Highly Active Iminopyridyl Iron-Based Catalysts for the Polymerization of Isoprene
Abstract
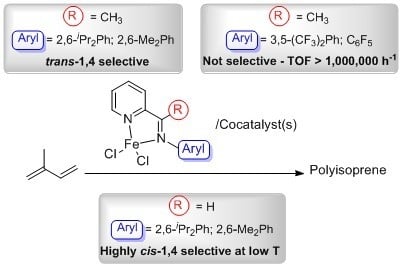
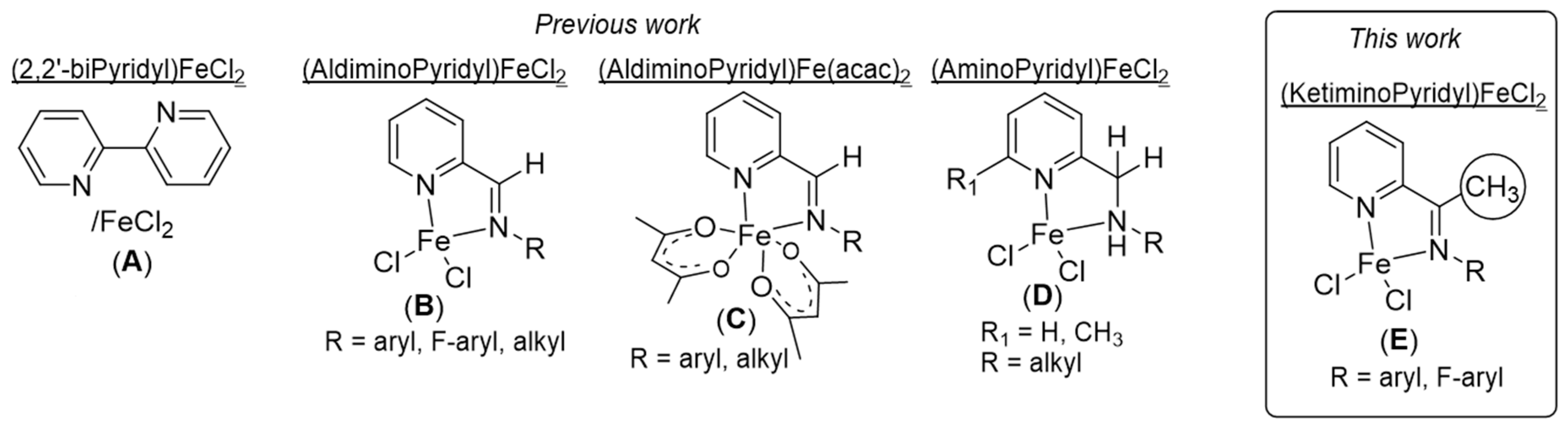
1. Introduction
2. Results and Discussion
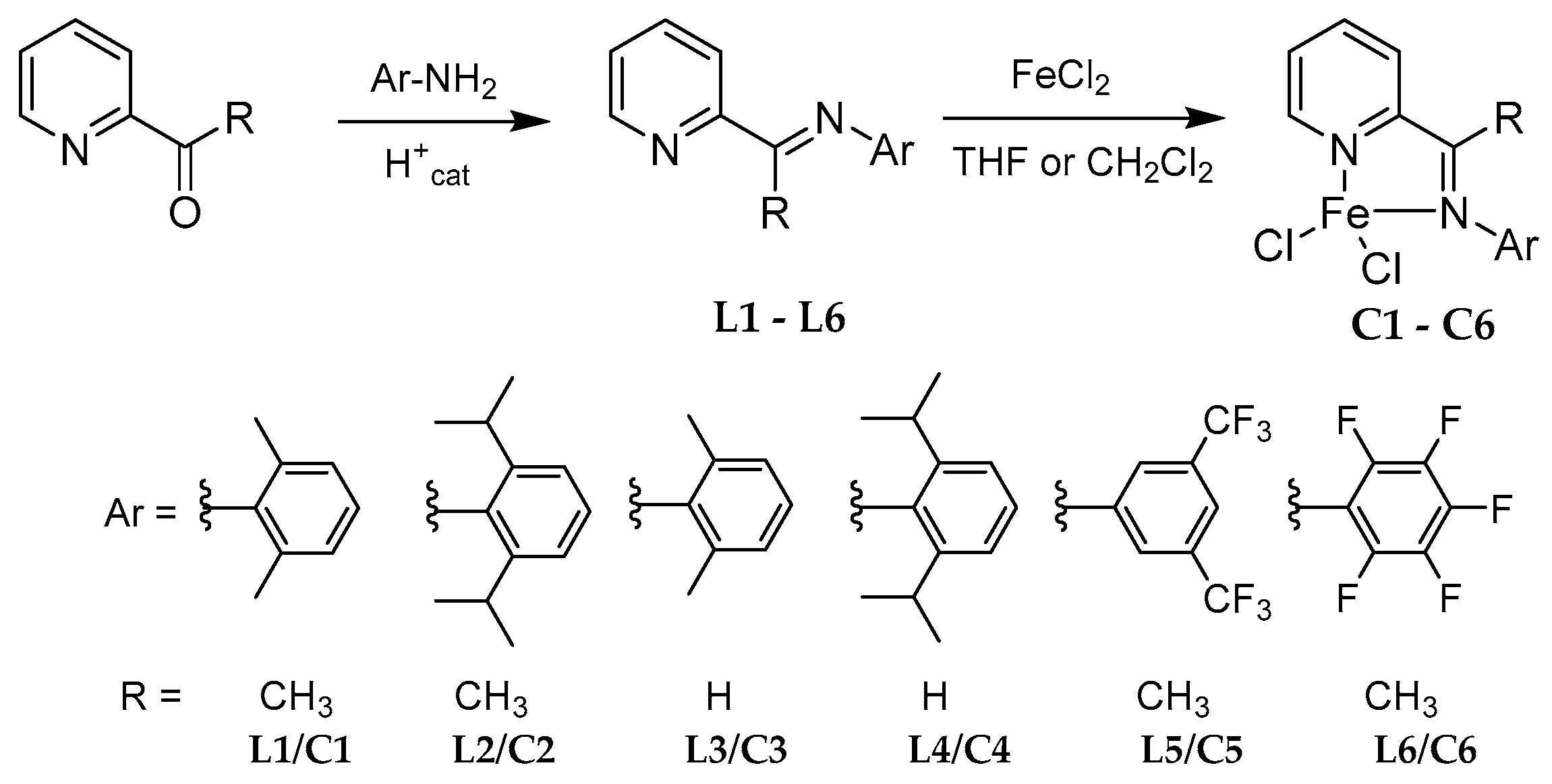
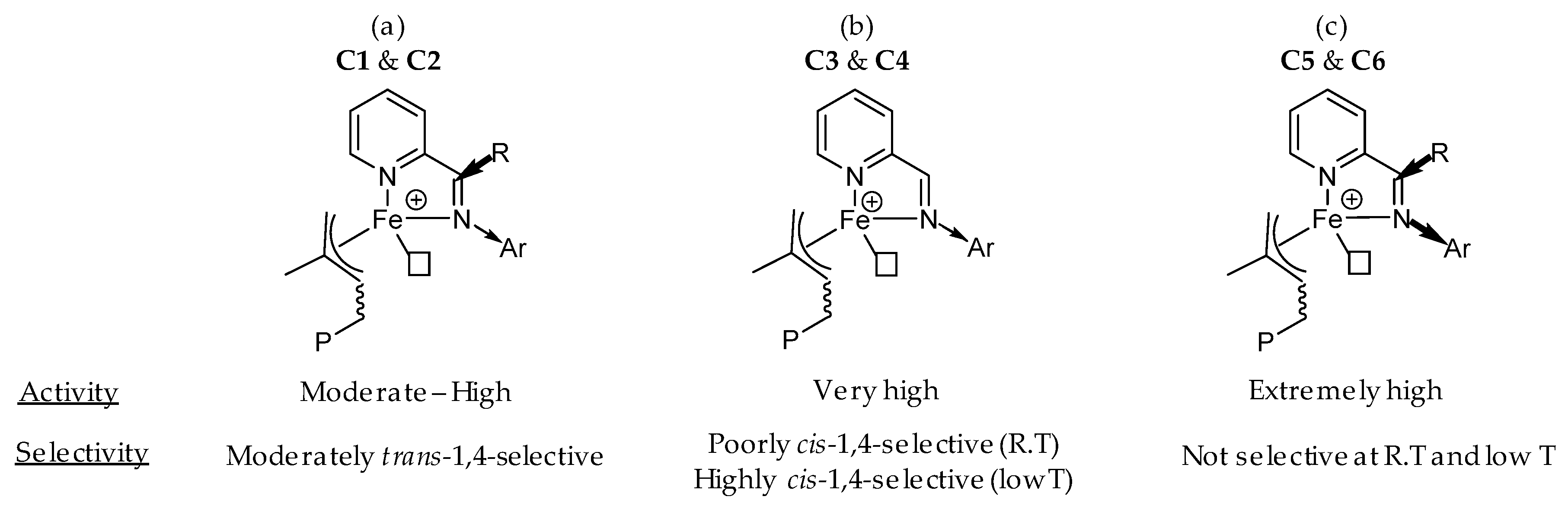
2.1. Synthesis of Pro-Ligands (L1–L6) and Their Corresponding Iron-Based Complexes (C1–C6)
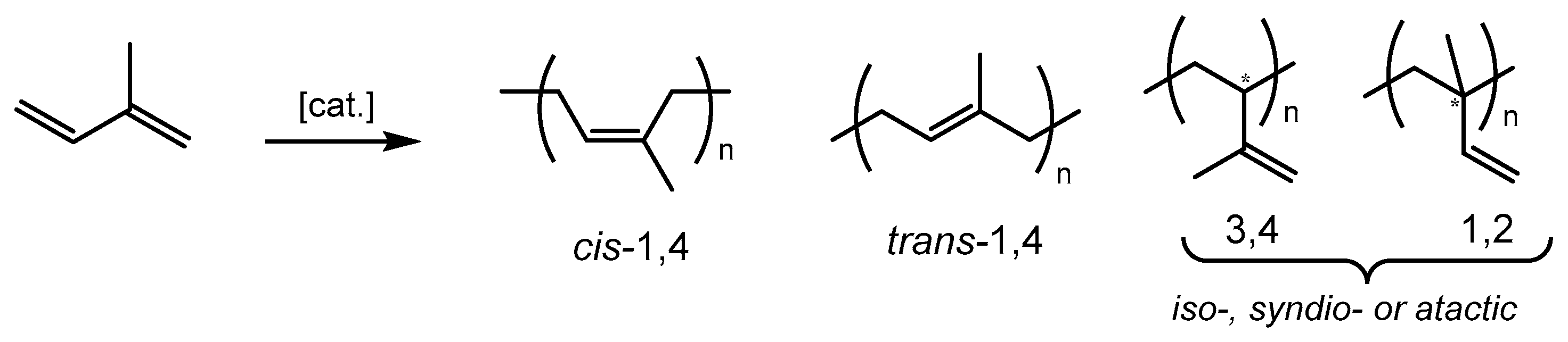
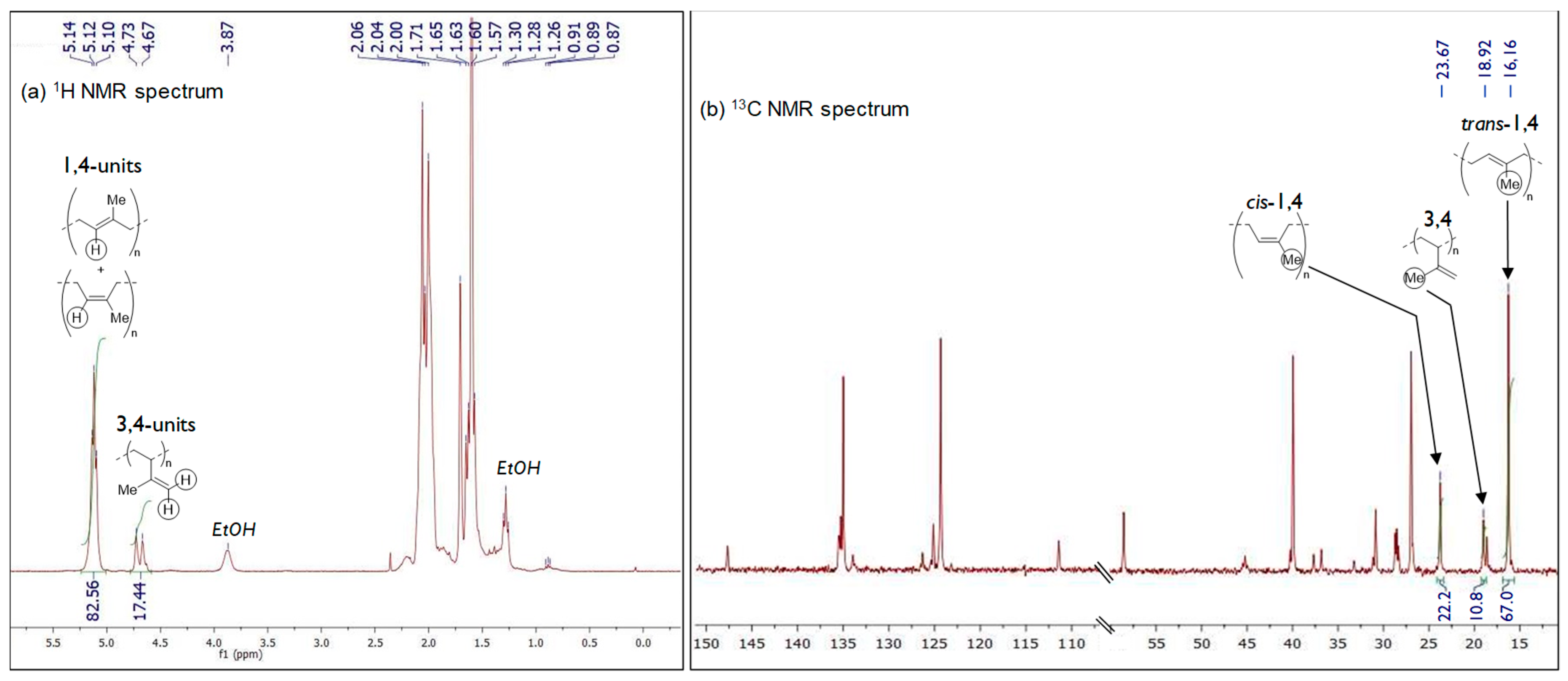
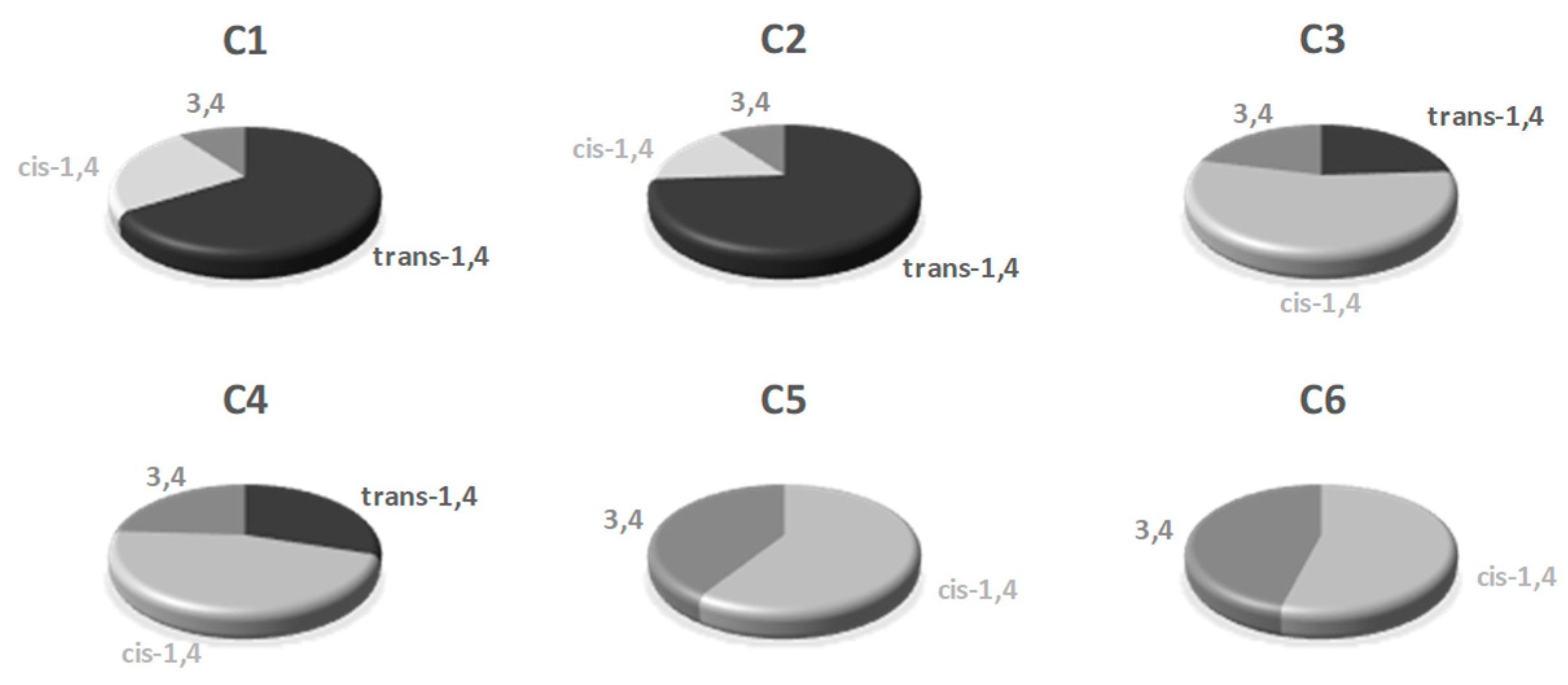
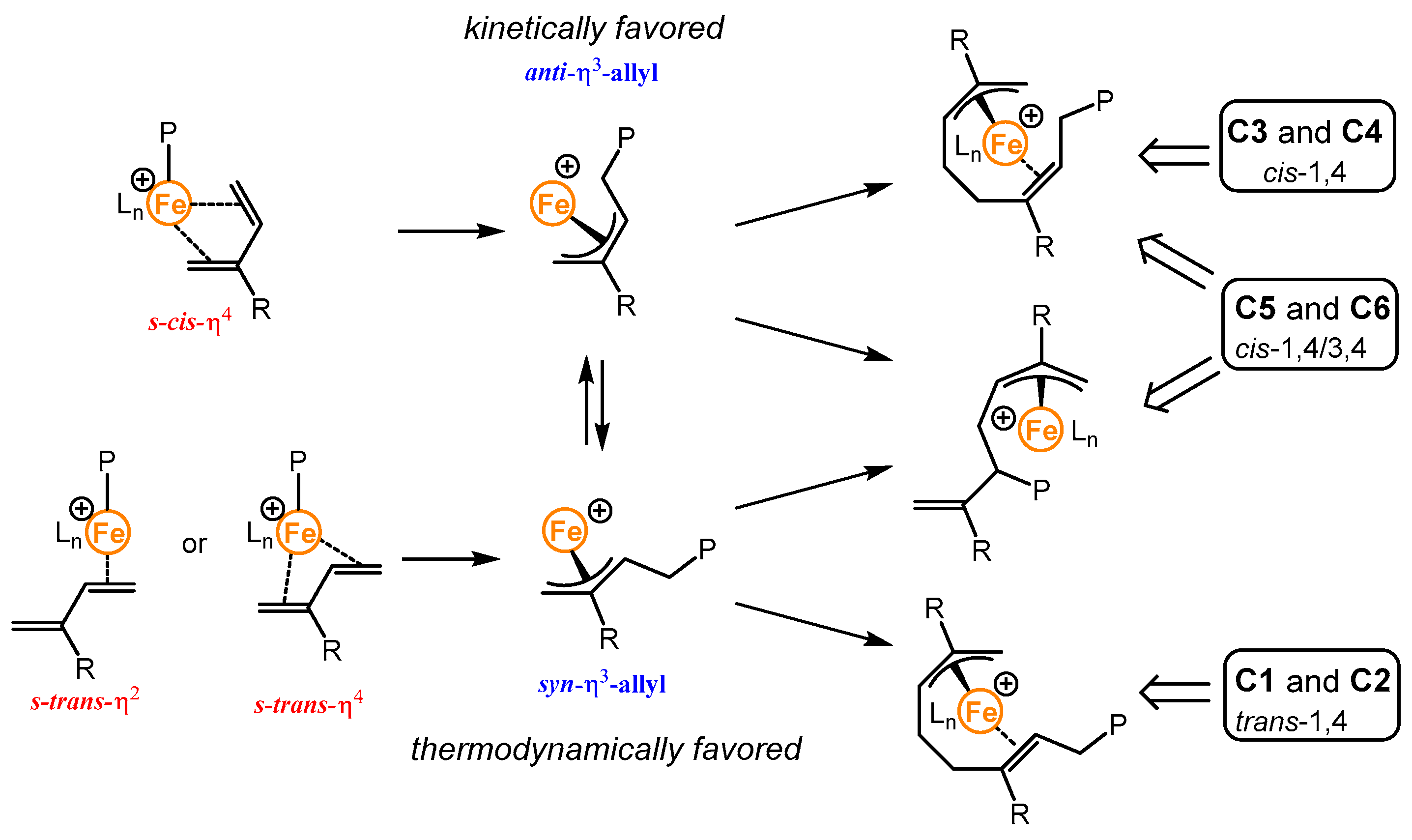
2.2. Polymerization of Isoprene with Iron-Based Complexes C1–C6
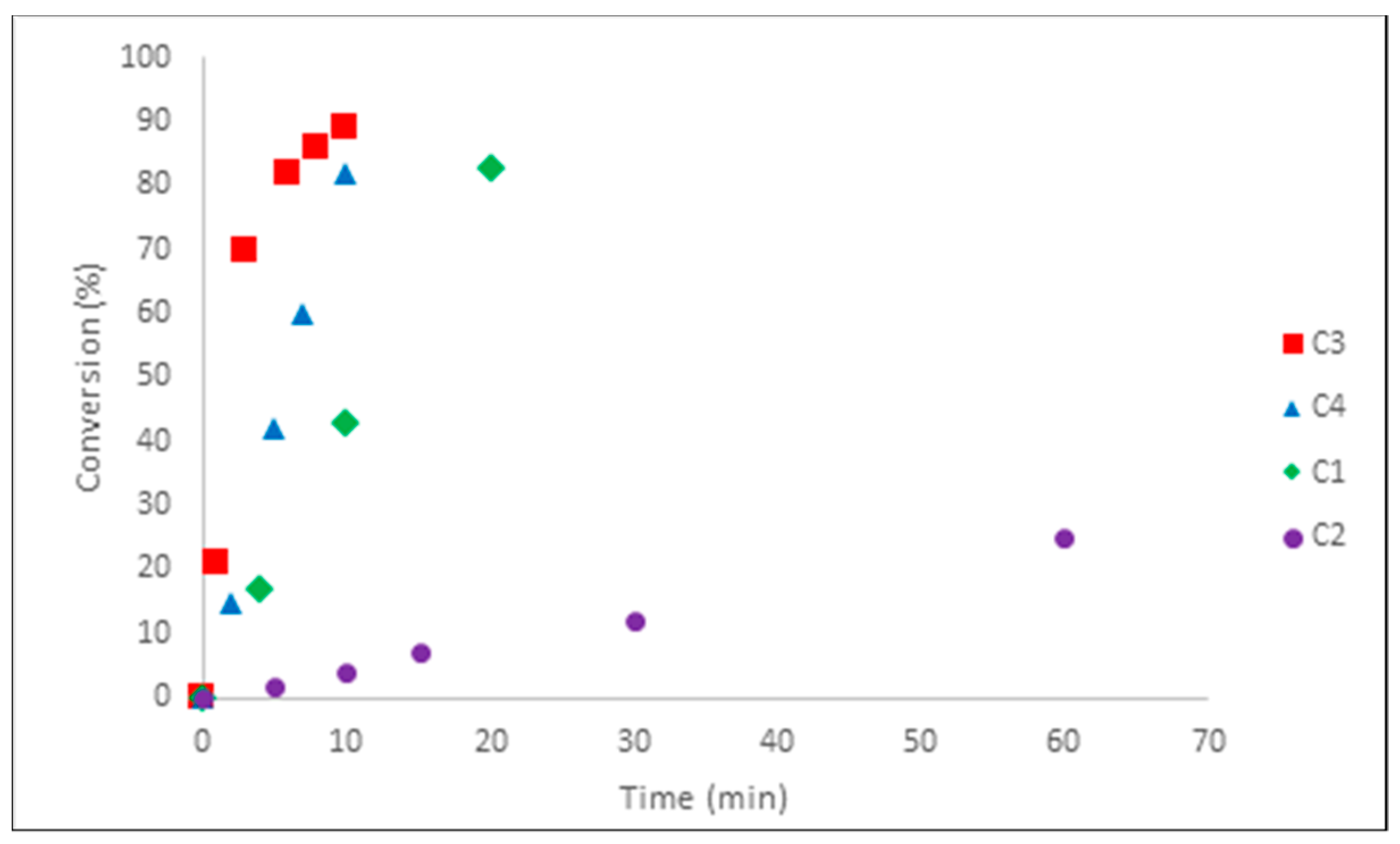
2.3. Kinetic Studies of the Polymerization of Isoprene with the Iron-Based Complexes C1–C6
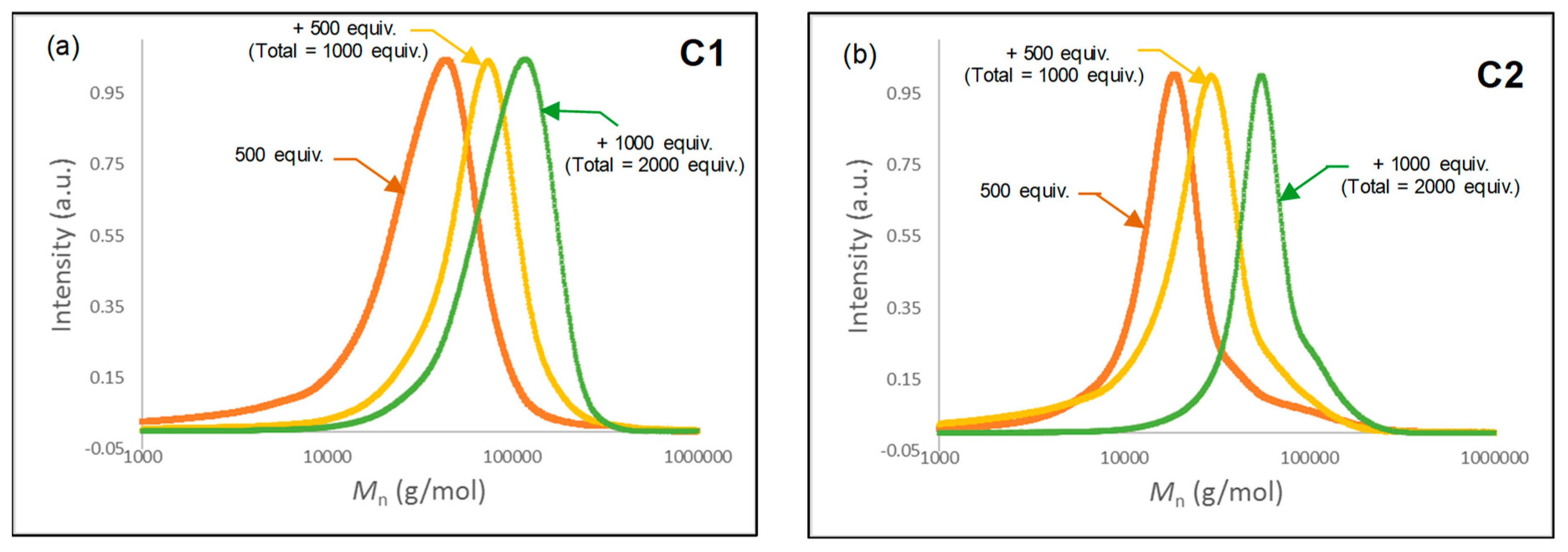
2.4. Sequential Polymerization of Isoprene with C1–C2/AliBu3/[Ph3C][B(C6F5)4]
2.5. Polymerization of Isoprene with Complexes C3–C6 at Lower Temperatures
3. Concluding Remarks
4. Experimental
4.1. General Information
4.2. General Procedure for the Synthesis of Ligands L1–L4
4.3. General Procedure for the Synthesis of Complexes C1–C6
4.4. General Procedure for Isoprene Polymerization
4.5. Calculation of Microstructure Contents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Britovsek, G.J.; Gibson, V.C.; McTavish, S.J.; Solan, G.A.; White, A.J.; Williams, D.J.; Kimberley, B.S.; Maddox, P.J. Novel Olefin Polymerization Catalysts Based on Iron and Cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Burcher, B.; Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H. Iron-Catalyzed Oligomerization and Polymerization Reactions. Top. Organomet. Chem. 2015, 50, 217–257. [Google Scholar]
- Li, L.; Gomes, P.T.; Bianchini, C.; Cole-Hamilton, D.J.; van Leeuwen, P.W.N.M. Olefin upgrading catalysis by nitrogen-based metal complexes II, Catalysis by metal complexes; Springer: Dordrecht, The Netherlands, 2012; Volume 36, pp. 77–197. [Google Scholar]
- Coates, G.W. Precise Control of Polyolefin Stereochemistry Using Single-Site Metal Catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Polymerization catalysis at the millennium: Frontiers in stereoselective, metal-catalyzed polymerization. J. Chem. Soc. Dalton Trans. 2002, 467–475. [Google Scholar] [CrossRef]
- Breuil, P.-A.; Magna, L.; Olivier-Bourbigou, H. Role of Homogeneous Catalysis in Oligomerization of Olefins: Focus on Selected Examples Based on Group 4 to Group 10 Transition Metal Complexes. Catal. Lett. 2015, 145, 173–192. [Google Scholar] [CrossRef]
- Champouret, Y.; Hashmi, O.H.; Visseaux, M. Discrete Iron-Based Complexes: Applications in Homogeneous Coordination-Insertion Polymerization Catalysis. Coord. Chem. Rev. 2019, 390, 127–170. [Google Scholar] [CrossRef]
- Kwag, G.; Kim, P.; Han, S.; Choi, H. Ultra high cis polybutadiene by monomeric neodymium catalyst and its tensile and dynamic properties. Polymer 2005, 46, 3782–3788. [Google Scholar] [CrossRef]
- Zhao, J.; Ghebremeskel, G.N. A review of some of the factors affecting fracture and fatigue in SBR and BR vulcanizates. Rubber Chem. Technol. 2001, 74, 409–427. [Google Scholar] [CrossRef]
- Khodzhaeva, I.D.; Kislinovskaja, N.V.; Smurova, E.V. Stability of cis-1,4-Polyisoprene and Its Vulcanizates in Aqueous and Biologically Active Media. Int. J. Polym. Mater. Polym. Biomater. 1994, 25, 107–115. [Google Scholar] [CrossRef]
- Song, J.S.; Huang, B.C.; Yu, D.S. Progress of Synthesis and Application of trans-1,4-Polyisoprene. J. Appl. Polym. Sci. 2001, 82, 81–89. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Z. A comparative study on the properties of Eucommia ulmoides gum and synthetic trans-1,4-polyisoprene. Polym. Test. 2011, 30, 753–759. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Cui, D.; Liu, X. Precisely Controlled Polymerization of Styrene and Conjugated Dienes by Group 3 Single-Site Catalysts. ChemCatChem 2018, 10, 42–61. [Google Scholar] [CrossRef]
- Jothieswaran, J.; Fadlallah, S.; Bonnet, F.; Visseaux, M. Recent Advances in Rare Earth Complexes Bearing Allyl Ligands and Their Reactivity towards Conjugated Dienes and Styrene Polymerization. Catalysts 2017, 7, 378. [Google Scholar] [CrossRef]
- Srivastava, V.K.; Maiti, M.; Basak, G.C.; Jasra, R.V. Role of catalysis in sustainable production of synthetic elastomers. J. Chem. Sci. 2014, 126, 415–427. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, C.; Bo, L.; Liu, D.; Yang, Y. Polymerization of 1,3-Conjugated Dienes with Rare-Earth Metal Precursors. Struct. Bond. 2010, 137, 49–108. [Google Scholar]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-Defined Transition Metal Complexes with Phosphorus and Nitrogen Ligands for 1,3-Dienes Polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-Based Ziegler/Natta Catalysts and Their Application in Diene Polymerization. In Neodymium Based Ziegler Catalysts – Fundamental Chemistry; Nuyken, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 204, pp. 1–154. [Google Scholar]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Metal Compounds: Knowledge and Myths. Organometallics 2017, 36, 4071–4090. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L. Diethylbis (2, 2′-Bipyridine) Iron/MAO. A Very Active and Stereospecific Catalyst for 1, 3-Diene Polymerization. Macromol. Rapid Commun. 2002, 23, 922–927. [Google Scholar] [CrossRef]
- Ricci, G. Polymerization of 1,3-Dienes with Iron Complexes Based Catalysts Influence of the Ligand on Catalyst Activity and Stereospecificity. J. Mol. Cat. A: Chem. 2003, 204–205, 287–293. [Google Scholar] [CrossRef]
- Luo, L.; Kang, X.; Zhou, G.; Chen, S.; Luo, G.; Qu, J.; Luo, Y. Mechanistic Insights into Regioselective Polymerization of 1,3-Dienes Catalyzed by a Bipyridine-Ligated Iron Complex: A DFT Study. Int. J. Quant. Chem. 2016, 116, 1274–1280. [Google Scholar]
- Raynaud, J.; Wu, J.Y.; Ritter, T. Iron-Catalyzed Polymerization of Isoprene and Other 1,3-Dienes. Angew. Chem. Int. Ed. 2012, 51, 11805–11808. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jing, X.; Xiong, S.; Liu, W.; Liu, Y.; Liu, Z.; Chen, C. Influences of Alkyl and Aryl Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers 2016, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, X.; Zhao, M.; Wang, L.; Jing, C.; Wang, P.; Wang, X.; Wang, Q. Influences of Fluorine Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers 2018, 10, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, L.; Mahmood, Q.; Jing, C.; Zhu, G.; Zhang, X.; Wang, X.; Wang, Q. Controlled Isoprene Polymerization Mediated by Iminopyridine-Iron (II) Acetylacetonate Pre-Catalysts. Appl. Organomet. Chem. 2019, e4836. [Google Scholar] [CrossRef]
- Jing, C.; Wang, L.; Mahmood, Q.; Zhao, M.; Zhu, G.; Zhang, X.; Wang, X.; Wang, Q. Synthesis and characterization of aminopyridine iron(II) chloride catalysts for isoprene polymerization: Sterically controlled monomer enchainment. Dalton Trans. 2019, 48, 7862–7874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, G.; Mahmood, Q.; Zhao, M.; Wang, L.; Jing, C.; Wang, X.; Wang, Q. Iminoimidazole-Based Co(II) and Fe(II) Complexes: Syntheses, Characterization, and Catalytic Behaviors for Isoprene Polymerization. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 767–775. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Jia, X.-Y.; Liu, L.; Bi, J.-F.; Zhang, C.-Y.; Zhao, L.-P.; Bai, C.-X.; Hu, Y.-M.; Zhang, X.-Q. Synthesis, Characterization, and 1,3-Butadiene Polymerization Studies of Co(II), Ni(II), and Fe(II) Complexes Bearing 2-(N-Arylcarboximidoylchloride)Quinoline Ligand. J. Mol. Cat. A: Chem. 2014, 391, 25–35. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Tan, P.T.; Fazzini, P.-F.; Hungria, T.; Durand, J.; Lachaize, S.; Sun, W.-H.; Respaud, M.; Soulantica, K.; et al. Isoprene Polymerization on Iron Nanoparticles Confined in Carbon Nanotubes. Chem. Eur. J. 2015, 21, 17437–17444. [Google Scholar] [CrossRef] [PubMed]
- Laine, T.V.; Piironen, U.; Lappalainen, K.; Klinga, M.; Aitola, E.; Leskelä, M. Pyridinylimine-Based Nickel (II) and Palladium (II) Complexes: Preparation, Structural Characterization and Use as Alkene Polymerization Catalysts. J. Organomet. Chem. 2000, 606, 112–124. [Google Scholar] [CrossRef]
- Bianchini, C.; Man Lee, H.; Mantovani, G.; Meli, A.; Oberhauser, W. Bis-Alkoxycarbonylation of Styrene by Pyridinimine Palladium Catalysts. New J. Chem. 2002, 26, 387–397. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Zhang, L.; Zhang, D.; Leng, X.; Huang, Z. Selective Synthesis of Secondary Benzylic (Z)-Allylboronates by Fe-Catalyzed 1,4-Hydroboration of 1-Aryl-Substituted 1,3-Dienes. Org. Chem. Front. 2014, 1, 1101–1106. [Google Scholar] [CrossRef]
- Laine, T.V.; Klinga, M.; Leskelä, M. Synthesis and X-Ray Structures of New Mononuclear and Dinuclear Diimine Complexes of Late Transition Metals. Eur. J. Inorg. Chem. 1999, 959–964. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, W.; Yang, J.; Du, H. A Continuously Regenerable Chiral Ammonia Borane for Asymmetric Transfer Hydrogenations. Angew. Chem. Int. Ed. 2018, 57, 12111–12115. [Google Scholar] [CrossRef] [PubMed]
- Nienkemper, K.; Kotov, V.V.; Kehr, G.; Erker, G.; Fröhlich, R. Chelate [2-(Iminoethyl)PyridineN-Oxide]Metal Complexes - Synthesis and Structural Comparison with Their Chemically Related 2-(Iminoethyl)Pyridine-Derived Systems. Eur. J. Inorg. Chem. 2006, 2006, 366–379. [Google Scholar] [CrossRef]
- Wu, J.Y.; Moreau, B.; Ritter, T. Iron-Catalyzed 1,4-Hydroboration of 1,3-Dienes. J. Am. Chem. Soc. 2009, 131, 12915–12917. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; O’Reilly, R.K.; Wass, D.F.; White, A.J.; Williams, D.J. Iron Complexes Bearing Iminopyridine and Aminopyridine Ligands as Catalysts for Atom Transfer Radical Polymerisation. Dalton Trans. 2003, 14, 2824–2830. [Google Scholar] [CrossRef]
- Tobisch, S. The Stereospecific Polymerization of 1,3-Butadiene Mediated by Early and Late Transition-Metal Catalysts. Towards a Deeper Understanding of the Catalytic Structure–reactivity Relationships from Computational-Mechanistic Studies. J. Mol. Struct. THEOCHEM 2006, 771, 171–179. [Google Scholar] [CrossRef]
- Tobisch, S. Mechanistic Insight into the Selective Trans.-1,4-Polymerization of Butadiene by Terpyridine–iron(II) Complexes—A Computational Study. Can. J. Chem. 2009, 87, 1392–1405. [Google Scholar] [CrossRef]
- Beebe, D.H. Structure of 3,4-(Cis-1,4-)Trans-1,4-Polyisoprene by NMR. Polymer 1978, 19, 231–233. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, H.; Seimiya, T. 13C-NMR of Polyisoprenes: Sequence Distribution of Cis-1,4 and Trans.-1,4 Units. Polymer J. 1975, 7, 264–266. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Not available. |
| Entry a | Complex | Conv. (%) | Mn(exp)b (g/mol) | Đb | Microstructure c (%) | |
|---|---|---|---|---|---|---|
| 1,4 (trans/cis) | 3,4 | |||||
| 1 | C1 | >99 | 44,000 | 1.5 | 90 (67/22) | 11 |
| 2 | C2 | >99 | 44,000 | 1.7 | 90 (74/16) | 10 |
| 3 | C3 | >99 | 101,000 | 2.7 | 79 (24/55) | 21 |
| 4 | C4 | >99 | 84,000 | 1.5 | 76 (30/46) | 24 |
| 5 | C5 | >99 | 513,000 | 2.4 | 59 (0/59) | 41 |
| 6 | C6 | >99 | 382,000 | 1.8 | 54 (0/54) | 46 |
| Entry | Complex | Conv. (%) | Time (min) | TOF (h−1) | Mn(exp)b (g/mol) | Đb | Microstructure c (%) | |
|---|---|---|---|---|---|---|---|---|
| 1,4 (trans/cis) | 3,4 | |||||||
| 1 | C1 | 83 | 20 | 12,450 | 142,000 | 1.9 | 90 (60/30) | 10 |
| 2 | C2 | 25 | 60 | 1250 | 87,000 | 1.4 | 84 (69/18) | 13 |
| 3 | C3 | 89 | 10 | 26,700 | 105,000 | 1.8 | 79 (25/54) | 21 |
| 4 | C4 | 82 | 10 | 23,400 | 163,000 | 1.6 | 74 (26/48) | 26 |
| 5 | C5 | >99 | < 1 | > 300,000 | 323,000 | 1.7 | 58 (0/58) | 42 |
| 6 | C6 | >99 | < 1 | > 300,000 | 213,000 | 1.6 | 54 (0/54) | 46 |
| Entry | Complex | Total Monomer (intermediate addition of monomer) (equiv./Fe) | Time (min) | Conv. (%) | Mn(exp)b (g/mol) | Đb |
|---|---|---|---|---|---|---|
| 1 | C1 | 500 (500) | 15 | >99 | 36,500 | 1.6 |
| 1000 (+500) | 30 (+15) | >99 | 69,000 | 1.5 | ||
| 2000 (+1000) | 60 (+30) | >99 | 102,000 | 1.3 | ||
| 2 | C2 | 500 (500) | 30 | >99 | 23,000 | 2.4 |
| 1000 (+500) | 60 (+30) | >99 | 32,000 | 2.0 | ||
| 2000 (+1000) | 120 (+60) | >99 | 62,000 | 1.3 |
| Entry | Complex | T (°C) | Time (min) | Conv. (%) | TOF (h−1) | Mn(exp)b (g/mol) | Đb | Microstructure c (%) | |
|---|---|---|---|---|---|---|---|---|---|
| 1,4 (trans/cis) | 3,4 | ||||||||
| 1 | C5 | 0 | 10 | >99 | 3000 | 294,000 | 1.2 | 58 (0/58) | 42 |
| 2 | –20 | 10 | >99 | 3000 | 251,000 | 1.3 | 58 (0/58) | 42 | |
| 3 | –40 | 10 | >99 | 3000 | 173,000 | 1.3 | 58 (0/58) | 42 | |
| 4 | –78 | 480 | traces | - | - | - | - | - | |
| 5 | C6 | –40 | 10 | >99 | 3000 | 274,500 | 1.2 | 54 (0/54) | 46 |
| 6 | –78 | 180 | traces | - | - | - | - | - | |
| 7 | C3 | –40 | 300 | >99 | 100 | 169,000 | 1.3 | 89 (0/89) | 11 |
| 8 | C4 | –40 | 300 | >99 | 100 | 216,000 | 1.6 | 88 (0/88) | 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashmi, O.H.; Champouret, Y.; Visseaux, M. Highly Active Iminopyridyl Iron-Based Catalysts for the Polymerization of Isoprene. Molecules 2019, 24, 3024. https://doi.org/10.3390/molecules24173024
Hashmi OH, Champouret Y, Visseaux M. Highly Active Iminopyridyl Iron-Based Catalysts for the Polymerization of Isoprene. Molecules. 2019; 24(17):3024. https://doi.org/10.3390/molecules24173024
Chicago/Turabian StyleHashmi, Obaid H., Yohan Champouret, and Marc Visseaux. 2019. "Highly Active Iminopyridyl Iron-Based Catalysts for the Polymerization of Isoprene" Molecules 24, no. 17: 3024. https://doi.org/10.3390/molecules24173024
APA StyleHashmi, O. H., Champouret, Y., & Visseaux, M. (2019). Highly Active Iminopyridyl Iron-Based Catalysts for the Polymerization of Isoprene. Molecules, 24(17), 3024. https://doi.org/10.3390/molecules24173024