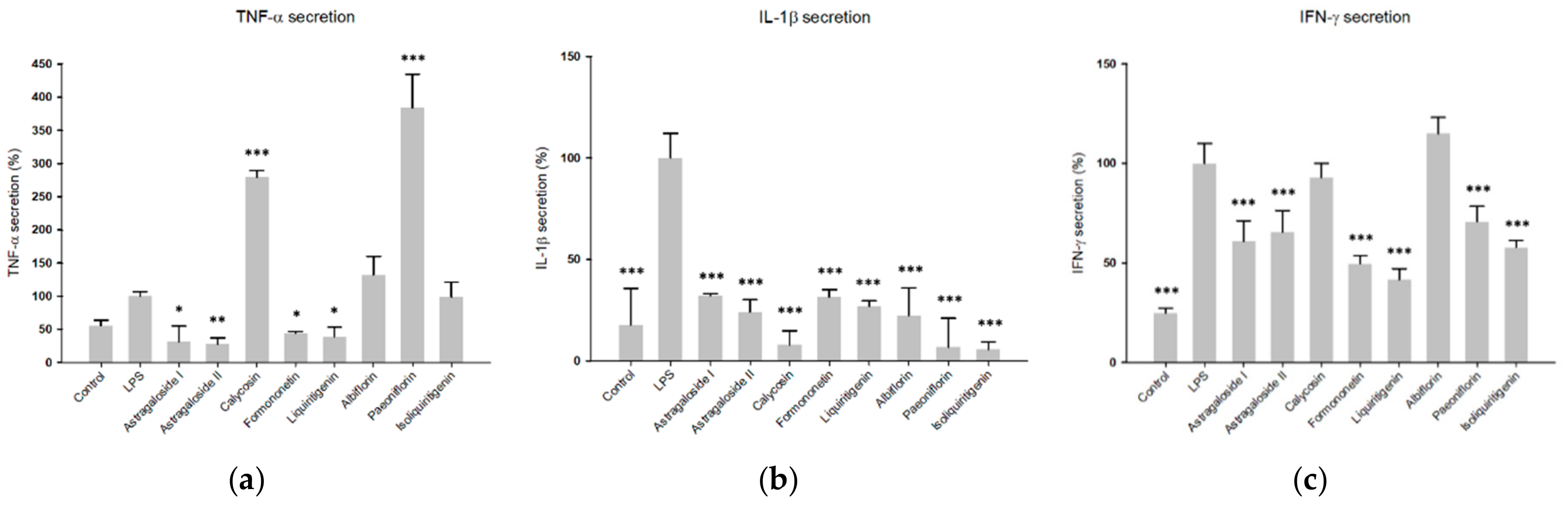
2.1. Fractions of HQZT Decoction and Its Single Herbs Exert Distinct Effects on Pro-Inflammatory Cytokines
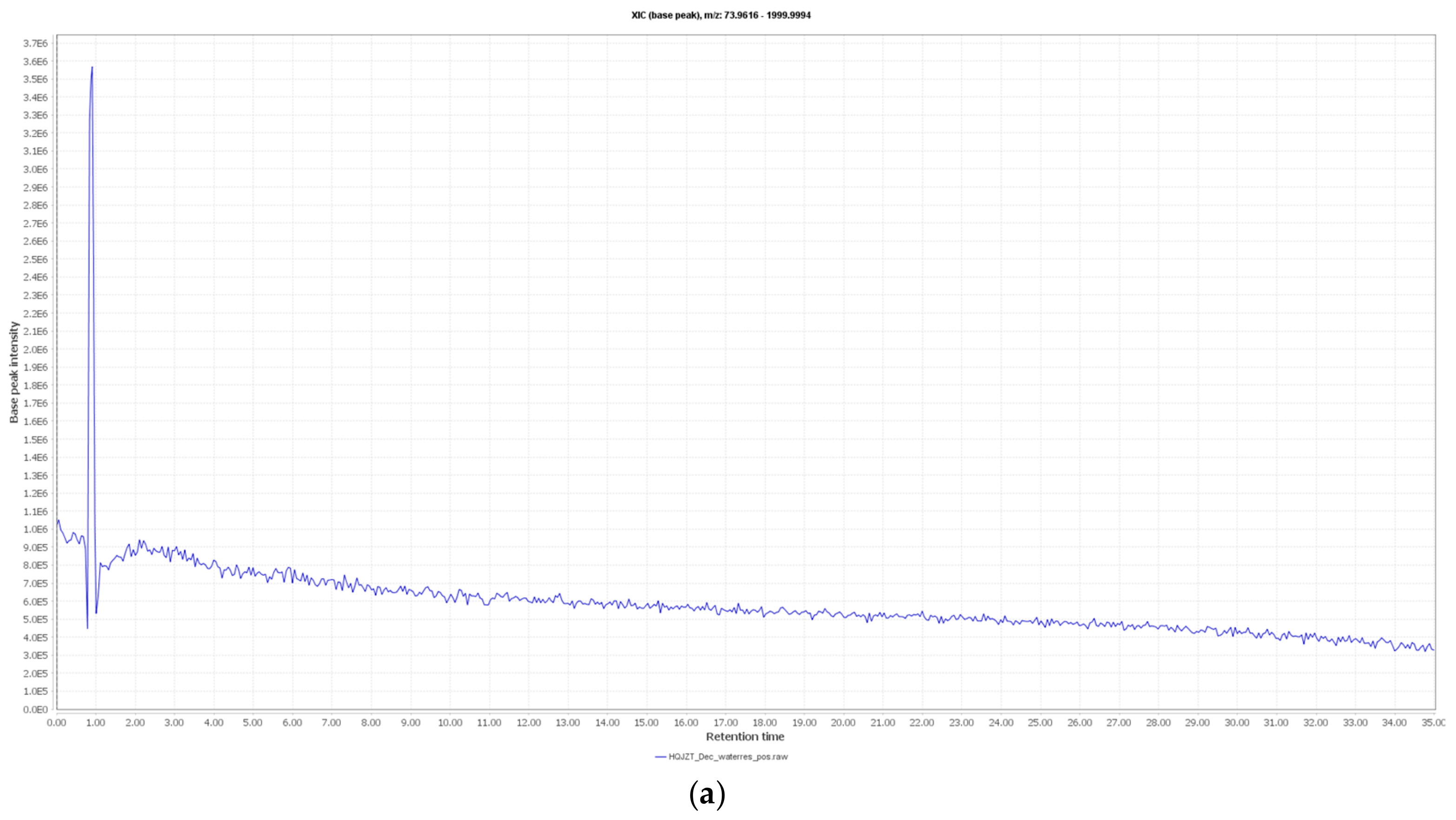
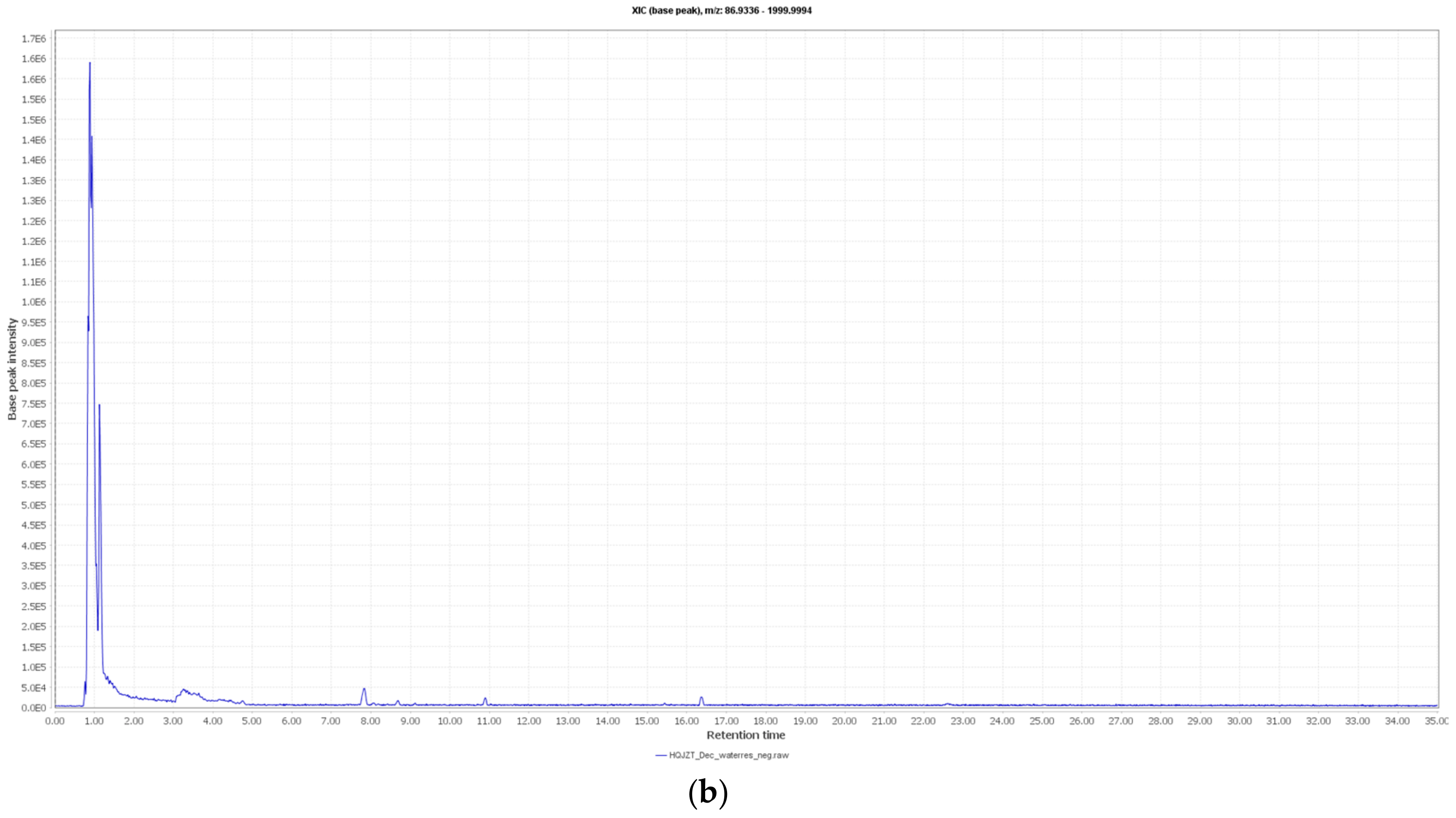
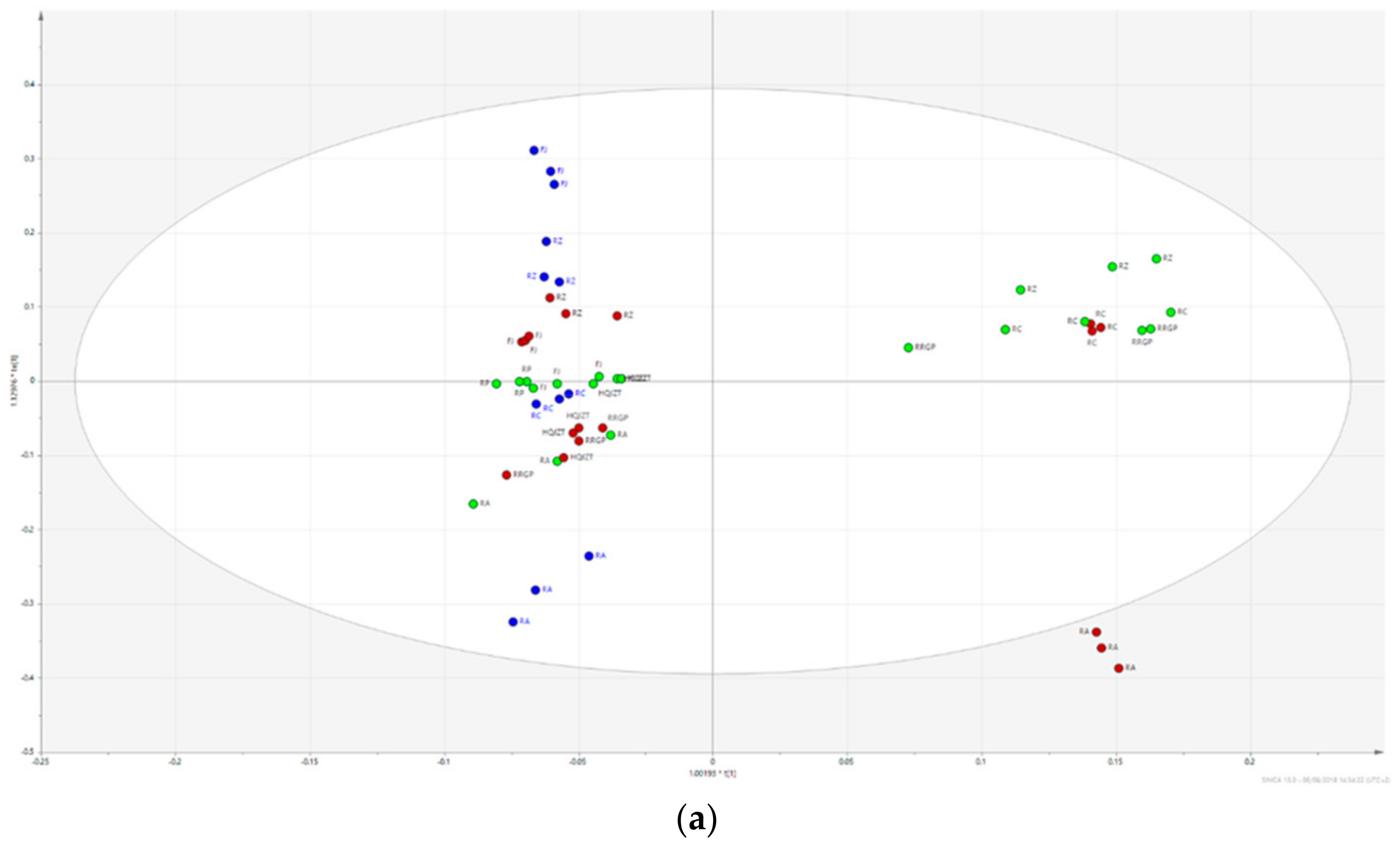
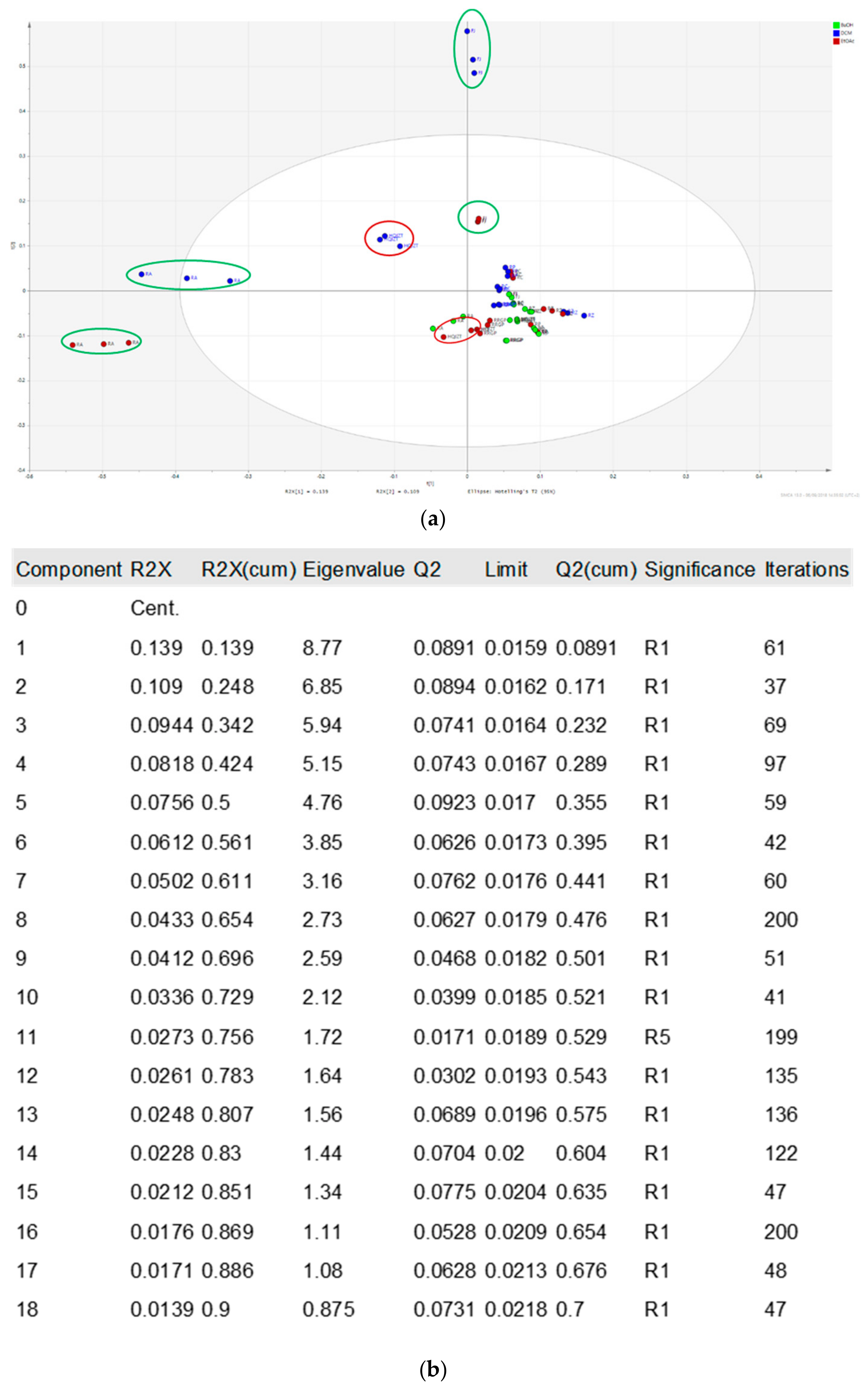
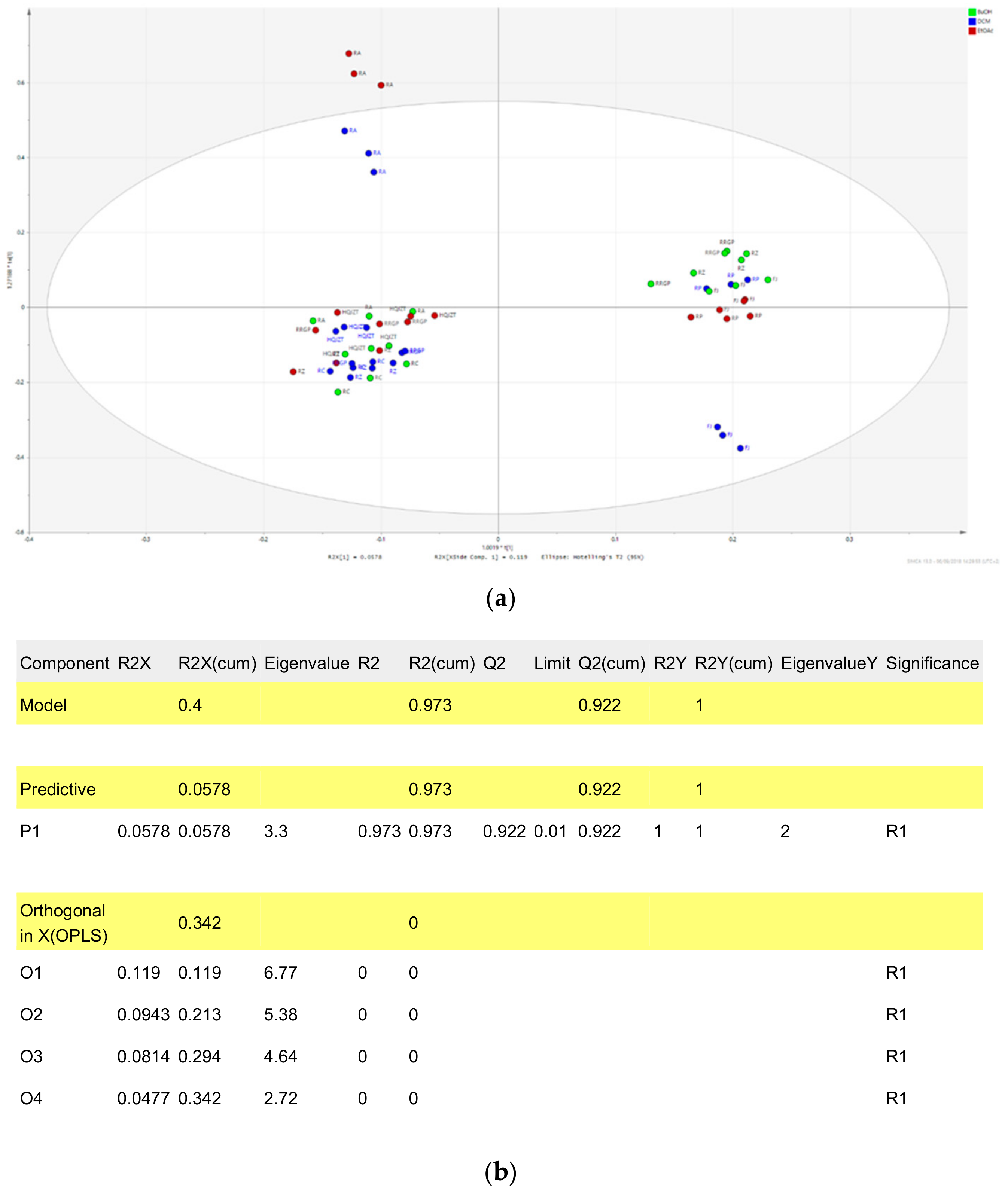
2.2. Fractions of HQZT Decoction and Its Single Herbs Possess Distinct UPLC-HRMS Profiles
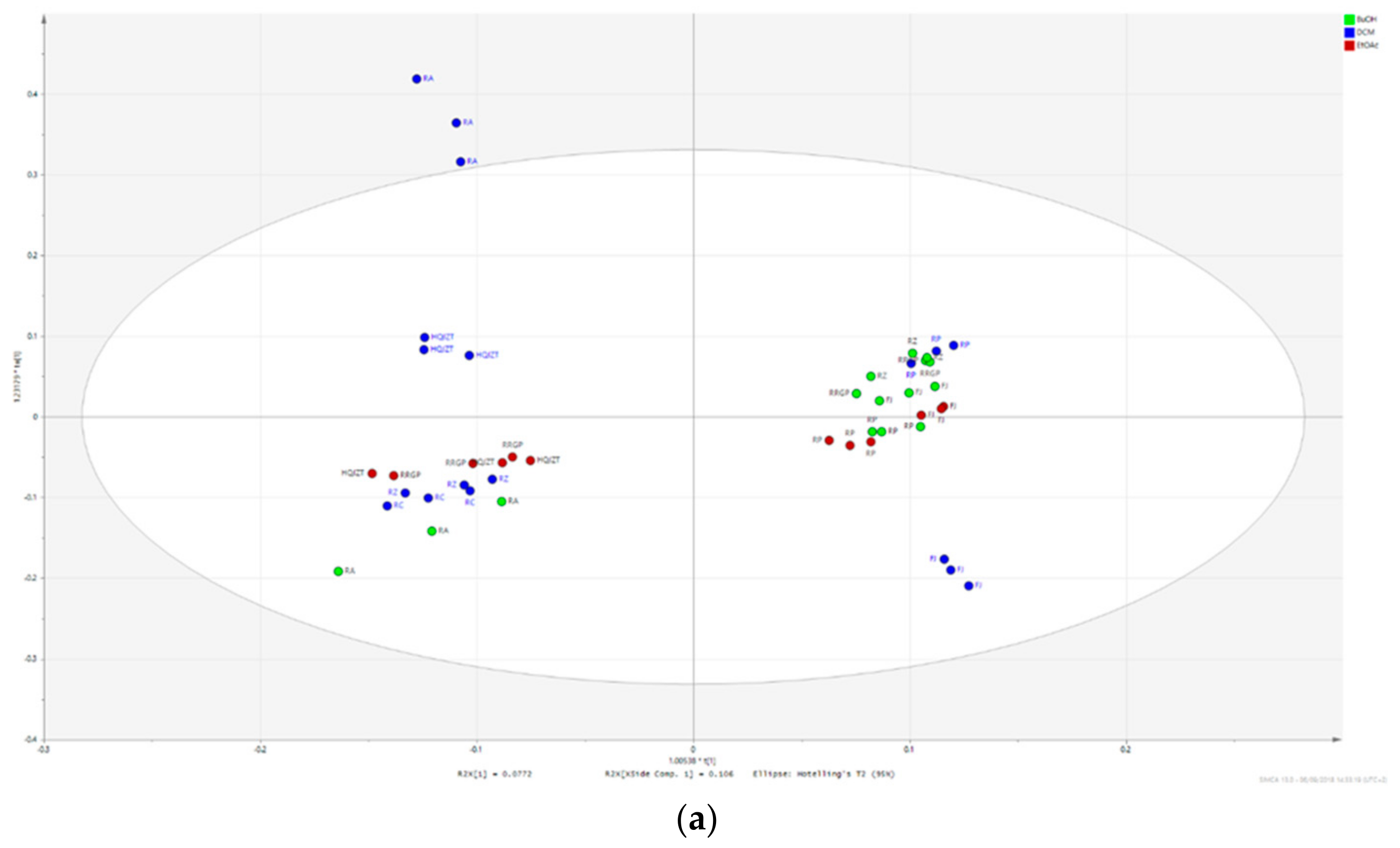
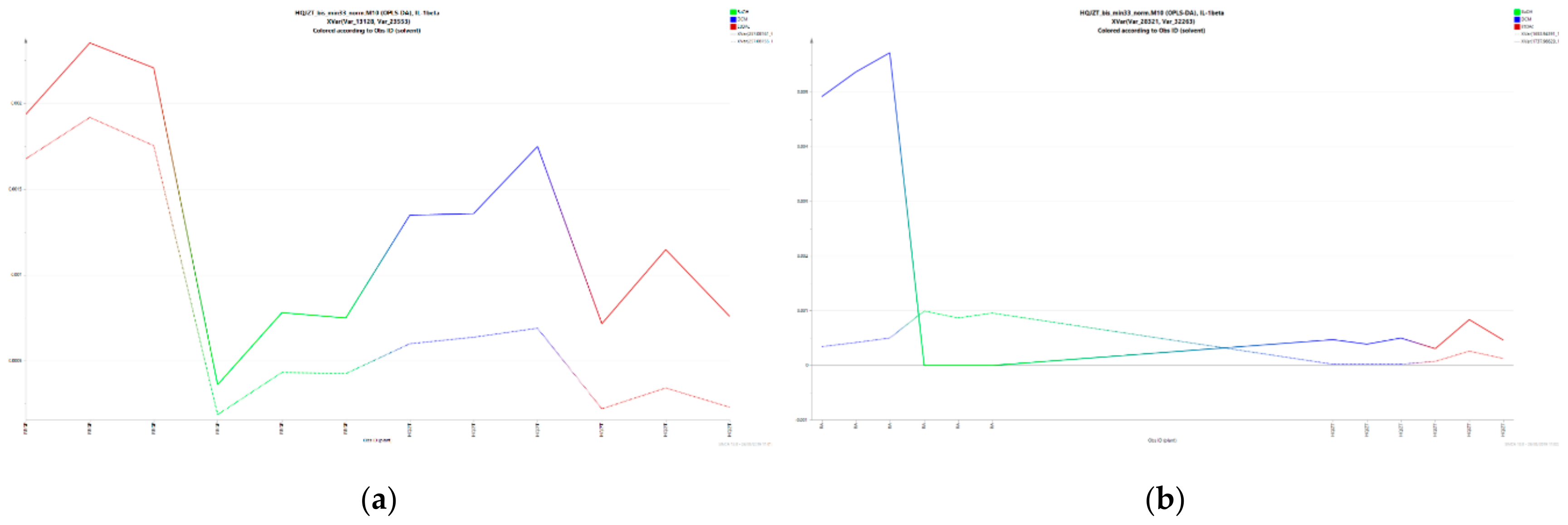
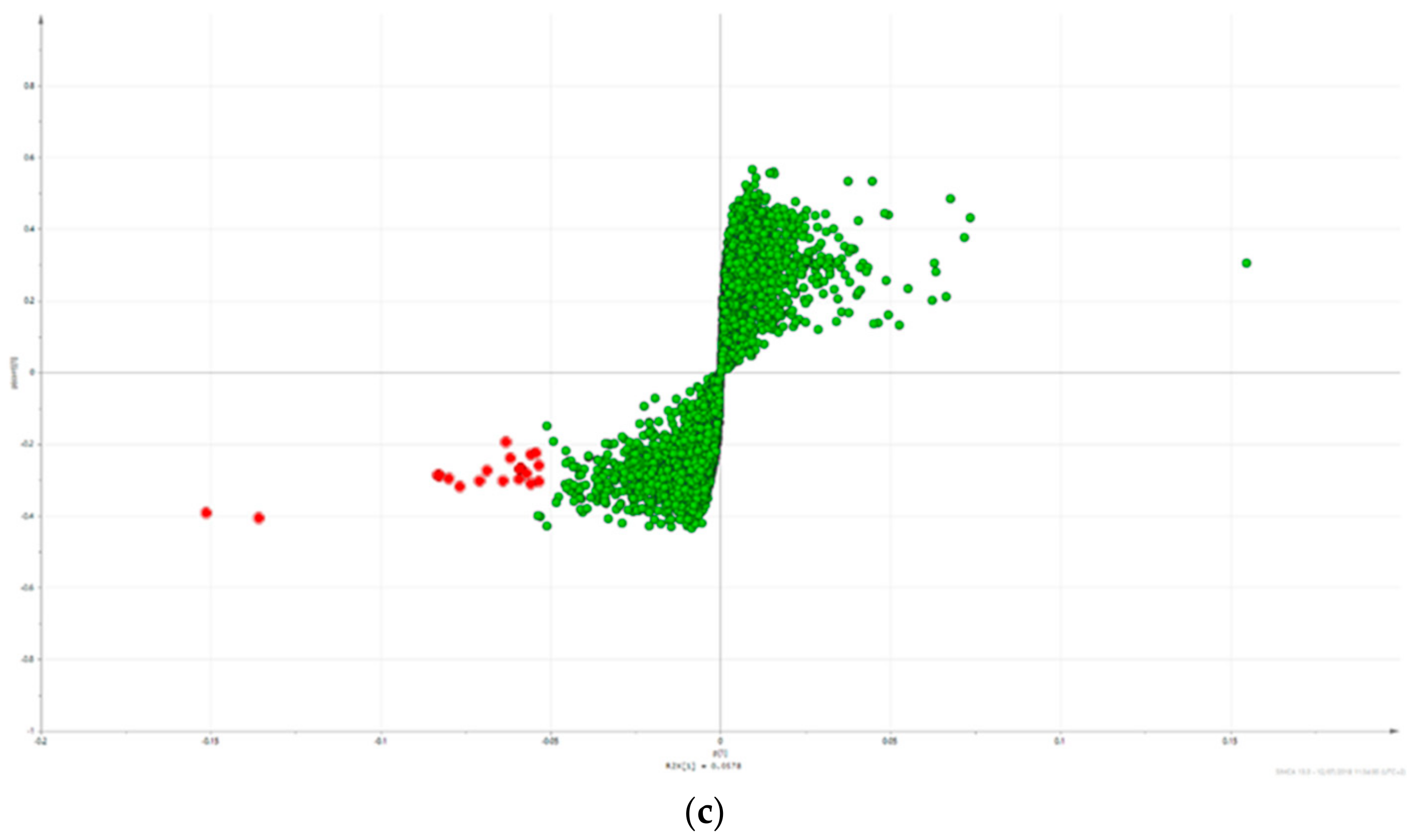
2.3. Triterpene Glycosides, Flavonoids and Monoterpene Glycosides are Most Strongly Correlated with Inhibitory Activity on Production of Pro-Inflammatory Cytokines
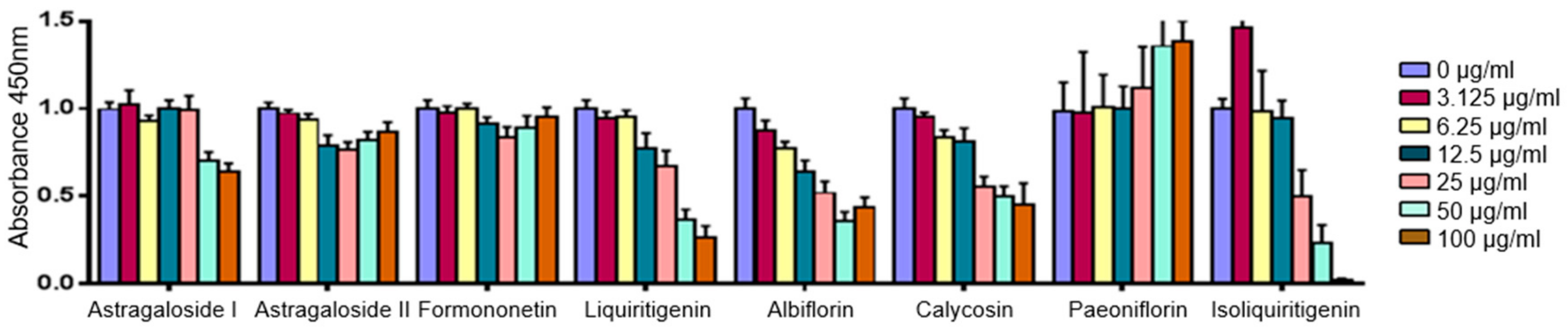
2.4. Predicted Pharmacological Activities Can Be Partially Verified for the Identified OPLS-DA Hit Compounds
3. Discussion
4. Materials and Methods
4.1. Plant Material, Chemicals and Reagents
4.2. Sample Preparation
4.3. UPLC-MS Analysis
4.4. Pharmalogical Studies
4.5. Data Processing and Multivariate Data Analysis
4.6. Metabolite Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Plant | Initial Weight Crude Drug (g) | Yield n-Hexane Extract (mg) | Yield DCM Extract (mg) | Yield EtOAc Extract (mg) | Yield n-Butanol Extract (mg) | Yield Water Residue (g) |
|---|---|---|---|---|---|---|
| Huangqin Jianzhong Tang | 45 | 34.1 | 63.6 | 209.0 | 962.9 | 7.8 |
| Radix Astragali | 45 | 12.3 | 93.6 | 104.6 | 803.6 | 13.7 |
| Ramulus Cinnamomi | 20 | 33.7 | 56.1 | 51.3 | 161.6 | 1.0 |
| Radix et Rhizoma Glycyrrhizae praeparata cum melle | 20 | 22.4 | 46.0 | 147.3 | 609.2 | 6.8 |
| Fructus Jujubae | 45 | 19.4 | 28.2 | 45.1 | 178.9 | 8.6 |
| Radix Paeoniae albae | 20 | 19.8 | 43.2 | 245.9 | 723.7 | 3.7 |
| Rhizoma Zingiberis recens | 45 | 39.3 | 17.0 | 6.3 | 55.4 | 1.0 |
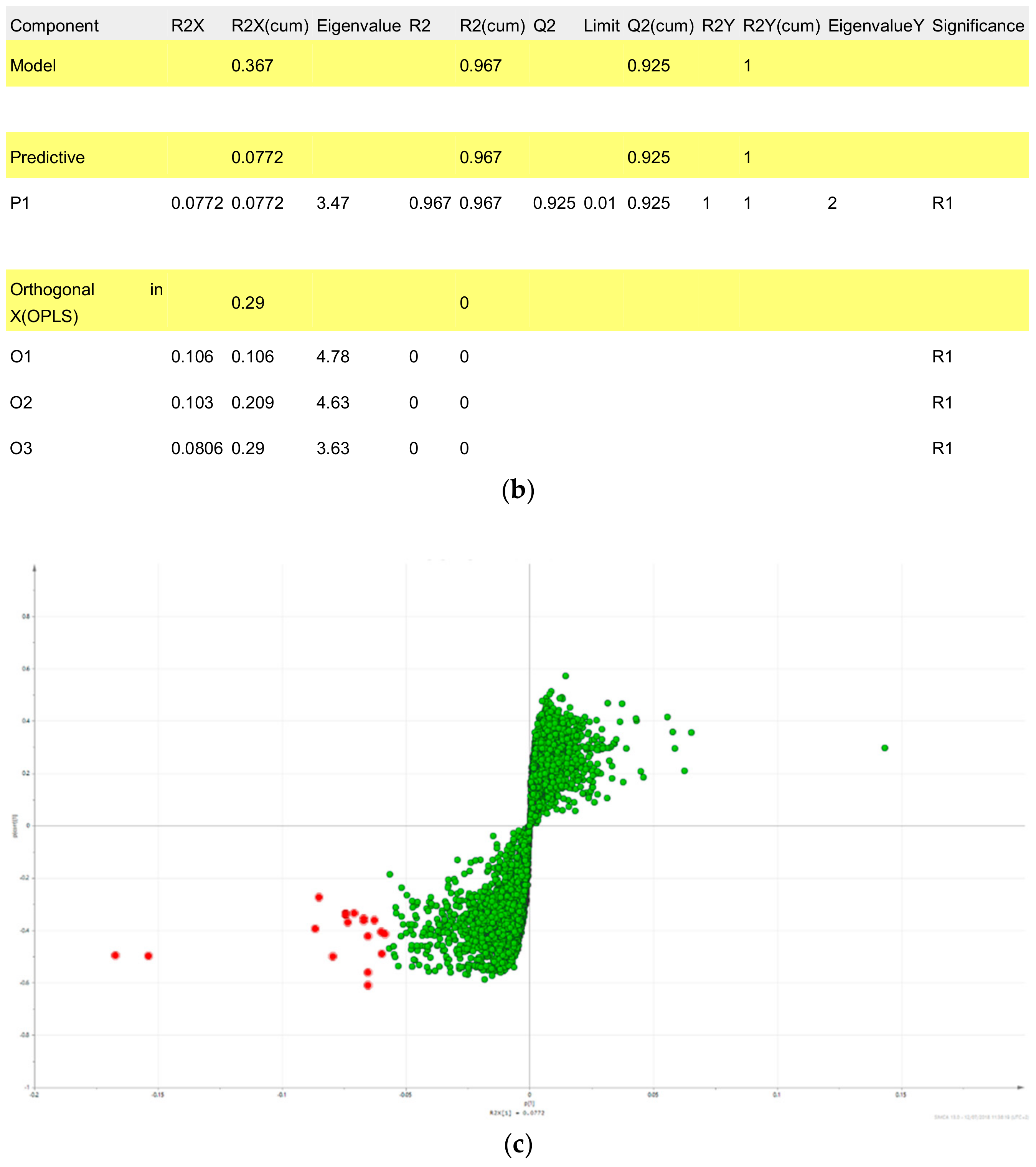
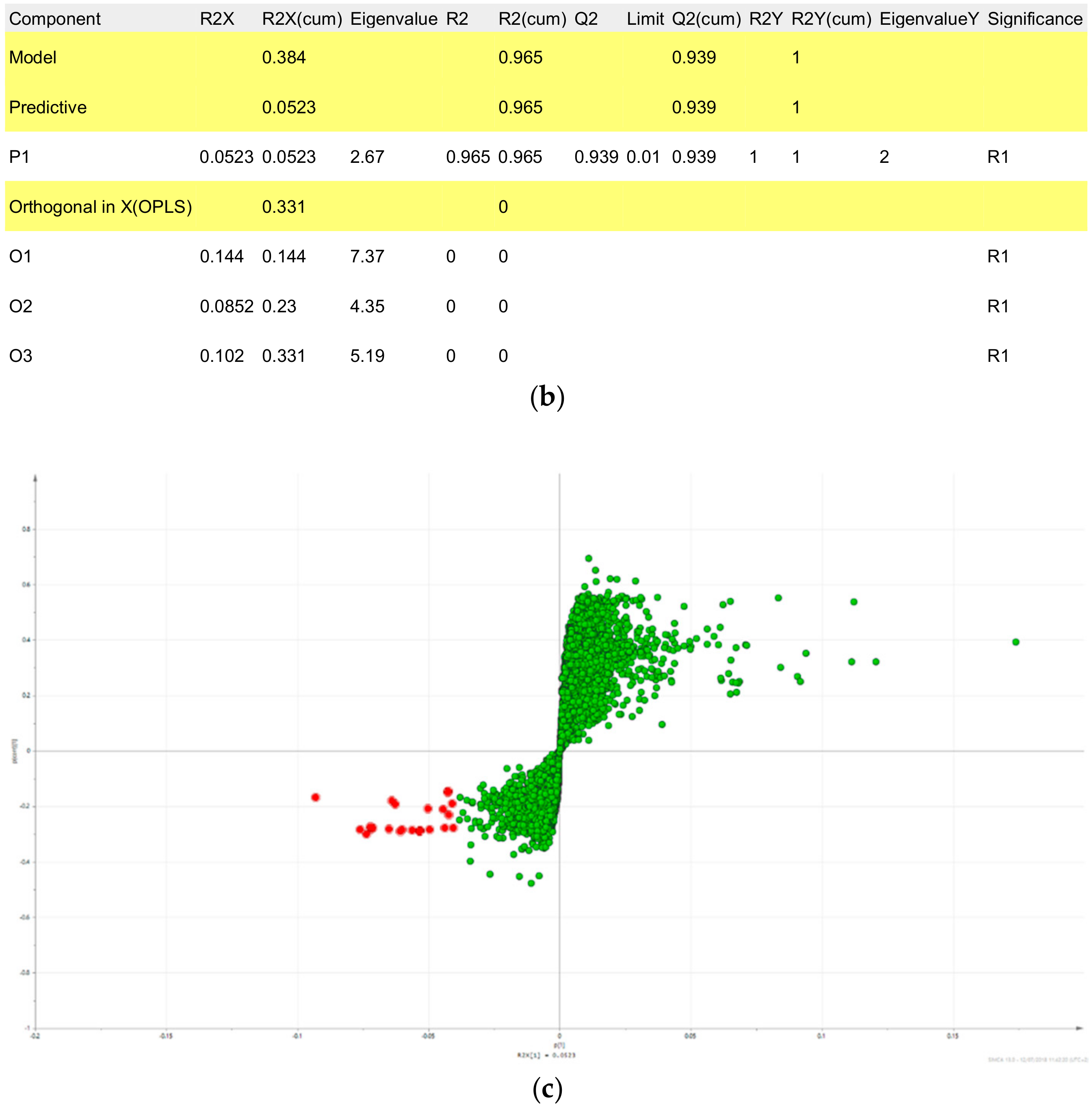
| m/z_RT | p[1] | po[1] | Origin | Compound | Fragment Ions | Literature |
|---|---|---|---|---|---|---|
| 285.07656_10.514 | −0.151342 | 0.247407 | RA | Calycosin * | 253, 225, 137 | [24,25] |
| 269.08165_13.771 | −0.135887 | 0.108774 | RA | Formononetin * | 269, 253, 237, 226, 197, 181, 169, 137, 118 | Metlin, [26] |
| 1737.96629_18.398 | −0.0831685 | 0.219044 | RA | Astragaloside I * (dimer) | 689, 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] |
| 1737.96335_19.162 | −0.0827036 | 0.216071 | RA | Astragaloside I isomer (dimer) | 689, 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] |
| 355.11759_16.953 | −0.0798714 | −0.0373342 | RRGP | n.i. | ||
| 301.10751_10.011 | −0.0767608 | 0.176659 | RA | 3-Hydroxy-9,10-dimethoxypterocarpan or isomer | 269, 191, 167, 152, 133, 147, 123, 105 | [25] |
| 371.10109_32.627 | −0.0708791 | 0.069922 | All | n.i. | ||
| 447.12852_6.654 | −0.0687443 | 0.132943 | RA | Calycosin-7-O-β-d-glucoside or isomer | 270, 253, 225, 137 | [24] |
| 355.06981_32.607 | −0.0640716 | 0.124033 | RA | n.i. | ||
| 714.41099_18.603 | −0.0632131 | −0.0573972 | RZ | n.i. | ||
| 411.22785_13.841 | −0.0617407 | −0.0464029 | RZ | n.i. | ||
| 508.25367_16.894 | −0.0592386 | −0.0425658 | RZ | n.i. | ||
| 438.23858_13.465 | −0.0591264 | −0.0471358 | RZ | n.i. | ||
| 447.12854_6.484 | −0.0589284 | 0.116497 | RA | Calycosin-7-O-β-d-glucoside or isomer | 270, 253, 225, 137 | [24] |
| 1737.96324_20.222 | −0.0571481 | 0.146308 | RA | Astragaloside I isomer (dimer) | 851, 833, 689, 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] |
| 1653.94391_15.879 | −0.0558838 | 0.154538 | RA | Astragaloside II or isomer (dimer) | 647, 629, 491, 473, 455, 437, 419 | [24,26] |
| 353.10204_16.488 | −0.0557885 | −0.0258396 | RRGP | n.i. | ||
| 471.34640_24.718 | −0.0542538 | −0.0348534 | RRGP | 18β-Glycyrrhetinic acid * | 453, 235, 217, 189, 175189 | Metlin |
| 301.10773_14.151 | −0.0535515 | 0.0891372 | RA | 3-Hydroxy-9,10-dimethoxypterocarpan or isomer | 269, 191, 167, 152, 147, 133, 123, 105 | [25] |
| 257.08161_9.822 | −0.0535154 | −0.0175027 | RRGP | Liquiritigenin * | 261, 215, 159, 147, 137, 119, 91, 81 |
| m/z RT | p[1] | po[1] | Origin | Compound | Fragment Ions | Literature |
|---|---|---|---|---|---|---|
| 285.07656_10.514 | −0.167255 | 0.258227 | RA | Calycosin * | 270, 253, 241, 229, 225, 137 | [24,25] |
| 269.08165_13.771 | −0.153853 | 0.218006 | RA | Formononetin * | 237, 225, 213, 209, 136 | Metlin, [24] |
| 355.11759_16.953 | −0.0865657 | −0.002337 | RRGP | n.i. | ||
| 714.41099_18.603 | −0.0851547 | −0.0579323 | RZ | n.i. | ||
| 301.10751_10.011 | −0.0794242 | 0.113695 | RA | 3-Hydroxy-9,10-dimethoxypterocarpan | 269, 191, 167, 152, 147, 133, 123, 105 | [25] |
| 1737.96335_19.162 | −0.0743328 | 0.178967 | RA | Astragaloside I isomer (dimer) | 851, 833, 689, 671, 653, 491, 473, 455, 437, 419 | [24,26] |
| 1737.96629_18.398 | −0.0743063 | 0.1784 | RA | Astragaloside I * (dimer) | 851, 833, 689, 671, 653, 491, 473, 455, 437, 419 | [24,26] |
| 301.10773_14.151 | −0.0733961 | 0.166495 | RA | 3-Hydroxy-9,10-dimethoxypterocarpan | 269, 191, 179, 167, 152, 133, 147, 123, 105 | [25] |
| 371.10109_32.627 | −0.0708126 | 0.0542131 | All | n.i. | ||
| 508.25367_16.894 | −0.0670202 | −0.0193137 | RZ | n.i. | ||
| 355.06981_32.607 | −0.0669833 | 0.135838 | RA | n.i. | ||
| 891.46950_19.156 | −0.0653422 | 0.0481451 | RA | Astragaloside I isomer | 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] |
| 257.08161_9.822 | −0.0652777 | −0.0002341 | RRGP | Liquiritigenin * | 261, 215, 159, 147, 137, 119 | |
| 891.47006_18.395 | −0.0652695 | 0.0662892 | RA | Astragaloside I * [M + Na]+ | 689, 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] |
| 447.12852_6.654 | −0.0627192 | −0.0564573 | RA | Calycosin-7-O-β-d-glucoside | 270, 253, 225, 137 | [24] |
| 353.10204_16.488 | −0.059812 | 0.0144688 | All | n.i. | ||
| 473.14387_12.038 | −0.0597473 | 0.0753214 | RA | n.i. | ||
| 315.08661_10.947 | −0.0583033 | 0.116079 | RA | Odoratin | 300, 283, 259, 255, 244, 167 | Metlin |
| m/z RT | p[1] | po[1] | Origin | Compound | Fragment Ions | Literature |
|---|---|---|---|---|---|---|
| 265.12345_8.988 | −0.0930574 | 0.175785 | FJ | n.i. | ||
| 481.17020_5.056 | −0.0759404 | −0.013529 | RP | Albiflorin * | 503 [M + Na], 197, 151, 133 | [22,23] |
| 415.21121_18.824 | −0.0736124 | 0.0616802 | FJ | n.i. | ||
| 503.15230_5.509 | −0.0722717 | −0.0109856 | RP | Paeoniflorin * [M + Na]+ | 498 [M + NH4], 179, 151, 133 | [22,23] |
| 503.15227_5.348 | −0.0719782 | −0.0112909 | RP | Paeoniflorin * [M + Na]+ | 498 [M + NH4], 179, 151, 133 | [22,23] |
| 498.19688_5.504 | −0.0712826 | −0.0135778 | RP | Paeoniflorin * [M + NH4]+ | 503 [M + Na], 179, 151, 133 | [22,23] |
| 481.17023_4.821 | −0.0650068 | −0.0111313 | RP | Albiflorin * | 503 [M + Na], 197, 151, 133 | [22,23] |
| 714.41099_18.603 | −0.0639971 | 0.0657522 | RZ | n.i. | ||
| 452.32137_22.770 | −0.0626265 | 0.0403574 | FJ | n.i. | ||
| 503.15215_5.060 | −0.0608256 | −0.0112778 | RP | Albiflorin * [M + Na]+ | 498 [M + NH4], 197, 151, 133 | [22,23] |
| 498.19685_5.348 | −0.0600451 | −0.0104305 | RP | Paeoniflorin * | 503 [M + Na], 179, 151, 133 | [22,23] |
| 503.15220_8.397 | −0.0562873 | −0.0109413 | RP | Albiflorin isomer | 219, 197, 133, 105 | [22,23] |
| 282.14928_9.203 | −0.0533495 | 0.0556512 | FJ | n.i. | ||
| 503.15228_4.813 | −0.0531328 | −0.0098537 | RP | Albiflorin * [M + Na]+ | 498 [M + NH4], 197, 151, 133 | [22,23] |
| 438.23858_13.465 | −0.0501564 | 0.0505945 | RZ | n.i. | ||
| 983.31365_5.058 | −0.0496619 | −0.0078355 | RP | Albiflorin * | 503 [M + Na], 481, 197, 151, 133, 105 | [22,23] |
| 379.24752_22.864 | −0.0443945 | 0.0688323 | FJ | n.i. | ||
| 464.24899_9.989 | −0.0438006 | −0.005821 | RP | Pinen-10-yl-ß-vicianoside | 133, 127, 115 | |
| 265.12281_9.203 | −0.0422487 | 0.010489 | RZ | n.i. | ||
| 983.31391_4.812 | −0.040604 | −0.0057421 | RP | Albiflorin * | 503 [M + Na], 481, 197, 151, 133, 105 | [22,23] |
| Compound | Chemical Structure | Predicted Activity | Verified Activity |
|---|---|---|---|
| Astragaloside I |  | TNF-α, IL-1β | TNF-α, IL-1β, IFN-γ |
| Astragaloside II | 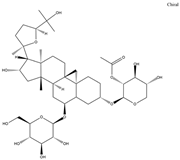 | TNF-α | TNF-α, IL-1β, IFN-γ |
| Calycosin | 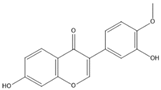 | TNF-α, IL-1β | IL-1β |
| Formononetin | 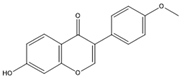 | TNF-α, IL-1β | TNF-α, IL-1β, IFN-γ |
| Liquiritigenin |  | TNF-α, IL-1β | TNF-α, IL-1β, IFN-γ |
| Albiflorin | 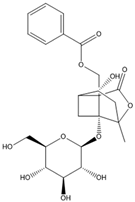 | IFN-γ | IL-1β, |
| Paeoniflorin |  | IFN-γ | IL-1β, IFN-γ |
| Isoliquiritigenin |  | IL-1β, IFN-γ |
References
- Bosma-den Boer, M.M.; van Wetten, M.-L.; Pruimboom, L. Chronic inflammatory diseases are stimulated by current lifestyle: How diet, stress levels and medication prevent our body from recovering. Nutr. Metab. 2012, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S. Chronic Diseases Caused by Chronic Inflammation Require Chronic Treatment: Anti-inflammatory Role of Dietary Spices. J. Clin. Cell Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Hou, J.P.; Jin, Y. The Healing Power of Chinese Herbs and Medicinal Recipes; Haworth Integrative Healing Pr: New York, NY, USA; London, UK; Oxford, UK, 2005; ISBN 978-0789022028. [Google Scholar]
- Nikles, S. Metabolic and Pharmacological Profiling of Three Classical TCM Formulations Used for Chronic Inflammatory Diseases with Immune Dysfunction. Ph.D. Dissertation, Karl-Franzens-Universität, Graz, Austria, 2016. [Google Scholar]
- Zhang, Z.; Luo, X. Synopsis of Prescriptions of the Golden Chamber with 300 Cases. A Classic of Traditional Chinese Medicine with Ancient and Contemporary Case Studies; New World Press: Beijing, China, 1995; ISBN 978-7800052910. [Google Scholar]
- Chen, K.-T.; Su, C.-H.; Hsin, L.-H.; Su, Y.-C.; Su, Y.-P.; Lin, J.-G. Reducing fatigue of athletes following oral administration of huangqi jianzhong tang. Acta Pharmacol. Sin. 2002, 23, 757–761. [Google Scholar] [PubMed]
- Nikles, S.; Monschein, M.; Zou, H.; Liu, Y.; He, X.; Fan, D.; Lu, A.; Yu, K.; Isaac, G.; Bauer, R. Metabolic profiling of the traditional Chinese medicine formulation Yu Ping Feng San for the identification of constituents relevant for effects on expression of TNF-α, IFN-γ, IL-1β and IL-4 in U937 cells. J. Pharm. Biomed. Anal. 2017, 145, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Y.; Pan, C.-S.; Liu, Y.-Y.; Wei, X.-H.; Zhou, C.-M.; Sun, K.; He, K.; Li, C.; Yan, L.; Fan, J.-Y.; et al. Huang Qi Jian Zhong pellet attenuates TNBS-induced colitis in rats via mechanisms involving improvement of energy metabolism. Evidence-Based. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, L.X.; Yin, S.J.; An, J.; Wei, Q.; Yang, J.X. Huangqi Jianzhong Tang for Treatment of Chronic Gastritis: A Systematic Review of Randomized Clinical Trials. Evid. Based Complement. Alternat. Med. eCAM 2015, 878164. [Google Scholar] [CrossRef]
- Chung, K.F. Cytokines. Asthma and COPD; Elsevier: Amsterdam, The Netherlands, 2009; pp. 327–341. ISBN 9780123740014. [Google Scholar]
- Udalova, I.; Monaco, C.; Nanchahal, J.; Feldmann, M. Anti-TNF Therapy. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Yuliana, N.D.; Khatib, A.; Choi, Y.H.; Verpoorte, R. Metabolomics for bioactivity assessment of natural products. Phytother. Res. 2011, 25, 157–169. [Google Scholar] [CrossRef]
- Commisso, M.; Strazzer, P.; Toffali, K.; Stocchero, M.; Guzzo, F. Untargeted metabolomics: An emerging approach to determine the composition of herbal products. Comput. Struct. Biotechnol. J. 2013, 4, e201301007. [Google Scholar] [CrossRef]
- Alonso, A.; Marsal, S.; Julià, A. Analytical methods in untargeted metabolomics: State of the art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts: Editors’ Choice IX. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Khan, I.A.; Combrinck, S.; Sandasi, M.; Chen, W.; Viljoen, A.M. 1H-NMR and UPLC-MS metabolomics: Functional tools for exploring chemotypic variation in Sceletium tortuosum from two provinces in South Africa. Phytochemistry 2018, 152, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.M.; Zhao, J.; Sandasi, M.; Chen, W.; Khan, I.A. Phytochemical distinction between Pelargonium sidoides (“Umckaloabo”) and P. reniforme through 1H-NMR and UHPLC–MS metabolomic profiling. Metabolomics 2015, 11, 594–602. [Google Scholar] [CrossRef]
- Afzan, A.; Kasim, N.; Ismail, N.H.; Azmi, N.; Ali, A.M.; Mat, N.; Wolfender, J.-L. Differentiation of Ficus deltoidea varieties and chemical marker determination by UHPLC-TOFMS metabolomics for establishing quality control criteria of this popular Malaysian medicinal herb. Metabolomics 2019, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Taketa, A.T.; Pereda-Miranda, R.; Choi, Y.H.; Verpoorte, R.; Villarreal, M.L. Metabolic profiling of the Mexican anxiolytic and sedative plant Galphimia glauca using nuclear magnetic resonance spectroscopy and multivariate data analysis. Planta Med. 2008, 74, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.; Pereira, F.; Grilo, I.R.; Godinho, C.C.; Paulino, M.; Almeida, V.; Gobbo-Neto, L.; Prieto-Davo, A.; Sobral, R.G.; Lopes, N.P.; et al. Intra-clade metabolomic profiling of MAR4 Streptomyces from the Macaronesia Atlantic region reveals a source of anti-biofilm metabolites. Environ. Microbiol. 2019, 21, 1099–1112. [Google Scholar] [CrossRef]
- Gong, C.; Yang, H.; Wei, H.; Qi, C.; Wang, C.-H. Pharmacokinetic comparisons by UPLC-MS/MS of isomer paeoniflorin and albiflorin after oral administration decoctions of single-herb Radix Paeoniae Alba and Zengmian Yiliu prescription to rats. Biomed. Chromatogr. 2015, 29, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wan, M.; Zhou, D.; Gao, J.; Zhu, Y.; Bi, K. LC-MS/MS determination and pharmacokinetic study of albiflorin and paeoniflorin in rat plasma after oral administration of Radix Paeoniae Alba extract and Tang-Min-Ling-Wan. Biomed. Chromatogr. 2010, 24, 1324–1331. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Song, F.; Liu, Z.; Liu, S. Studies on principal components and antioxidant activity of different Radix Astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta 2009, 78, 1090–1101. [Google Scholar] [CrossRef]
- Xiao, H.B.; Krucker, M.; Albert, K.; Liang, X.M. Determination and identification of isoflavonoids in Radix astragali by matrix solid-phase dispersion extraction and high-performance liquid chromatography with photodiode array and mass spectrometric detection. J. Chromatogr. A 2004, 1032, 117–124. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, H.; Shi, Z.; Feng, Y.; Rui, W. Rapid and reliable method for analysis of raw and honey-processed astragalus by UPLC/ESI-Q-TOF-MS using HSS T3 columns. Anal. Methods 2014, 6, 8045–8054. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. TNF-Alpha as a Therapeutic Target in Inflammatory Diseases, Ischemia- Reperfusion Injury and Trauma. CMC 2009, 16, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, R.; ten Hagen, T.L.M.; Eggermont, A.M.M. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.-I.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-α: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, F.; Beutler, B. The Tumor Necrosis Factor Ligand and Receptor Families. N. Engl. J. Med. 1996, 334, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Akdis, M.; Burgler, S.; Crameri, R.; Eiwegger, T.; Fujita, H.; Gomez, E.; Klunker, S.; Meyer, N.; O’Mahony, L.; Palomares, O.; et al. Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011, 127, 701–721. [Google Scholar] [CrossRef]
- Payne, S. Chapter 6-Immunity and Resistance to Viruses. In Viruses; Payne, S., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 61–71. ISBN 978-0-12-803109-4. [Google Scholar]
- The Autoimmune Diseases, 5th ed.; Rose, N.R., Mackay, I.R., Eds.; Academic Press: Boston, MA, USA, 2014; ISBN 978-0-12-384929-8. [Google Scholar]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Zhang, J. Yin and yang interplay of IFN-γ in inflammation and autoimmune disease. J. Clin. Investig. 2007, 117, 871–873. [Google Scholar] [CrossRef]
- Sang, A.; Yin, Y.; Zheng, Y.-Y.; Morel, L. Animal Models of Molecular Pathology: Systemic Lupus Erythematosus. In Progress in Molecular Biology and Translational Science: Animal Models of Molecular Pathology; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 321–370. ISBN 1877-1173. [Google Scholar]
- Lin, F.-C.; Young, H.A. The talented interferon-gamma. ABB 2013, 4, 6–13. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, H.-Y.; Xu, L.; Wang, S.-S.; Zhang, X.-M. In vivo and in vitro immunomodulatory and anti-inflammatory effects of total flavonoids of astragalus. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Huang, Q.; Chen, J.; Wang, M.; Pan, H.; Wang, R.; Zhou, H.; Zhou, Z.; Liu, J.; Yang, F.; et al. Calycosin suppresses expression of pro-inflammatory cytokines via the activation of p62/Nrf2-linked heme oxygenase 1 in rheumatoid arthritis synovial fibroblasts. Pharmacol. Res. 2016, 113, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Lee, Y.M.; Koh, Y.-S.; Kim, Y.H. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharmacal. Res. 2014, 37, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Yesilada, E.; Bedir, E.; Çalış, İ.; Takaishi, Y.; Ohmoto, Y. Effects of triterpene saponins from Astragalus species on in vitro cytokine release. J. Ethnopharmacol. 2005, 96, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Y.; Ha, Y.J.; Kim, K.-M.; Jung, Y.-S.; Jung, J.-C.; Oh, S. Anti-Inflammatory Activities of Licorice Extract and Its Active Compounds, Glycyrrhizic Acid, Liquiritin and Liquiritigenin, in BV2 Cells and Mice Liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Zhao, R.J.; Park, S.J.; Lee, J.R.; Cho, I.J.; Yang, C.H.; Kim, S.G.; Kim, S.C. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharm. 2008, 154, 165–173. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zhang, Y.; Wang, Y.; Zhu, D.X.; Xu, L.Q.; Fang, H.; Wu, W. The Beneficial Effect of Total Glucosides of Paeony on Psoriatic Arthritis Links to Circulating Tregs and Th1 Cell Function. Phytother. Res. 2014, 28, 372–381. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, R.; Zhou, F.; Cheng, H.; Xia, B. Total Glucosides of Peony Attenuates 2,4,6-Trinitrobenzene Sulfonic Acid/Ethanol-Induced Colitis in Rats Through Adjustment of Th1/Th2 Cytokines Polarization. Cell Biochem. Biophys. 2014, 68, 83–95. [Google Scholar] [CrossRef]
- Chang, Y.; Wei, W.; Zhang, L.; Xu, H.-M. Effects and mechanisms of total glucosides of paeony on synoviocytes activities in rat collagen-induced arthritis. J. Ethnopharmacol. 2009, 121, 43–48. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Wei, W. Total glucosides of paeony suppresses adjuvant arthritis in rats and intervenes cytokine-signaling between different types of synoviocytes. Int. Immunopharmacol. 2005, 5, 1560–1573. [Google Scholar] [CrossRef]
- Bi, X.; Han, L.; Qu, T.; Mu, Y.; Guan, P.; Qu, X.; Wang, Z.; Huang, X. Anti-Inflammatory Effects, SAR, and Action Mechanism of Monoterpenoids from Radix Paeoniae Alba on LPS-Stimulated RAW 264.7 Cells. Molecules 2017, 22, 715. [Google Scholar] [CrossRef]
Sample Availability: All cited extracts and samples of the compounds formononetin, calycosin, astragaloside I, astragaloside II, liquiritigenin, isoliquiritigenin, 18ß-glycyrrhetinic acid, paeoniflorin and albiflorin are available from the authors. |


| Activity Class | TNF-α Inhibition (%) | IL-1β Inhibition (%) | IFN-γ Inhibition (%) |
|---|---|---|---|
| 1 (active) | 70–100 | 60–100 | 60–100 |
| 2 (moderately active) | 50–69 | 45–59 | 45–59 |
| 3 (inactive) | 0–49 | 0–44 | 0–44 |
| RT (min) | Detected m/z (ESI+) | (Tentative) Identification | Origin | Mono-Isotopic Mass (g/mol) | Molecular Formula | MS/MS Fragment Ions | Literature for Identification | Pharmacological Activity Predicted by OPLS-DA | p[1] | po[1] |
|---|---|---|---|---|---|---|---|---|---|---|
| 4.82 | 481.17023 | Albiflorin * | RP | 480.163 | C23H28O11 | 197, 151, 133 | [22,23] | IFN-γ | −0.0650068 | −0.0111313 |
| 5.34 | 503.15227 | Paeoniflorin * (Na-Adduct) | RP | 480.163 | C23H28O11 | 179, 151, 133 | [22,23] | IFN-γ | −0.0719782 | −0.0112909 |
| 6.48 | 447.1285 | Calycosin-7-O-β-d-glucoside or isomer | RA | 446.121 | C22H22O10 | 270, 253, 225, 137 | [24] | TNF-α | −0.0687443 | 0.132943 |
| 8.39 | 503.1522 | Albiflorin isomer (Na-Adduct) | RP | 480.163 | C23H28O11 | 219, 197, 133, 105 | [22,23] | IFN-γ | −0.0562873 | −0.0109413 |
| 9.82 | 257.08161 | Liquiritigenin * | RRGP | 256.074 | C15H12O4 | 137, 147, 119, 261, 91, 81, 215, 159 | TNF-α IL-1β | −0.0535154 −0.0652777 | −0.0175027 −0.0002341 | |
| 9.98 | 464.2489 | Pinen-10-yl-ß-vicianoside (NH4-Adduct) | RP | 446.215 | C21H34O10 | 133, 127, 115 | IFN-γ | −0.0438006 | −0.005821 | |
| 10.01 | 301.1075 | 3-Hydroxy-9,10-dimethoxypterocarpan or isomer | RA | 300.1 | C17H16O5 | 269, 191, 167, 152, 147, 133, 123, 105 | [25] | TNF-α IL-1β | −0.0767608 −0.0794242 | 0.176659 0.113695 |
| 10.51 | 285.07656 | Calycosin * | RA | 284.068 | C16H12O5 | 270, 253, 225, 229, 241, 137 | [24,25] | TNF-α, IL-1β | −0.151342 −0.167255 | 0.247407 0.258227 |
| 10.94 | 315.0866 | Odoratin | RA | 314.079 | C17H14O6 | 300, 283, 259, 255, 244, 167 | Metlin | IL-1β | −0.0583033 | 0.116079 |
| 13.77 | 269.08165 | Formononetin * | RA | 268.074 | C16H12O4 | 237, 209, 213, 225, 136 | Metlin, [24] | TNF-α IL-1β | −0.135887 −0.153853 | 0.108774 0.218006 |
| 14.15 | 301.1077 | 3-Hydroxy-9,10-dimethoxypterocarpan or isomer | RA | 300.1 | C17H16O5 | 269, 191, 167, 152, 147, 133, 123, 105 | [25] | TNF-α IL-1β | −0.0535515 −0.0733961 | 0.0891372 0.166495 |
| 15.87 | 1653.9439 | Astragaloside II (dimer) | RA | 798.44 | C41H66O15 | 647, 629, 491, 473, 455, 437, 419 | [24,26] | TNF-α | −0.0558838 | 0.154538 |
| 18.39 | 1737.9663 | Astragaloside I * (dimer) | RA | 868.482 | C45H72O16 | 689, 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] | TNF-α IL-1β | −0.0831685 −0.0743063 | 0.219044 0.1784 |
| 19.15 | 1737.9633 | Astragaloside I isomer (dimer) | RA | 868.482 | C45H72O16 | 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] | TNF-α IL-1β | −0.0827036 −0.0743328 | 0.216071 0.178967 |
| 20.22 | 1737.9632 | Astragaloside I isomer (dimer) | RA | 868.482 | C45H72O16 | 851, 833, 689, 671, 653, 491, 473, 455, 437, 419, 297 | [24,26] | TNF-α | −0.0571481 | 0.146308 |
| 24.71 | 471.3464 | 18β-Glycyrrhetinic acid * | RRGP | 470.34 | C30H46O4 | 453, 235, 217, 189, 175, 189 | Metlin | TNF-α | −0.0542538 | −0.0348534 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nöst, X.; Pferschy-Wenzig, E.-M.; Nikles, S.; He, X.; Fan, D.; Lu, A.; Yuk, J.; Yu, K.; Isaac, G.; Bauer, R. Identification of Constituents Affecting the Secretion of Pro-Inflammatory Cytokines in LPS-Induced U937 Cells by UHPLC-HRMS-Based Metabolic Profiling of the Traditional Chinese Medicine Formulation Huangqi Jianzhong Tang. Molecules 2019, 24, 3116. https://doi.org/10.3390/molecules24173116
Nöst X, Pferschy-Wenzig E-M, Nikles S, He X, Fan D, Lu A, Yuk J, Yu K, Isaac G, Bauer R. Identification of Constituents Affecting the Secretion of Pro-Inflammatory Cytokines in LPS-Induced U937 Cells by UHPLC-HRMS-Based Metabolic Profiling of the Traditional Chinese Medicine Formulation Huangqi Jianzhong Tang. Molecules. 2019; 24(17):3116. https://doi.org/10.3390/molecules24173116
Chicago/Turabian StyleNöst, Xuehong, Eva-Maria Pferschy-Wenzig, Stefanie Nikles, Xiaojuan He, Danping Fan, Aiping Lu, Jimmy Yuk, Kate Yu, Giorgis Isaac, and Rudolf Bauer. 2019. "Identification of Constituents Affecting the Secretion of Pro-Inflammatory Cytokines in LPS-Induced U937 Cells by UHPLC-HRMS-Based Metabolic Profiling of the Traditional Chinese Medicine Formulation Huangqi Jianzhong Tang" Molecules 24, no. 17: 3116. https://doi.org/10.3390/molecules24173116
APA StyleNöst, X., Pferschy-Wenzig, E.-M., Nikles, S., He, X., Fan, D., Lu, A., Yuk, J., Yu, K., Isaac, G., & Bauer, R. (2019). Identification of Constituents Affecting the Secretion of Pro-Inflammatory Cytokines in LPS-Induced U937 Cells by UHPLC-HRMS-Based Metabolic Profiling of the Traditional Chinese Medicine Formulation Huangqi Jianzhong Tang. Molecules, 24(17), 3116. https://doi.org/10.3390/molecules24173116






