Abstract
The reactions of dialkyl acetylenedicarboxylates with various 2-oxo-acenaphthoquinylidene- and 4-acetyl[2.2]paracyclophanylidene-thiosemicarbazones were investigated. Using simple experimental procedures, 1,3-Thiazolidin-4-ones derived from acenaphthequinone or [2.2]paracyclophane were obtained as major products in good yields. In the case of allyl derivative of acenaphthoquinylidene-thiosemicarbazones, a complex structure of tetramethyl 5-(2-(((Z,E)-N-allyl-N′-(2-oxoacenaphthylen-1(2H)-ylidene)carbamohydrazonoyl)thio)-1,2,3-tris-(methoxycarbonyl)-cyclopropyl)-4-methoxy-7-oxabicyclo[2.2.1]hepta-2,5-diene-1,2,3,6-tetracarboxylate was formed. Single crystal X-ray analysis was used as an efficient tool to confirm the structure of the synthesized compounds as well as different spectroscopic data (1H-NMR, 13C-NMR, 2D-NMR, mass spectrometry and elemental analysis). The mechanism of the obtained products was discussed.
1. Introduction
Of interest due to their high biological activities, 1,3-Thiazolidin-4-one derivatives are very important core structures of many heterocyclic compounds. Compounds with a thiazolidinone structure have been reported as anti-inflammatory and analgesic [1], antioxidants [2], antitubercular [1,3], antimicrobial [2,4] and antifungal [4], antiviral (especially as anti-HIV agents [5]), anticancer [1], anticonvulsants [6], and antidiabetic agents [6,7]. Recently, various synthetic routes were used for the synthesis of different thiazolidinones from several thiosemicarbazides and thiocarbohydrazides by using different reagents, and their biological evaluation was also discussed [8,9]. Thiosemicarbazones were used as a starting material for the synthesis of different heterocyclic compounds, i.e., naphtho-thiazole and naphtho-thiadiazepines [10], 4-thiazolidinone derivatives [11,12], 1,3,4-thiadiazoles [13] which possess high biological activities, e.g., anticancer [14], antioxidants [15], antifungal [16], and antibacterial [17].
Acenaphthequinone (acenaphthylene-1,2-dione) is a quinone derivative, widely used as significant starting material for the synthesis of various heterocycles, and as a key intermediate in organic synthesis for different reactions and pharmaceutical applications [18,19,20,21]. Some derivatives of acenaphthequinone have been used as biological active compounds, dyes, pharmaceuticals, drugs, pesticides, and therapeutic agents [22,23,24]. Acenaphthylene-1,2-dione is one of the important reagents for constructing new heterocycles, e.g., spirooxadiazoline [25], spiroacenaphthyl-3,4′-pyranopyrazoles [26], fused acenaphthopyrrolizines and spiroacenaphthene pyrrole [27], dispiropyrrolidines [28,29], and polycyclic heterocycles [29,30] via reactions such as the Huisgen reaction, 1,3-dipolar cycloaddition, and [3+2]cycloaddition reactions. One pot synthesis of 2-oxo-1,2-dihydroacenaphthylphosphonates was obtained by using a Knoevenagel-phospha-Michael reaction of acenaphthequinone, malononitrile, and diethylphosphite catalyzed by a 1,4-diazabicyclo-[2.2.2]octane (DABCO)-based ionic liquid [31].
Recently, three component reactions were widely used as one of the most efficient routes for the synthesis of many heterocyclic rings. Consequently, three component reactions of acenaphthequinone, proline derivatives and dialkyl acetylenedicarboxylates gave azocinobenzoisoquinolone-dicarboxylates through [3+2]cycloaddition of azomethine ylides followed by ring expansion [32]. In addition, the domino three component reaction of isoquinolinium salts, acenaphthequinone and indanedione in basic media yielded 2′-acenaphthylidenespiro[indane-2,1′-pyrrolo[2,1-a]isoquinolones] [33]. Using a similar synthetic strategy, bis-spiro-oxoacenaphthalenes were obtained via the reaction of acenaphthalene-1,2-dione, active alkynes, and 4-cyanopyridine in 1,2-dimethoxyethane (DME) at room temperature [34]. The reaction of dialkyl acetylenedicarboxylate with acenaphthalenedione and CS2 in t-butylphosphine afforded oxoindolin-3-ylidene-1,3-dithioles [35]. Also, spirodihydropyridine derivatives were obtained in high yields from the reaction of acenaphthylidene malononitrile and Zwitter ionic intermediate (generated from primary amine and dimethyl acetylenedicarboxylate (DMAD) [36].
Since the maturation of the synthetic methodologies, with the possibilities for the fine-tuning of structural and functional properties, [2.2]paracyclophane (PC) chemistry has evolved—from functional molecules to functional materials [37]—and is rapidly growing from a structural and synthetic curiosity to a prevalent scaffold in asymmetric synthesis [37], π-stacked polymers, energy materials, and functional perylene coatings that find broad applications in bio-material science and as natural products [38,39,40]. Aly et al. prepared various heterocycles derived from [2.2]paracyclophane such as a five-membered ring (i.e., imidazolone and pyrrole [41,42,43,44] and six-membered ring (i.e., pyridine [45]). Additionally, we reported on the synthesis of various paracyclophanyl-substituted thiosemicarbazones, thiocarbazones and thioureas then studied their complexation towards tridentate and bidentate copper complexes [46]. Inspired by the aforementioned, we aim here to synthesize various thiazolidinone derivatives of both acenaphthequinone and [2.2]paracyclophane.
2. Results and Discussion
Acenaphthequinones conjugated with NH, SH, CH=N and NH2 groups are of considerable interest in organic synthesis and were produced from the condensation of thiosemicarbazide and 4-substituted thiosemicarbazides with acenaphthylene-1,2-dione [47]. A literature survey revealed, that acenaphthequinone (1) was reacted with thiosemicarbazide 2a either in ethanol at room temperature and catalyzed by acidic ionic liquid furnished spiro-1,2,4-triazolidine-3-thiones 3a [48] or in refluxing chloroform in the presence of glacial acetic acid (AcOH) yielded acenaphtho[1,2-e]-1,2,4-triazine-9(8H)-thione 4 (Scheme 1) [49].
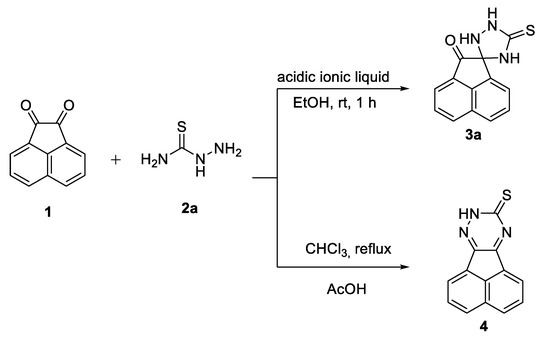
Scheme 1.
Previous work on acenaphthalene-1,2-dione 1 with thiosemicarbazide 2a.
2.1. Preparation of Compounds 5a–e
It was obvious that the reaction conditions, e.g., solvent, temperature and/or catalyst play a vital role on the reaction efficiency and product obtained. Various conditions were investigated and the most efficient method was the condensation of thiosemicarbazide derivatives with acenaphthalene-1,2-dione in refluxing ethanol in the presence of Et3N to give (Z)-N-unsubstituted/substituted-2-(2-oxoacenaphthylen-1(2H)-ylidene)hydrazinecarbothioamide derivatives (Scheme 2) instead of the aforementioned spiro-1,2,4-triazolidine-3-thione (3a) [48] and 1,2,4-triazine-3-thione 4 [49]. Although (Z)-2-(2-oxoacenaphthylen-1(2H)-ylidene)hydrazinecarbothioamide 5a was previously reported [50], its structure was confirmed herein using single crystal X-ray analysis (Figure 1).
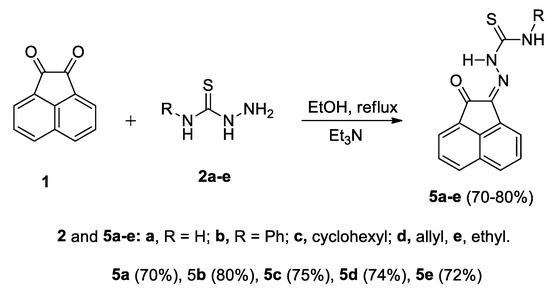
Scheme 2.
Reactions of acenaphthoqinone 1 with thiosemicarbazides 2a–e.
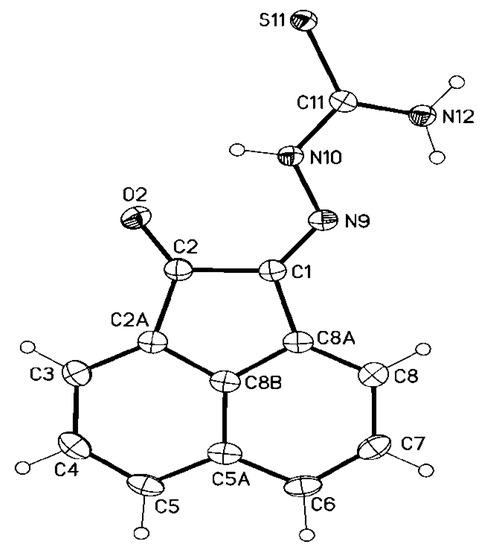
Figure 1.
Molecular structure of one of the two crystallographic independent molecules of 5a (displacement parameters are drawn at 50% probability level).
2.2. Reaction of Compounds 5a–e with Dimethyl Acetylenedicarboxylate (DMAD, 6)
A mixture of dimethyl acetylenedicarboxylate (DMAD, 6a) and (Z)-N-unsubstituted/substituted-2-(2-oxoacenaphthylen-1-(2H)-ylidene)thiosemicarbazones 5a–e, was refluxed in absolute EtOH for 20–30 min. The reaction revealed the complete disappearance of compounds 5a–e and 6a to give compounds 7a–e in 70–76% yields (Scheme 3). Surprisingly, only in the case of allyl derivative 5d, an unexpected compound 8d was obtained in 10% yield (Scheme 3). Different spectroscopic data were insufficient to determine completely the correct structure of this compound.
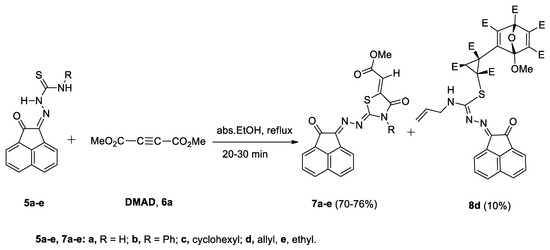
Scheme 3.
Reaction of 2-oxo-acenapthoquinylidene-thiosemicarbazones 5a–e with DMAD, 6a.
Different nucleophilic sites on thiosemicarbazone derivatives 5a–e were expected to participate in the reaction and formation of the product. Nucleophilic attack of lone pair of electrons of the sulfur atom in the thione group in 5a–e to the acetylenic carbon in 6a would give the Zwitter ions 9a–e. Neutralization of 9a–e would give the intermediate 10a–e. Subsequently another nucleophilic attack from a lone pair of electrons of 2NH or 4NH on CO2Me; would yield the corresponding 4-thiazolidinones 7a–e and/or 12a–e via the loss of methanol (Scheme 4). The 1H and 13C-NMR spectral data (see details in Supplementary Materials) were used as an important tool for excluding some alternative structures. Furthermore, in the case of 7a–e and 12a–e, X-ray analyses were used as a useful tool to determine the correct structure and to prove that the 1,3-thiazolidine-4-ones 7a–e were formed rather than 12a–e.
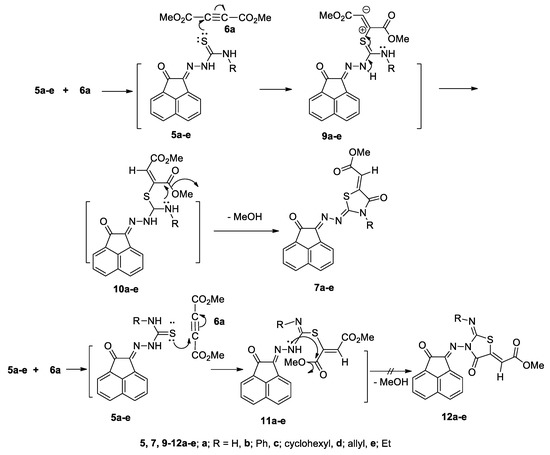
Scheme 4.
The mechanism describes the formation of compounds 7a–e and 12a–e.
For example, the IR spectrum of 7a showed absorption bands at ν = 3489 cm−1 for the NH group, 1715, 1696 cm−1 for C=O as well as 1623 and 1609 cm−1 for C=N and Ar-C=C groups. The 1H-NMR spectrum of 7a clearly proved that there are two singlets one at δ = 3.81 ppm with 3H integral corresponding to one methoxy group, the other at δ = 6.73 ppm with 1H integral relating to vinyl-CH group. Acenaphthequinone protons resonated at δ = 7.85–8.35 ppm due to six aromatic protons. A broad NH group also resonated as a singlet at 13.46 ppm. Further, 13C-NMR of 7a showed further signals at δ = 52.49, 115.27, 142.07, (151.66 and 154.23), 165.78, 166.01 and 183.77 corresponding to OCH3, -CH=, C5-thiazole, (C2-thiazole and C=N), cyclic-C=O, CO2Me, C=O. The 2D-NMR further exhibited different correlations between C, H, N (Table 1 and Figure 2). The elemental analysis of 7a suggests that it has C18H11N3O4S formula. This was further investigated from mass spectrum giving a molecular ion at m/z = 365 (10%).

Table 1.
NMR spectroscopic data of 7a.
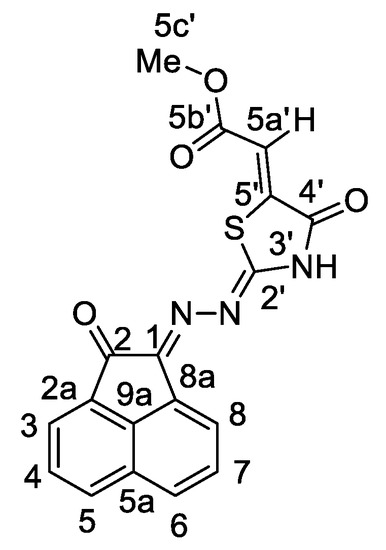
Figure 2.
The distinctive carbons in compound 7a.
Additionally, the structure of (Z)-methyl 2-((Z)-3-allyl-4-oxo-2-((E)-(2-oxoacenaphthylen-1(2H)-ylidene)hydrazono)thiazolidin-5-ylidene)acetate (7d) has been unambiguously confirmed via a single-crystal X-ray structure analysis (Figure 3). The characteristic properties of compound 7d are that the C2-N13 and C15-N14, double bonds (note that the crystallographic numbering does not correspond to the systematic IUPAC numbering rules) exhibit bond lengths of 1.2912 (16) Å and 1.2892 (16) Å, respectively, that are a little shorter than C = N δ bonds due to high resonance in 7d. The dihedral angle of N19-C15-N14-N13 is 178.82 (9)°, showing that it has a trans configuration relating to N19. Bond lengths C1-C2 and N13-N14 are 1.5345 (16) and 1.3950 (14), respectively, which are shorter than the corresponding C-C and N-N single bonds due to high resonance in this compound. From X-ray analysis, it was also observed that the thiazolidine C = O has a cisoid geometry regarding to vinyl CH [torsion angle O18–C18–C17–C23 −2.2 (2)°].
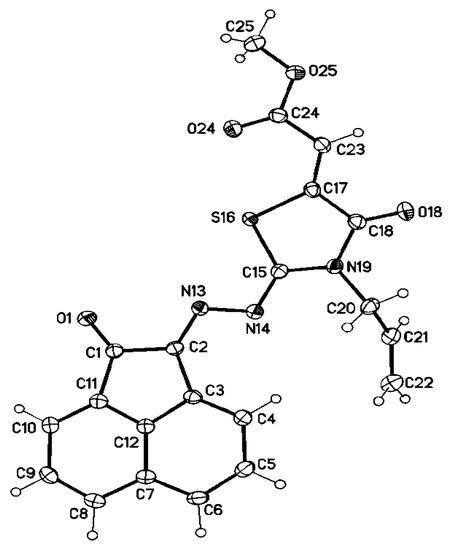
Figure 3.
Molecular structure of 7d (displacement parameters are drawn at 50% probability level).
A literature survey revealed that, the tetramerization reaction is one of the most fascinating reactions of both dialkyl acetylenedicarboxylic acid derivatives occurring at 25 °C for several days or by heating it alone or in solution for several hours [51,52,53]. Furthermore, trimer [54] and dimer [55] forms were reported for compound 6a. Recently, Huang et al. [56] have reported the reaction of 2,2′-bis(azaphosphindole) with four equivalents of 6a in THF at room temperature to afford a tetramer complex of 6a with 2,2′-bis(azaphosphindole).
The structure of 8d was resolved by using single crystal X-ray analysis and it was found that it is tetramethyl 5-(2-(((Z,E)-N-allyl-N′-(2-oxoacenaphthylen-1(2H)-ylidene)carbamo-hydrazonoyl)-thio)-1,2,3-tris(methoxycarbonyl)cyclopropyl)-4-methoxy-7-oxabicyclo-[2.2.1]hepta-2,5-diene-1,2,3,6-tetracarboxylate (8d) (Figure 4). To our best knowledge, it would be the first X-ray structure of that tetramer of 6a. It is interesting to note that only one diastereomer was isolated (at least being isolated).
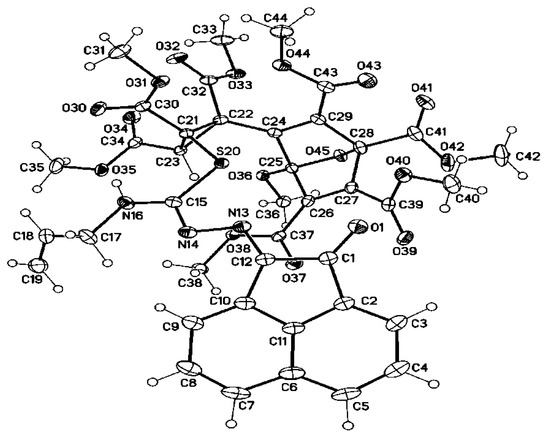
Figure 4.
Molecular structure of one of the two crystallographic independent molecules of 8d (displacement parameters are drawn at 30% probability level).
The unusual reactivity of 6a towards allyl derivative of thiosemicarbazones 5d obviously involves four molecules of 6 reacting with one molecule of 5d. The mechanism presumably begins via dimerization of 6a (acting as dienophile and as 1,3-dipole) to afford 13, which then rearranged to give 14. Addition of the third molecule of 6a to the intermediate 14 would give the intermediate 15, which would be neutralized to give cyclopropene 16 (Scheme 5). Diels-Alder reaction of 16 and the fourth molecule of 6a, yield the complex 17 [56] (Scheme 5). Subsequently, a nucleophilic attack of thione-lone pair of thiosemicarbazone 5d to the double bond of the cyclopropene ring would give the Zwitter ion 18, which would be neutralized to give compound 8d (Scheme 5).
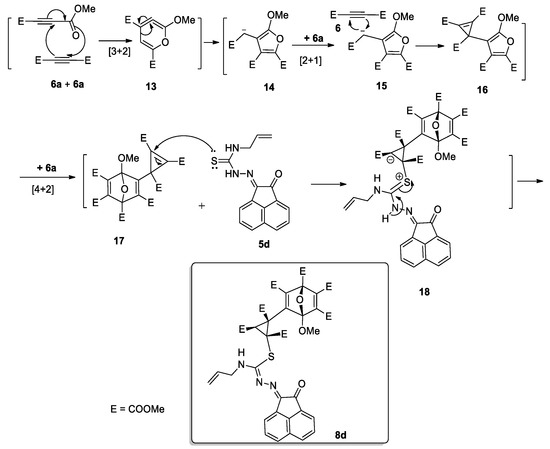
Scheme 5.
Plausible mechanism for the synthesis of compound 8d.
The formation of a complex structure may be further used as a Supplementary tool for the formation of 1,3-thiazolidinone derivative 7d, which might be attributed to the instability of 8d during the course of a reaction. It can be also suggested that another nucleophilic attack of NH lone-pair of compound 8d to one of the carbonyl ester group of the cyclopropane ring afforded the intermediate 19 that would be rearranged giving the 4-thiazolidinone 7d and 20 (Scheme 6). However, compound 20 was unfortunately not isolated.
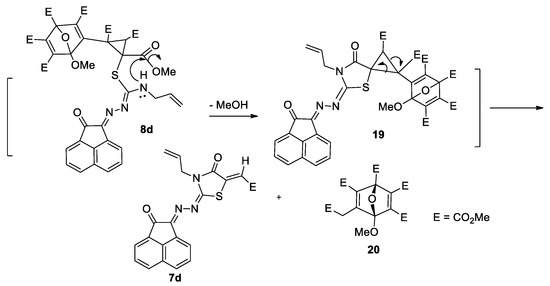
Scheme 6.
Formation of thiazolidinone 7d from compound 8d.
On the other hand, the synthesis of 4-acetyl[2.2]paracyclophanylidene thiosemicarbazone (22a) was established from the reaction between 4-acetyl[2.2]paracyclophane (21) and thiosemicarbazide (2a) and by applying the procedure described by Aly et al. [46].
Similarly, the reaction of 21 with 2f gave 22f in 60% yield (Scheme 7). If two equivalents of 21 were reacted with one equivalent of thiocarbohydrazide (2f), the reaction gave bis-4-acetyl-[2.2]paracyclophanylidene-hydrazine-1-carbothiohydrazone (23) in 88% yield. In addition, refluxing of the two equivalents of N-cyclohexylhydrazinecarbothioamide (2c) with 4,15-diformyl-2.2]paracyclophane (24) yielded the desired 2,2′-(1,4(1,4)-dibenzen-acyclohexaphane-12,43-diylbis(methane-ylylidene))bis-(N-cyclohexylhydrazine-1-carbothioamide (25) in 80% yield (Scheme 7). The X-ray structure analysis of compound 25 is as shown in Figure 5.
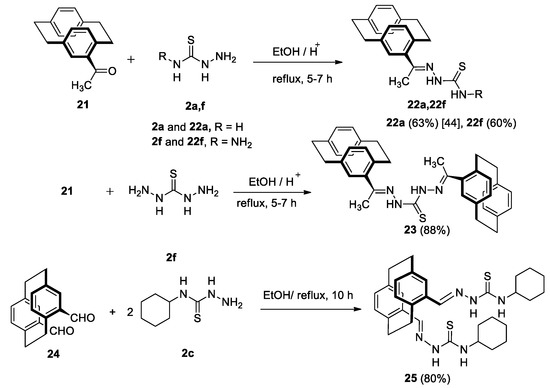
Scheme 7.
Synthesis of paracyclophane thiosemicarbazone derivatives 22a,b, 23 and 25.
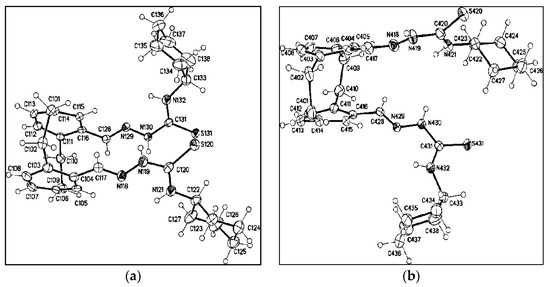
Figure 5.
Molecular structure of two of the four crystallographic independent molecules of 25 showed the two different conformations in the crystal (displacement parameters, are drawn at 30% probability level).
We aimed to synthesize thiazolidinones bearing a paracyclophanyl moiety; accordingly, we reacted 4-acetyl[2.2]paracyclophanylidene-thiosemicarbazone derivatives 22a and 22f with DMAD, 6a and DEAD, 6b (Scheme 8). By adaptation of the previously mentioned procedure, the expected 1,3-thiazolidinones 26–29 were obtained in good yields (Scheme 8). Depending on 1H-NMR and 13C-NMR, various alternative structures were ruled out. In order to distinguish between the expected 1,3-thiazolidinone derivatives, a single crystal of 28 was obtained as ethyl 2-((Z)-2-(((E)-1-(1,4(1,4)-dibenzenacyclohexaphane-12-yl)ethylidene)hydrazine-ylidene)-4-oxothiazolidin-5-yl)acetate (Figure 6).
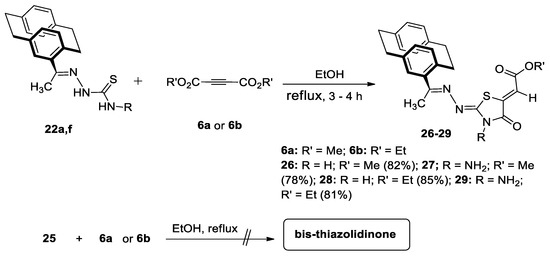
Scheme 8.
Reaction of [2.2]paracyclophanylidene thiosemicarbazones 22a,f with 6a and 6b.
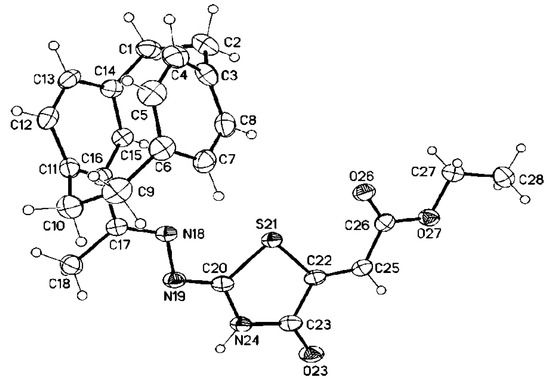
Figure 6.
Molecular structure of 28 (displacement parameters are drawn at 30% probability level).
Prolonged heating of 25 with either 6a or 6b under the aforementioned conditions failed to give either a mono- or a bis-thiazolidinone structure. Either the sensitivity of such layered molecules towards light and heat to undergo dimerization and/or steric factors might cause the reaction not to occur.
By analogy, the mechanism of formation of compounds 7a–e would be similar to that for the formation of compounds 26–29 (Scheme 9). Nucleophilic attack from sulfur lone pair in 22a,f to the triple bond of either 6a or 6b would give the intermediate 30, which would be neutralized as described before, to give 31. The intermediate 31 would be easily cyclized to give the corresponding thiazolidinones 26–29 (Scheme 8), via another nucleophilic attack from NH in the formed intermediate 32 to the ester-C=O and liberation of methanol or ethanol (Scheme 9).
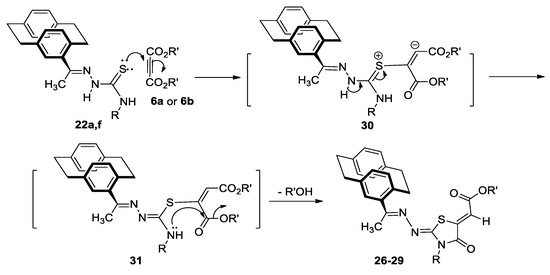
Scheme 9.
Mechanism described the formation of compounds 26–29.
3. Material and Methods
3.1. Chemistry
Melting points (mp’s) were recorded on a Gallenkamp melting point apparatus (Gallenkamp, UK) using open capillaries and were uncorrected. NMR data were recorded on Bruker AM 400 or AV400 spectrometers (Bruker, Rheinstetten, Germany), at 400 MHz for 1H and 100 MHz for 13C. Chemical shifts were reported in ppm from tetramethylsilane using solvent resonance in CDCl3 or DMSO-d6 solutions as the internal standard. The 13C-NMR signals were assigned on the basis of DEPT 135/90 spectra. The mass spectra were obtained on Finnigan MAT 312) (Germany) instrument using electron impact ionization (70 eV). The IR spectra were recorded on Bruker Alpha FT-IR instrument (Germany) with samples prepared as potassium bromide pellets. Thin-layer chromatography (TLC) was performed on precoated plates (silica gel 60 PF254), and zones were visualized with ultraviolet (UV) light. Elemental analyses for C, H, N were carried out with Elementar 306.
3.1.1. Starting Materials: Acenaphthequinone, 1 Was Bought and Bought from Aldrich, whereas [2.2]Paracyclophane Was Commercially Available
Preparation of 2-Oxoacenaphthylidene Thiosemicarbazones 5a–e
Compounds 5a–e were synthesized by refluxing solutions of acenaphthequinone 1 (1.82 g, 10 mmol) in absolute EtOH (100 mL) containing triethylamine (0.5 mL) and different solutions of thiosemicarbazide derivatives 2a–e for 3–5 h. The products were left to stand, and then collected by filtration after precipitation with ethanol. The resulting solid was recrystallized from the stated solvents to give yellow to orange crystals.
(Z)-2-(2-Oxoacenaphthylen-1-(2H)-ylidene)hydrazinecarbothioamide (5a). Yield: (1.58 g, 70%); orange crystals (EtOH); m.p.: 214–216 °C [50].
(Z)-2-(2-Oxoacenaphthylen-1-(2H)-ylidene)-N-phenylhydrazinecarbothioamide (5b). Yield: (2.65 g, 80%); yellow crystals (MeOH); m.p.: 208–210 °C [47].
(Z)-N-Cyclohexyl-2-(2-oxoacenaphthylen-1-(2H)-ylidene)hydrazinecarbothioamide (5c). Yield: (2.80 g, 75%); yellow crystals (CH3CN); m.p.: 202–204 °C. IR (KBr): ν = 3295 (NH’s), 2931 (Ali-CH), 1681 (CO), 1620 (C=N), 1609 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 1.17 (m, 1H, cyclic-CH), 1.33 (m, 2H, cyclic-CH2), 1.53 (m, 2H, cyclic-CH2), 1.66 (m, 1H, cyclic-CH), 1.79 (m, 2H, cyclic-CH2), 1.95 (m, 2H, cyclic-CH2), 4.23 (m, 1H, cyclic-CH), 7.85 (t, 1H, Ar-H, J = 7.9 Hz), 7.89 (t, 1H, Ar-H, J = 8.1 Hz), 8.09 (d, 1H, Ar-H, J = 8.2 Hz), 8.10 (d, 1H, Ar-H, J = 8.2 Hz), 8.15 (d, 1H, Ar-H, J = 8.3 Hz), 8.38 (d, 1H, Ar-H, J = 8.2 Hz), 8.97 (d, 1H, cyclohexyl-NH), 12.62 (s, 1H, = N-NH). 13C-NMR: (100 MHz, DMSO-d6): δ = 24.87, 25.05, 31.44 (cyclic-CH2), 53.84 (cyclic-CH), 118.61, 122.43, 127.05, 128.54, 128.79, 132.76 (Ar-CH), 129.88, 129.99, 130.43, 139.09 (Ar-C), 137.24 (C=N), 176.04 (C=S), 188.51 (C=O). MS: m/z (%) = 387 (M+, 100), 180 (53), 154 (59), 136 (40), 107 (13). Anal. Calcd. for C19H19N3OS (337.44); C, 67.63; H, 5.68; N, 12.45; S, 9.50. Found: C, 67.55; H, 5.75; N, 12.35; S, 9.57.
(Z)-N-Allyl-2-(2-oxoacenaphthylen-1-(2H)-ylidene)hydrazinecarbothioamide (5d). Yield: (2.18 g, 74%); light orange crystals (EtOH), m.p.: 190–191 °C [57].
(Z)-N-Ethyl-2-(2-oxoacenaphthylen-1(2H)-ylidene)hydrazinecarbothioamide (5e). Yield: (2.03 g, 72%); yellow crystals (EtOH), m.p.: 195–196 °C; [47].
Reactions of 2-Oxoacenaphthylidene Thiosemicarbazone Derivatives with 6a
A mixture of 2-oxoacenaphthylidene thiosemicarbazones (5a–e, 1 mmol) in absolute ethanol (50 mL) was refluxed with 6a for 20–30 min and the reaction was monitored by TLC analysis. A yellow precipitate of 7a–e was formed, and then the reaction mixture was filtered and washed with a small amount of ethanol. The obtained precipitates were crystalized in ethanol to give yellow crystals of 1,3-thiazolidin-4-ones 7a–d. In addition, the filtrate was left to stand at room temperature after separation of the precipitates 7a–e and, in the case of 7d, fine crystals of 8d were collected after filtration).
(Z)-Methyl 2-((Z)-4-oxo-2-((E)-(2-oxoacenaphthylen-1-(2H)-ylidene)hydrazono)thiazolidin-5-ylidene)acetate (7a). Yield: (0.277 g, 76%); yellow crystals (EtOH); m.p.: 252–254 °C. IR (KBr): ν = 3489 (NH), 3150 (Ar-CH), 2960 (Ali-CH), 1715, 1696 (CO), 1623 (C=N), 1604 (Ar-C=C) cm−1. NMR see Table 1. MS, m/z (%) = 365 (M+, 10), 307 (35), 154 (100), 137 (65), 107 (17). Anal. Calcd. for C18H11N3O4S (365.36); C, 59.17; H, 3.03; N, 11.50; S, 8.78. Found: C, 59.26; H, 3.07; N, 11.57; S, 8.67.
(Z)-Methyl 2-((Z)-4-oxo-2-((E)-(2-oxoacenaphthylen-1-(2H)-ylidene)hydrazono)-3-phenyl-thiazolidin-5-ylidene)acetate (7b). Yield: (0.321 g, 73%); yellow crystals (EtOH); m.p.: 266–268 °C. IR (KBr): ν = 3120 (Ar-CH), 2952 (Ali-CH), 1713, 1690 (CO), 1630 (C=N), 1589 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 3.87 (s, 3H, OCH3), 6.95 (s, 1H, vinyl-CH), 7.49 (t, 1H, Ar-H, J = 7.7 Hz), 7.66 (m, 3H, Ar-H), 7.69 (m, 2H, Ar-H), 7.72 (d, 1H, Ar-H, J = 7.0 Hz), 7.86 (t, 1H, Ar-H, J = 8.0 Hz), 8.07 (d, 1H, Ar-H, J = 6.9 Hz), 8.13 (d, 1H, Ar-H, J = 8.3 Hz), 8.32 (d, 1H, Ar-H, J = 8.2 Hz). 13C-NMR (100 MHz, DMSO-d6): δ = 52.72 (OCH3), 116.42 (vinyl-CH), 125.72, 127.19, 128.08, 128.21, 128.29, 128.74, 129.15, 129.20, 129.41 (Ar-CH), 130.06, 130.86, 132.14, 134.13, 140.69 (Ar-C), 140.77 (thiazole-C5), 155.55, 163.52 (thiazole-C2, C = N), 165.77 (cyclic-C = O), 167.12 (ester C = O), 188.41 (C = O). MS, m/z (%) = 441 (M+, 30), 306 (35), 180 (17), 154 (100), 136 (63), 107 (20). Anal. Calcd. for C24H15N3O4S (441.46); C, 65.30; H, 3.42; N, 9.52; S, 7.26. Found: C, 65.23; H, 3.38; N, 9.57; S, 7.37.
(Z)-Methyl 2-((Z)-3-cyclohexyl-4-oxo-2-((E)-(2-oxoacenaphthylen-1(2H)-ylidene)-hydrazono)-thiazolidin-5-ylidene)acetate (7c). Yield: (0.322 g, 72%); yellow crystals (EtOH); m.p.: 284–286 °C. IR (KBr): ν = 3110 (Ar-CH), 2955 (Ali-CH), 1709, 1683 (CO), 1618 (C=N), 1591 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 1.35–1.37 (m, 2H, cyclic-CH2), 1.72–1.75 (m, 4H, cyclic-CH2), 1.86–1.88 (m, 4H, cyclic-CH2), 2.32 (m, 1H, cyclic-CH), 3.83 (s, 3H, OCH3), 6.83 (s, 1H, vinyl-CH), 7.85 (t, 1H, Ar-H, J = 8.0 Hz), 7.88 (t, 1H, Ar-H, J = 7.8 Hz), 7.93 (d, 1H, Ar-H, J = 7.1 Hz), 8.05 (d, 1H, Ar-H, J = 7.8 Hz), 8.13 (d, 1H, Ar-H, J = 8.3 Hz), 8.26 (d, 1H, Ar-H, J = 8.3 Hz). 13C-NMR (100 MHz, DMSO-d6): δ = 27.61, 30.72, 35.93 (cyclic-CH2), 53.07 (OCH3), 59.92 (cyclic-CH), 116.42 (vinyl-CH), 119.03, 121.72, 128.33, 128.44, 128.92, 132.10 (Ar-CH), 131.23, 133.44, 131.93, 139.50 (Ar-C), 142.19 (thiazole-C5), 152.18, 155.56 (thiazole-C2, C=N), 166.37 (cyclic-C=O), 166.69 (ester-C=O), 183.87 (C=O). MS, m/z (%) = 447 (M+, 5), 306 (35), 154 (100) 137 (67), 107 (17). Anal. Calcd. for C24H21N3O4S (447.51); C, 64.41; H, 4.73; N, 9.39; S, 7.17. Found: C, 64.32; H, 4.80; N, 9.34; S, 7.09.
(Z)-Methyl 2-((Z)-3-allyl-4-oxo-2-((E)-(2-oxoacenaphthylen-1-(2H)-ylidene)hydrazono)-thiazolidin-5-ylidene)acetate (7d). Yield: (0.303 g, 75%); yellow crystals (EtOH); m.p.: 218–220 °C. IR (KBr): ν = 3085 (Ar-CH), 2970 (Ali-CH), 1710, 1695 (CO), 1620 (C=N), 1609 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 3.86 (s, 3H, OCH3), 4.75 (m, 2H, allyl-CH2N), 5.34 (m, 2H, allyl-CH2=), 6.06 (m, 1H, allyl-CH=), 6.89 (s, 1H, vinyl-CH), 7.78 (t, 1H, Ar-H, J = 8.2 Hz), 7.81 (t, 1H, Ar-H, J = 7.9 Hz), 7.98 (d, 1H, Ar-H, J = 7.1 Hz), 8.04 (d, 1H, Ar-H, J = 7.8 Hz), 8.12 (d, 1H, Ar-H, J = 8.1 Hz), 8.58 (d, 1H, Ar-H, J = 8.1 Hz). 13C-NMR (100 MHz, DMSO-d6): δ = 47.72 (allyl-CH2N) 52.61 (OCH3), 115.28 (vinyl-CH), 118.58 (allyl-CH2=), 118.90, 121.62, 128.35, 128.70, 129.06, 131.91 (Ar-CH), 131.21, 131.63, 132.40, 138.9 (Ar-C), 134.85 (allyl-CH=), 142.23 (thiazole-C5), 151.91, 155.36 (thiazole-C2, C = N) 165.41 (cyclic-C=O), 166.54 (ester C=O), 183.93 (C=O). MS, m/z (%) = 405 (M+, 10), 306 (40), 289 (16), 154 (100), 137 (65), 107 (15). Anal. Calcd. for C21H15N3O4S (405.43); C, 62.21; H, 3.73; N, 10.36; S, 7.91. Found: C, 62.25; H, 3.68; N, 10.45; S, 7.97.
(Z)-Methyl 2-((Z)-3-ethyl-4-oxo-2-((E)-(2-oxoacenaphthylen-1-(2H)-ylidene)hydrazono)-thiazolidin-5-ylidene)acetate (7e). Yield: (0.290 g, 74%); yellow crystals (EtOH); m.p.: 200–202 °C. IR (KBr): ν = 3143 (Ar-CH), 2944 (Ali-CH), 1714, 1698 (CO), 1607 (C=N), 1590 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 1.36 (t, 3H, CH3, J = 6.72 Hz), 3.75 (q, 2H, CH2, J = 6.72 Hz), 3.82 (s, 3H, OCH3), 6.86 (s, 1H, vinyl-CH), 7.82 (t, 1H, Ar-H, J = 8.1 Hz), 8.01 (d, 1H, Ar-H, J = 7.2 Hz), 8.30 (t, 1H, Ar-H, J = 7.9 Hz), 8.45 (d, 1H, Ar-H, J = 7.8 Hz), 8.65 (d, 1H, Ar-H, J = 8.2 Hz), 8.36 (d, 1H, Ar-H, J = 8.0 Hz). 13C-NMR (100 MHz, DMSO-d6): δ = 12.29 (CH3), 31.5 (CH2), 52.52 (OCH3), 116.37 (vinyl-CH), 126.02, 127.46, 128.85, 129.66, 130.03, 131.30 (Ar-CH), 132.37, 135.30, 139.91, 140.74 (Ar-C), 144.24 (thiazole-C5), 154.92, 160.66 (thiazole-C2, C=N), 164.13 (cyclic-C=O), 165.66 (ester C=O), 187.49 (C=O). MS, m/z (%) = 393 (M+, 20), 306 (40), 153 (100), 136 (60), 107 (17). Anal. Calcd. for C20H15N3O4S (393.42); C, 61.06; H, 3.84; N, 10.68; S, 8.15. Found: C, 61.16; H, 3.81; N, 10.75; S, 8.20.
Tetramethyl 5-(2-(((Z)-N-allyl-N′-((E)-2-oxoacenaphthylen-1-(2H)-ylidene)carbamo-hydrazonoyl)thio)-1,2,3-tris(methoxycarbonyl)cyclopropyl)-4-methoxy-7-oxabicyclo-[2.2.1]hepta-2,5-diene-1,2,3,6-tetracarboxylate (8d). Yield: (0.086 g, 10%); orange crystals (EtOH); m.p.: 224–226 °C. IR (KBr): ν = 3401 (NH), 3100 (Ar-CH), 2953 (Ali-CH), 1717, 1698 (CO), 1623 (C=N), 1590 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 3.13 (s, 1H, cyclopropane-H), 3.40 (s, 3H, OCH3), 3.46, 3.55, 3.65, 3.73, 3.80, 3.85, 3.95 (s, 3H, OCH3), 4.40 (m, 2H, allyl-CH2N), 5.36 (m, 2H, allyl-CH2=), 6.08 (m, 1H, allyl-CH=), 7.77 (t, 1H, Ar-H, J = 8.2 Hz), 7.81 (t, 1H, Ar-H, J = 7.91 Hz), 8.00 (d, 1H, Ar-H, J = 7.1 Hz), 8.05 (d, 1H, Ar-H, J = 7.7 Hz), 8.11 (d, 1H, Ar-H, J = 8.3 Hz), 8.24 (d, 1H, Ar-H, J = 8.1 Hz), 9.40 (s, IH, NH-allyl). 13C-NMR (100 MHz, DMSO-d6): δ = 22.23, 24.90 (cyclopropane-C), 25.60 (cyclopropane-CH), 46.81 (allyl-CH2N), 52.00, 52.20, 52.60, 53.07, 53.19, 53.53, 53.65, 54.17 (OCH3), 118.19 (allyl-CH2=), 134.22 (allyl-CH=), 119.91, 121.80, 128.52, 128.29, 130.00, 132.32 (Ar-CH), 94.66, 121.66, 131.44, 134.58, 136.84, 138.01, 138.22, 138.93, 140.41, 142.82 (Ar-C), 152.63, 154.82 (C=N), 165.21, 167.53, 168.91, 171.68, 172.00, 173.51, 175.44 (ester-C=O), 185.16 (C=O). MS, m/z (%) = 863 (M+, 10), 805 (5), 612 (20), 307 (35), 154 (100), 137 (67), 107 (18). Anal. Calcd. for C40H37N3O17S (863.80); C, 55.62; H, 4.32; N, 4.86; S, 3.71. Found: C, 55.69; H, 4.27; N, 4.79; S, 3.61.
Preparation of 4-Acetyl-[2.2]Paracyclophanylidene-Thiosemicarbazones 22a,f
4-Acetyl[2.2]paracyclophanylidene-thiosemicarbazones 22a,f were synthesized via refluxing solutions of 4-acetyl[2.2]paracyclophane (21) (2.50 g, 10 mmol) in absolute ethanol (100 mL) containing glacial acetic acid (0.5 mL) and thiosemicarbazide 2a (0.91 g, 10 mmol) or (1.06 g, 10 mmol) of thiocarbohydrazide 2f for 6 h. Compounds 22a,f were precipitated, then filtered and washed with dry ethanol to get the crude as colorless crystals. On the other hand, Bis-4-acetyl[2.2]-paracyclophanylidene-hydrazine-1-carbothiohydrazide (23) was obtained efficiently after treating 21 (2.50 g, 10 mmol) with 2f (0.53 g, 5 mmol) in absolute EtOH (50 mL) under the same condition mentioned before (Scheme 7).
(E)-2-(4-([2.2]Paracyclophanylethylidene)-1-carbothiohydrazide (22f). Yield: (2.03 g, 60%); colorless crystals (EtOH/DMF); m.p.: 188–189 °C. IR (KBr): ν = 3450–3344 (NH), 2926–2853 (Ali-CH), 1642 (C=N), 1617 (Ar-C=C), 1354 (C=S) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 2.28 (s, 3H, CH3), 2.79 (m, 2H, PC-CH2), 2.98 (m, 4H, 2 PC-CH2), 3.40 (m, 2H, PC-CH2), 4.99 (b, 2H, NH2), 6.49 (m, 6H, PC-H), 6.86 (s, 1H, PC-H), 9.37 (s, 1H, NH), 10.10 (s, 1H, = N-NH). 13C-NMR (100 MHz, DMSO-d6): δ = 18.04 (CH3), 34.38, 34.71, 34.84 (Pc-CH2), 130.56, 131.62, 132.23, 132.92, 132.50, 132.41, 135.61 (Pc-CH), 137.89, 138.97, 139.15, 139.45 (Pc-C), 150.68 (C = N), 177.17 (C = S). MS: m/z (%) = 338 [M+, 50], 306 (30), 153 (100), 135 (75), 106 (26). Anal. Calcd. for C19H22N4S (338.47); C, 67.42; H, 6.55; N, 16.55; S, 9.47. Found: C, 67.46; H, 6.50; N, 16.49; S, 9.53.
Bis-4-acetyl[2.2]paracyclophanylidene-hydrazine-1-carbothiohydrazide (23). Yield: (4.14 g, 88%); colorless crystals (EtOH/ DMF); m.p.: 224–226 °C. IR (KBr): ν = 3450–3344 (NH), 2926–2853 (Ali-CH), 1642 (C=N), 1617 (Ar-C=C), 1354 (C=S) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 2.29 (s, 6H, 2CH3), 2.35 (m, 4H, PC-CH2), 2.97 (m, 8H, PC-CH2), 3.10 (m, 4H, PC-CH2), 6.48–6.56 (m, 4H, PC-H), 6.82 (s, 2H, PC-H), 7.08–7.09 (m, 8H, PC-H), 10.25 (s, 2H, 2=N-NH). 13C-NMR (100 MHz, DMSO-d6): δ = 18.56, 18.69 (CH3), 34.38, 34.59, 34.71, 34.83 (PC-CH2), 130.57, 131.60, 131.90, 132.23, 132.61, 133.20, 133.40 (PC-CH), 135.61, 135.75, 138.23, 139.15, 139.24 (PC-C), 150.93 (C=N), 177.32 (C = S). MS: m/z (%) = 570 [M+, 20], 363 (15), 351 (25), 307 (34), 154 (100), 106 (20), 98 (63). Anal. Calcd. for C37H38N4S (570.79); C, 77.86; H, 6.71; N, 9.82; S, 5.62. Found: C, 77.97; H, 6.63; N, 9.94; S, 5.75.
2,2′-(1,4(1,4)-Dibenzenacyclohexaphane-12,43-diylbis(methane-ylylidene))bis-(N-cyclohexyl-hydrazine-1-carbothioamide) (25). Yield: (4.59 g, 80%); colorless crystals (CH3CN); m.p.:142–143 °C. IR (KBr): ν = 3440 (NH), 2953 (Ali-CH), 1637 (C=N), 1610 (Ar-C=C), 1350 (C=S) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 1.39 (m, 4H, cyclohexyl-CH2), 1.75 (m, 8H, cyclohexyl-CH2), 1.92 (m, 8H, cyclohexyl-CH2), 2.55 (m, 2H, cyclohexyl-CH), 2.90 (m, 4H, 2 PC-CH2), 3.23 (m, 2H, PC-CH2), 3.55 (m, 2H, PC-CH2), 6.52 (m, 4H, PC-H), 6.75 (s, 2H, PC-H), 7.55 (d, 2H, NH-cyclohexyl), 8.15 (s, 2H, CH=N), 11.20 (s, 2H, =N-NH). 13C-NMR (100 MHz, DMSO-d6): δ = 25.92, 32.50, 34.60 (cyclic-CH2), 32.58, 34.65, 34.88, (PC-CH2), 130.07, 132.40, 133.24 (PC-CH), 135.52, 138.14, 138.80, 139.90 (PC-C), 142.50 (CH=N), 175.06 (C = S). MS: m/z (%) = 574 [M+, 3], 417 (4), 261 (13), 258 (18), 157 (4), 141 (29), 129 (98), 83 (33), 56 (100). Anal. Calcd. for C32H42N6S2 (574.29); C, 66.86; H, 7.36; N, 14.62; S, 11.16. Found: C, 66.90; H, 7.42; N, 14.64; S, 11.10.
Reactions of 4-Acetyl[2.2]Paracyclophanylidene-Thiosemicarbazones, 22a,f with 6a and 6b
A mixture of 22a,f (1 mmol) in absolute ethanol (50 mL) was refluxed with either 6a or 6b for 3 h and the reaction was monitored by TLC. The precipitate of 26–29 was formed after concentration the solvent under vacuum, was filtered and washed with cold ethanol (15 mL) and recrystallized from methanol.
Methyl 2-((Z)-2-(((E)-1-(1,4(1,4)-dibenzenacyclohexaphane-12-yl)ethylidene)hydrazine-ylidene)-4-oxothiazolidin-5-yl)acetate (26). Yield: (0.355 g, 82%); yellow crystals; m.p.: 256–258 °C. IR (KBr) ν = 3260 (NH), 3110 (Ar-CH), 2925 (Ali-CH), 1713, 1699 (CO), 1626 (C=N), 1600 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 2.37 (s, 3H, CH3), 2.89 (m, 8H, PC-CH2), 3.77 (s, 3H, OCH3), 6.54 (s, 1H, PC-H), 6.68 (m, 6H, PC-H), 6.74 (s, 1H, vinyl-CH), 12.89 (s, 1H, NH-thiazole). 13C-NMR (100 MHz, DMSO-d6): δ = 18.33 (CH3), 34.54, 34.60, 34.76, 35.02 (PC-CH2), 52.38 (OCH3), 113.92 (vinyl-CH), 130.81, 132.08, 132.37, 132.58, 132.70, 133.63, 135.82 (PC-CH), 138.23, 138.54, 138.97, 139.22 (PC-C), 143.54 (thiazole-C5), 159.05, 165.09 (thiazole-C2, C=N) 165.92 (cyclic-C=O), 165.99 (ester C=O). MS, m/z (%) = 433 (M+, 43), 306 (40), 289 (17), 153 (100) 136 (65), 106 (15). Anal. Calcd. for C24H23N3O3S (433.52); C, 66.49; H, 5.35; N, 9.69; S, 7.40. Found: C, 66.52; H, 5.46; N, 9.57; S, 7.33.
Methyl 2-((Z)-3-amino-2-(((E)-1-(1,4(1,4)-dibenzenacyclohexaphane-12-yl)ethylidene)-hydrazineylidene)-4-oxothiazolidin-5-yl)acetate (27). Yield: (0.349 g, 78%); yellow crystals (CH3OH); m.p.: 260–262 °C. IR (KBr) ν = 3337 (NH2), 3050 (Ar-CH), 2923 (Ali-CH), 1737, 1700 (CO), 1630 (C=N), 1603 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 2.35 (s, 3H, CH3), 2.90 (m, 8H, PC-CH2), 3.80 (s, 3H, OCH3), 6.65 (m, 6H, PC-H), 6.55 (s, 1H, PC-H), 6.75 (s, 1H, vinyl-CH), 6.90 (br, s, 2H, NH2). 13C-NMR (100 MHz, DMSO-d6): δ = 18.61 (CH3), 34.51, 34.57, 34.75, 34.89 (PC-CH2), 52.61 (OCH3), 114.19 (vinyl-CH), 130.48, 131.52, 132.24, 132.38, 132.58, 133.34, 135.68 (PC-CH), 138.11, 138.86, 139.97, 139.17 (PC-C), 144.51 (thiazole-C5), 152.36, 161.06 (thiazole-C2) 165.90 (cyclic-C=O), 166.77 (ester C = O). MS, m/z (%) = 448 (M+, 7), 306 (30), 289 (15), 153 (100) 136 (64), 106 (20). Anal. Calcd. for C24H24N4O3S (448.54); C, 64.27; H, 5.39; N, 12.49; S, 7.15. Found: C, 64.39; H, 5.48; N, 12.37; S, 7.31.
Ethyl 2-((Z)-2-(((E)-1-(1,4(1,4)-dibenzenacyclohexaphane-12-yl)ethylidene)hydrazine-ylidene)-4-oxothiazolidin-5-yl)acetate (28). Yield: (0.380, 85%); yellow plates (CH3OH); m.p. 200–202 °C. IR (KBr) ν = 3240 (NH), 3120 (Ar-CH), 2919 (Ali-CH), 1714, 1695 (CO), 1624 (C=N), 1592 (Ar-C=C) cm−1. 1H-NMR (DMSO-d6): δ = 1.27 (t, 3H, CH3, J = 7.1 Hz), 2.37 (s, 3H, CH3), 2.90–3.14 (m, 8H, PC-CH2), 4.24 (q, 2H, OCH2, J = 7.1 Hz), 6.56–6.61 (m, 6H, PC-H), 6.54 (s, 1H, PC-H), 6.70 (s, 1H, vinyl-CH), 12.90 (s, 1H, NH-thiazole). 13C-NMR (100 MHz, DMSO-d6): δ = 14.01, 18.36 (CH3), 34.53, 34.61, 34.75, 35.03 (PC-CH2), 61.25 (OCH2), 114.56 (vinyl-CH), 130.79, 132.09, 132.36, 132.58, 132.70, 133.70, 135.82 (PC-CH), 138.17, 138.55, 138.96, 139.22 (PC-C), 142.90 (thiazole-C5), 158.45, 165.27 (thiazole-C2, C=N), 165.32 (cyclic-C=O), 165.62 (ester C=O). MS, m/z (%) = 447 (M+, 62), 306 (32), 153 (100), 135 (65), 106 (20). Anal. Calcd. For C25H25N3O3S (447.55): C, 67.09; H, 5.63; N, 9.39; S, 7.16. Found: C, 67.12; H, 5.66; N, 9.45; S, 7.28.
Ethyl 2-((Z)-3-Amino-2-(((E)-1-(1,4(1,4)-dibenzenacyclohexaphane-12-yl)ethylidene)-hydrazine-ylidene)-4-oxothiazolidin-5-yl)acetate (29). Yield: (0.374 g, 81%); yellow crystals (CH3OH); m.p. 174–175 °C. IR (KBr): ν = 3344 (NH), 3100 (Ar-CH), 2924 (Ali-CH), 1735, 1720 (CO), 1622 (C=N), 1607 (Ar-C=C) cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 1.28 (t, 3H, CH3, J = 7.2 Hz), 2.33 (s, 3H, CH3), 2.92–3.04 (m, 8H, PC-CH2), 4.27 (q, 2H, OCH2, J = 7.2 Hz), 6.68 (m, 6H, PC-H), 6.57 (s, 1H, PC-H), 6.72 (s, 1H, vinyl-CH), 10.09 (b, s, 2H, NH2). 13C-NMR (100 MHz, DMSO-d6): δ = 14.38 (CH3), 18.62 (CH3), 34.57, 34.66, 34.76, 35.12, (PC-CH2), 54.40 (OCH3), 62.0 (OCH2), 115.46 (vinyl-CH), 131.81, 132.18, 132.40, 132.60, 132.73, 135.72, 134.86 (PC-CH), 138.27, 138.60, 138.99, 139.25 (PC-C), 143.56 (thiazole-C5), 159.17, 166.02 (thiazole-C2, C=N), 165.95 (cyclic-C=O), 176.02 (ester C=O). MS, m/z (%) = 462 (M+, 6), 307 (25), 248 (40), 155 (27), 154 (95), 143 (100), 135 (65), 106 (20). Anal. Calcd. for C25H26N4O3S (462.56): C, 64.91; H, 5.67; N, 12.11; S, 6.93. Found: C, 65.01; H, 5.75; N, 12.16; S, 7.02.
3.2. Single Crystal X-ray Structure Determination of 5a, 7d, 8d, 25 and 28
A single crystal of 5a was obtained by recrystallization from C2H5OH (ethanol), a single crystal of 7d was obtained by recrystallization from C2H5OH (ethanol), a single crystal of 8d was obtained from C2H5OH (ethanol), 25 from CH3CN (acetonitrile), and a single crystal of 28 was obtained acenaphthequinone recrystallization from CH3OH (methanol). The single-crystal X-ray analyses were carried out on a Bruker D8 Venture diffractometer with Photon100 or Photon II CPAD detector at 123K using Cu-Kα radiation (λ = 1.54178 Å). Direct Methods (SHELXS [58] or Dual Space Methods (SHELXT [59]) were used for structure solution and refinement was carried out using SHELXL-2014 (full-matrix least-squares on F2) [59]. Hydrogen atoms were refined using a riding model (H (N, O) free). Semi-empirical absorption corrections were applied.
Compound 5a (SB1184_HY): C13H9N3OS, Mr = 255.29 g mol−1, orange blocks, size 0.30 × 0.25 × 0.20 mm, monoclinic, space group P21/c (no.14), a = 6.3357 (2) Ǻ, b = 18.2558 (7) Ǻ, c = 20.0737 (7) Ǻ, β = 97.327 (1)°, V = 2302.83 (14) Ǻ3, Z = 8, Dcalcd = 1.473 Mg m−3, F (000) = 1056, μ (Cu-Kα) = 2.42 mm−1, Τ = 123 K, 19236 measured reflections (2θmax = 144.2 °), 4510 independent reflections [Rint = 0.023], 343 parameters, 6 restraints, R1 [for 4468 I › 2σ (I)] = 0.030, wR2 (for all data) = 0.079, S = 1.06, largest diff. peak and hole = 0.37 e Ǻ−3/−0.31 e Ǻ−3. It is a polymorph of EBINOV (2-(2-oxoacenaphthylen-1(2H)-ylidene)hydrazinecarbothioamide methanol solvate) [60].
Compound 7d (SB1186_HY): C21H15N3O4S, Mr = 405.42 g mol−1, yellow blocks, size 0.12 × 0.06 × 0.04 mm, monoclinic, space group P21/n (no.14), a = 7.0903 (2) Ǻ, b = 15.6914 (5) Ǻ, c = 16.4943 (5) Ǻ, β = 98.917 (1)°, V = 1812.92 (9) Ǻ3, Z = 4, Dcalcd = 1.485 Mg m−3, F (000) = 840, μ (Cu-Kα) = 1.90 mm−1, Τ = 123 K, 18041 measured reflections (2θmax = 144.4°), 3575 independent reflections [Rint = 0.024], 263 parameters, R1 [for 3402 I › 2σ (I)] = 0.029, wR2 (for all data) = 0.077, S = 1.06, largest diff. peak and hole = 0.28 e Ǻ−3/−0.30 e Ǻ−3.
Compound 8d (SB1185_HY): C40H37N3O17S, Mr = 863.78 g mol−1, orange plates, size 0.09 × 0.06 × 0.03 mm, triclinic, space group P-1 (no. 2), a = 12.8863 (5) Ǻ, b = 15.1023 (6) Ǻ, c = 22.3027 (8) Ǻ, α = 100.800 (2)°, β = 100.850 (2)°, γ = 104.460 (2)°, V = 4000.4 (3) Ǻ3, Z = 4, Dcalcd = 1.434 Mg m−3, F (000) = 1800, μ (Cu-Kα) = 1.43 mm−1, Τ = 123 K, 71781 measured reflections (2θmax = 144.4°), 15705 independent reflections [Rint = 0.039], 1113 parameters, 986 restraints, R1 [for 14,020 I › 2σ (I)] = 0.059, wR2 (for all data) = 0.148, S = 1.10, largest diff. peak and hole = 1.52 e Ǻ−3/−0.59 e Ǻ−3. In the 2nd molecule the vinyl moiety is disordered, disordered atoms refined isotropically. In the second molecule the oxacenaphthylene moiety shows high Uij-values, probably disordered, but the disorder is not resolved. Use of constraints (EADP) and restraints (SADI) for the refinement as well as a general RIGU restraint.
Compound 25 (SB1014_HY): C32H42N6S2 ∙ 0.5 C2H5N, Mr = 595.36 g mol−1, colorless blocks, size 0.40 × 0.18 × 0.06 mm, triclinic, space group P-1 (no.2), a = 13.7588 (6) Ǻ, b = 23.3483 (9) Ǻ, c = 23.3963 (9) Ǻ, α = 60.101 (2)°, β = 84.894 (3)°, γ = 84.516 (3)°, V = 6478.3 (5) Ǻ3, Z = 8, Dcalcd = 1.221 Mg m−3, F (000) = 2552, μ (Cu-Kα) = 1.74 mm−1, Τ = 123 K, 67389 measured reflections (2θmax = 145.4°), 24776 independent reflections [Rint = 0.067], 1485 parameters, 1254 restraints, R1 [for 16,926 I › 2σ (I)] = 0.115, wR2 (for all data) = 0.321, S = 1.01, largest diff. peak and hole = 1.33 e Ǻ−3/−0.79 e Ǻ−3. The compound was refined as a two-component pseudo merohedral twin. A general RIGU restraint is used for the refinement due to the bad quality of the data. Two solvent molecules acetonitrile per asymmetric unit disordered, the disordered atoms refined isotropically.
Compound 28 (SB1183_HY): C25H25N3O3S ∙ CH3OH, Mr = 479.58 g mol−1, yellow plates, size 0.16 × 0.14 × 0.02 mm, monoclinic, space group P21/c (no,.14), a = 19.5995 (5) Ǻ, b = 7.4181 (2) Ǻ, c = 18.2170 (5) Ǻ, β = 112.833 (2)°, V = 2441.04 (12) Ǻ3, Z = 4, Dcalcd = 1.305 Mg m−3, F (000) = 1016, μ (Cu-Kα) = 1.49 mm−1, Τ = 123 K, 25915 measured reflections (2θmax = 144.6°), 4808 independent reflections [Rint = 0.052], 315 parameters, two restraints, R1 [for 4146 I › 2σ (I)] = 0.064, wR2 (for all data) = 0.155, S = 1.17, largest diff. peak and hole = 0.46 e Ǻ−3/−0.32 e Ǻ−3.
CCDC 1937480 (5a–sb1184_hy), 1937481 (7d–sb1186_hy), 1937482 (8d–sb1185_hy), 1937483 (25–sb1014_hy), and 1937484 (28–sb1183_hy) contain the Supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (details in Supplementary Materials).
4. Conclusions
In conclusion, we prepared various derivatives of thiazolodinone derived by acenaphthequinone and [2.2]paracyclophane. We are able here to prove the structures of the obtained products via X-ray structure analysis. We could also, for the first time, get the X-ray structure analysis of the tetramer form resulting from DMAD.
Supplementary Materials
The following are available online.
Author Contributions
Writing—A.A.A.; revision—N.K.M., A.A.H., K.M.E.-S.; experimental work—M.M.M.; revision and submission—S.B.; X-ray—M.N.; NMR—A.B.B.
Funding
The authors thank the Science and Technology Development Fund, STDF, Egypt for its financial supporting of the Grant No. 22934. However this research received no other external funding. The NMR spectrometer at Florida Institute of Technology was purchased with the assistance of the U.S. National Science Foundation (CHE 03 42251).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suthar, S.K.; Jaiswal, V.; Lohan, S.; Bansal, S.; Chaudhary, A.; Tiwari, A.; Alex, A.T.; Joseph, A. Novel quinolone substituted thiazolidin-4-ones as anti-inflammatory, anticancer agents: Design, synthesis and biological screening. Eur. J. Med. Chem. 2013, 63, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Ishak, E.A.; El Malah, T.; Brown, A.B.; Elayat, W.M. Synthesis of potentially antioxidant and antibacterial biologically active thiazolidines. J. Heterocycl. Chem. 2015, 52, 1758–1764. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.K.; Kumar, N.; Dudhe, R. A review on various heterocyclic moieties and their antitubercular activity. Biomed. Pharmacother. 2011, 65, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Kumari, P.; Patel, N. Synthesis and biological evaluation of some thiazolidinones as antimicrobial agents. Eur. J. Med. Chem. 2012, 48, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.K.; Tripathi, R.; Katti, S.B.; Pannecouque, C.; de Clercq, E. Design, synthesis, and evaluation of 2-aryl-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Bioorg. Med. Chem. 2007, 15, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S.; Hugar, M.H. Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 2010, 45, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.; Nampurath, G.K.; Mathew, S.P.; Zachariah, R.T.; Potu, B.K.; Rao, M.S.; Valiathan, M.; Chamallamudi, M.R. Antidiabetic effect through islet cell protection in streptozotocin diabetes: A preliminary assessment of two thiazolidin-4-ones in Swiss albino mice. Chem. Biol. Interact. 2009, 177, 242–246. [Google Scholar] [CrossRef]
- Aly, A.A.; Brown, A.B.; El-Emary, T.I.; Ewas, A.M.M.; Ramadan, M. Hydrazinecarbothioamide group in the synthesis of heterocycles. Arkivoc 2009, 2009, 150–197. [Google Scholar]
- Hassan, A.A.; Mohamed, N.K.; Makhlouf, M.M.; Bräse, S.; Nieger, M. Reactions of dimethyl acetylenedicarboxylate with 2,5-dithiobiurea derivatives. Synthesis 2014, 46, 3097–3102. [Google Scholar] [CrossRef]
- Hassan, A.A.; Mohamed, N.K.; Makhlouf, M.M.; Bräse, S.; Nieger, M.; Höpf, H. (Hex-2-en-ylidene)-N-substituted hydrazonecarbothioamides and 2,3-dichloro-1,4-naphthoquinone: Nucleophilic substitution reactions and synthesis of naphtho[2,3-f][1,3,4]-triazepines and naphtho[2,3-d]thiazoles. Synthesis 2016, 48, 3134–3140. [Google Scholar] [CrossRef]
- Aly, A.A.; Brown, A.B.; Abdel-Aziz, M.; Abuo-Rahma, G.E.D.A.; Radwan, M.F.; Ramadan, M.; Gamal-Eldeen, A.M. Synthesis of new 4-oxo-thiazolidine-5-ylidenes of antitumor and antioxidant activities. J. Heterocycl. Chem. 2010, 47, 547–554. [Google Scholar] [CrossRef]
- Aly, A.A.; Brown, A.B.; Abdel-Aziz, M.; Abuo-Rahma, G.E.D.A.; Radwan, M.F.; Ramadan, M.; Gamal-Eldeen, A.M. An efficient synthesis of thiazolidin-4-ones with antitumor and antioxidant activities. J. Heterocycl. Chem. 2012, 49, 726–731. [Google Scholar] [CrossRef]
- Hassan, A.A.; Abdel-Latif, F.F.; Nour El-Din, A.M.; Mostafa, S.M.; Nieger, M.; Bräse, S. Synthesis of (E)-2,5-disubstituted 1,3,4-thiadiazolyl-2,3-diphenylpropenones from alkenylidene-hydrazinecarbothioamides. Tetrahedron 2012, 68, 8487–8492. [Google Scholar] [CrossRef]
- Hu, W.-X.; Zhou, W.; Xia, C.-N.; Wen, X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg. Med. Chem. Lett. 2006, 16, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Barbuceanu, S.-F.; Ilies, D.C.; Saramet, G.; Uivarosi, V.; Draghici, C.; Radulescu, V. Synthesis and antioxidant activity evaluation of new compounds from hydrazinecarbothioamide and 1,2,4-triazole class containing diarylsulfone and 2,4-difluorophenyl moieties. Int. J. Mol. Sci. 2014, 15, 10908–10925. [Google Scholar] [CrossRef] [PubMed]
- Paiva, R.O.; Kneipp, L.F.; Goular, C.M.; Albuquerque, M.A.; Echevarria, A. Antifungal activities of thiosemicarbazones and semicarbazones against mycotoxigenic fungi. Ciência Agrotecnol. 2014, 38, 531–537. [Google Scholar] [CrossRef]
- Reis, D.C.; Despaigne, A.A.R.; Da Silva, J.G.; Silva, N.F.; Vilela, C.F.; Mendes, I.C.; Takahashi, J.A.; Beraldo, H. Structural studies and investigation on the activity of imidazole-derived thiosemicarbazones and hydrazones against crop-related fungi. Molecules 2013, 18, 12645–12662. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Abdel Hamid, H.; Kassem, A.A.; Shoukry, M. Synthesis and reactions of acenaphthenequinones. Part 2. The reactions of acenaphthenequinones. Molecules 2002, 7, 155–188. [Google Scholar] [CrossRef]
- Mhaidat, I.; Mergos, J.A.; Hamilakis, S.; Kollia, C.; Loizos, Z.; Tsolomitis, A.; Dervos, C.T. Synthesis and reactions of acenaphthenequinones. Part 2. The reactions of acenaphthenequinones. Mater. Lett. 2009, 63, 2587–2590. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Hajiabbasi, P.; Gholamzadeh, P. Development of the acenaphthenequinone reactions. Heterocycles 2012, 85, 1869–1890. [Google Scholar] [CrossRef]
- Yavari, I.; Khajeh-Khezri, A. Recent advances in the synthesis of hetero- and carbocyclic compounds and complexes based on acenaphthylene-1,2-dione. Synthesis 2018, 50, 3947–3973. [Google Scholar] [CrossRef]
- Hyatt, J.L.; Wadkins, R.M.; Tsurkan, L.; Hicks, L.D.; Hatfield, M.J.; Edwards, C.C.; Ross, C.R., II; Cantalupo, S.A.; Crundwell, G.; Danks, M.K.; et al. Planarity and constrain of the carbonyl groups in 1,2-diones are determinants for selective inhibition of human carboxylesterase 1. J. Med. Chem. 2007, 50, 5727–5734. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Argüelles, M.C.; Ferrari, M.B.; Fava, G.G.; Pelizzi, C.; Pelosi, G.; Albertini, R.; Bonati, A.; Dall’Aglio, P.P.; Lunghi, P.; Pinelli, S. Acenaphthenequinone thiosemicarbazone and its transition metal complexes: Synthesis, structure, and biological activity. J. Inorg. Biochem. 1997, 66, 7–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Wu, G.; Li, Z.; Song, T.; Li, X.Q. Probing the difference between BH3 groove of Mcl-1 and Bcl-2 protein: Implications for dual inhibitors design. Eur. J. Med. Chem. 2011, 46, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- El-Alawi, Y.S.; McConkey, B.J.; Dixon, D.G.; Greenberg, B.M. Measurement of short- and long-term toxicity of polycyclic aromatic hydrocarbons using luminescent bacteria. Ecotoxicol. Environ. Saf. 2002, 51, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Biju, A.T.; Vinod, A.U.; Suresh, E. Reaction of Huisgen Zwitter ion with 1,2-benzoquinones and isatins: Synthesis of dihydro-1,2,3-benzoxadiazoles and spirooxadiazolines. Org. Lett. 2005, 7, 5139–5142. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, Y.-H.; Yan, C-G. Convenient synthesis of spiro (indoline-3,4′-pyrano[2,3-c]pyrazole) and spiro (acenaphthyl-3,4′-pyrano[2,3-c]pyrazoles via four-component reaction. Chin. Chem. Lett. 2015, 26, 889–893. [Google Scholar] [CrossRef]
- Yavari, I.; Baoosi, L.; Halvagar, M.R. A synthesis of fused acenaphthopyrrolizines via the 1,3-dipolar cycloaddition reaction of azomethine ylides with acetylenic esters. Mol. Divers. 2017, 21, 257–263. [Google Scholar] [CrossRef]
- Wei, A.C.; Ali, M.A.; Yoon, Y.K.; Ismail, R.; Choon, T.S.; Kumar, R.S. A facile three-component [3+2]-cycloaddition for the regioselective synthesis of highly functionalized dispiropyrrolidines acting as antimycobacterial agents. Bioorg. Med. Chem. Lett. 2013, 23, 1383–1386. [Google Scholar] [CrossRef]
- Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Perumal, S.; Ghabbour, H.A.; Fun, H.-K. A 1,3-dippolar cycloaddition-annulation protocol for the expedient region-, stereo-, and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron Lett. 2013, 54, 2515–2519. [Google Scholar] [CrossRef]
- Song, L.-L.; Yang, C.; Yu, Y.-Q.; Xu, D.-Z. A simple and green tandem Knoevenagel-phospha-Michael reaction for one-pot synthesis of 2-oxindol-3-yl-phosphonates catalyzed by a DABCO-based ionic liquid. Synthesis 2017, 49, 1641–1647. [Google Scholar]
- Yavari, I.; Baoosi, L.; Halvagar, M.R. A convenient synthesis of fused tetrahydroazocines from acenaphthylene-1,2-dione, proline, and acetylenic esters. Synlett 2018, 29, 635–639. [Google Scholar] [CrossRef]
- Wang, X.-H.; Yan, C.-G. Facile synthesis of spiro(indane-2,1′-pyrrolo[2,1-a]isoquinolines) via three-component reaction of isoquinolinium salts, indane-1,3-dione, and isatins. Synthesis 2014, 46, 1059–1066. [Google Scholar] [CrossRef]
- Gong, H.; Sun, J.; Yan, C.-G. Efficient synthesis of polycyclic dispirooxindoles via domino Diels-Alder cyclodimerization reaction. Tetrahedron 2014, 70, 6641–6650. [Google Scholar] [CrossRef]
- Ahadi, S.; Hosseini, G.; Bazgir, A. Synthesis of oxo-indolin-3-ylidene-1,3-dithioles. J. Iran. Chem. Soc. 2012, 9, 333–338. [Google Scholar] [CrossRef]
- Kiruthika, S.E.; Lakshmi, N.V.; Banu, B.R.; Perumal, P.T. A facile strategy for the one pot multicomponent synthesis of spiro dihydropyridines from amines and activated alkynes. Tetrahedron Lett. 2011, 52, 6508–6511. [Google Scholar] [CrossRef]
- Hopf, H. [2.2] Paracyclophanes in polymer chemistry and materials science. Angew. Chem. Int. Ed. 2008, 47, 9808–9812. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.E.; Knight, J.D. [2.2] Paracyclophane derivatives in asymmetric catalysis. Org. Biomol. Chem. 2003, 1, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Gulder, T.; Baran, P.S. Strained cyclophane natural products: Macrocyclization at its limits. Nat. Prod. Rep. 2012, 29, 899–934. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Spuling, E.; Knoll, D.M.; Bräse, S. Regioselective functionalization of [2.2] paracyclophanes: Recent synthetic progress and perspectives. Angew. Chem. Int. Ed. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Brown, A.B. Asymmetric and fused heterocycles based on [2.2] paracyclophane. Tetrahedron 2009, 65, 8055–8089. [Google Scholar] [CrossRef]
- Aly, A.A.; Hopf, H.; Ernst, L.; Dix, I.; Jones, P.G. New cycloadditions of (E)-N,α-dimethyl-α-(4-[2.2] paracyclophanyl)nitrone. Eur. J. Org. Chem. 2006, 2006, 3001–3006. [Google Scholar] [CrossRef]
- Aly, A.A. Cycloaddition of (E)-N-{2-([2.2]paracyclophan-4-yl)ethylidene}methylamine N-oxide with 2,3-diphenylcyclopropenones and dibenzoyl acetylene: Synthesis of new paracyclophanylpyrroles. J. Chem. Res. 2007, 2007, 451–454. [Google Scholar] [CrossRef]
- Aly, A.A.; Hopf, H.; Jones, P.G.; Dix, I. Cycloadditions of α-(4-[2.2]paracyclophane)-N-methyl nitrone. Tetrahedron 2006, 62, 4498–4505. [Google Scholar] [CrossRef]
- Hopf, H.; Aly, A.A.; Swaminathan, V.N.; Ernst, L.; Dix, I.; Jones, P.G. A simple route to a pyridinyl[2.2]paracyclophane. Eur. J. Org. Chem. 2005, 2005, 68–71. [Google Scholar] [CrossRef]
- Aly, A.A.; Bräse, S.; Weis, P. Tridentate and bidentate copper complexes of [2.2] paracyclophanyl-substituted thiosemicarbazones, thiocarbazones, hydrazones, and thioureas. J. Mol. Struct. 2019, 1178, 311–326. [Google Scholar] [CrossRef]
- Pascu, S.L.; Waghorn, P.A.; Churchill, G.C.; Sim, R.B. Synthesis of Metal Complexes with Thiosemicarbazone Derivatives for Use in Medical Imaging and Therapy. PCT International Application WO 2008025941 A2 20080306, 4 August 2008. [Google Scholar]
- Patil, P.B.; Patil, J.D.; Korade, S.N.; Kshirsagar, S.D.; Govindwar, S.P.; Pore, D.M. An efficient synthesis of anti-microbial 1,2,4-triazole-3-thiones promoted by acidic ionic liquid. Res. Chem. Intermed. 2016, 42, 4171–4180. [Google Scholar] [CrossRef]
- Mohammadi, M.K.; Firuzi, O.; Khoshneviszadeh, M.; Razzaghi-Asl, N.; Sepehri, S.; Miri, R. Novel 9-(alkylthio)-acenaphtho[1,2-e]-1,2,4-triazine derivatives: Synthesis, cytotoxic activity, and molecular docking studies on B-cell lymphoma 2 (Bcl-2). DARU J. Pharm. Sci. 2014, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Satheshkumar, A.; El-Mossalamy, E.H.; Manivannam, R.; Parthiban, C.; Al-Harbi, L.M.; Kosa, S.; Elango, K.P. Anion induced azo-hydrazine tautomerism for the selective colorimetric sensing of fluoride ion. Spectrochim. Acta A 2014, 128, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Kauer, J.C.; Simmons, H.E. Tetramers of acetylenedicarboxylic esters. J. Org. Chem. 1968, 33, 2720–2726. [Google Scholar] [CrossRef]
- Hocking, M.B.; van der Voort Maarschalk, F.W. X-ray structures of triphenylphosphine and 1,3,5-triphenylphosphole products with dimethyl acetylenedicarboxylate tetramer. Can. J. Chem. 1994, 72, 2428–2442. [Google Scholar] [CrossRef]
- Winterfeldt, E.; Giesler, G. Formation of trimethyl 2-methoxyfurantricarboxylate from dimethyl acetylenedicarboxylate. Angew. Chem. Int. Ed. 1966, 5, 579. [Google Scholar] [CrossRef]
- Banert, K.; Bochmann, S.; Ihle, A.; Plefka, O.; Taubert, F.; Walther, T.; Korb, M.; Rueffer, T.; Lang, H. Synthesis with perfect atom economy: Generation of furan derivatives by 1,3-dipolar cycloaddition of acetylenedicarboxylates at cyclooctynes. Molecules 2014, 19, 14022–14035. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Gonzalez-Gomez, A.; Dominguez, G.; Perez-Castells, J. Medium-sized and strained heterocycles from non-catalyzed and gold-catalyzed conversions of β-carbolines. Org. Biomol. Chem. 2012, 10, 7167–7176. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Luo, H.; Tao, G.; Cai, W.; Cao, J.; Duan, Z.; Mathey, F. Selective synthesis of (Z)-diazadiphosphafulvalene from 2,2′-bis-azaphosphindole. Org. Lett. 2018, 20, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, R.L.; Waghorn, P.A.; Jones, M.W.; Bauman, A.; Brayshaw, S.K.; Hu, Z.; Kociok-Köhn, G.; Mindt, T.L.; Tyrrell, R.M.; Botchway, S.W.; et al. Fluorescent gallium and indium bis(thiosemicarbazonates) and their radiolabeled analogues: Synthesis, structures, and cellular confocal imaging investigations. Dalton Trans. 2011, 40, 6238–6252. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Najjari, S.; Notash, B. Synthesis and crystal structure of a new thiosemicarbazone, acenaphthenequinone thiosemiscarbazone mono methanol. Iran. J. Crystallogr. Miner. 2015, 22, 109–114. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).