Understanding Conformational Preferences of Atropisomeric Hydrazides and Its Influence on Excited State Transformations in Crystalline Media
Abstract
1. Introduction
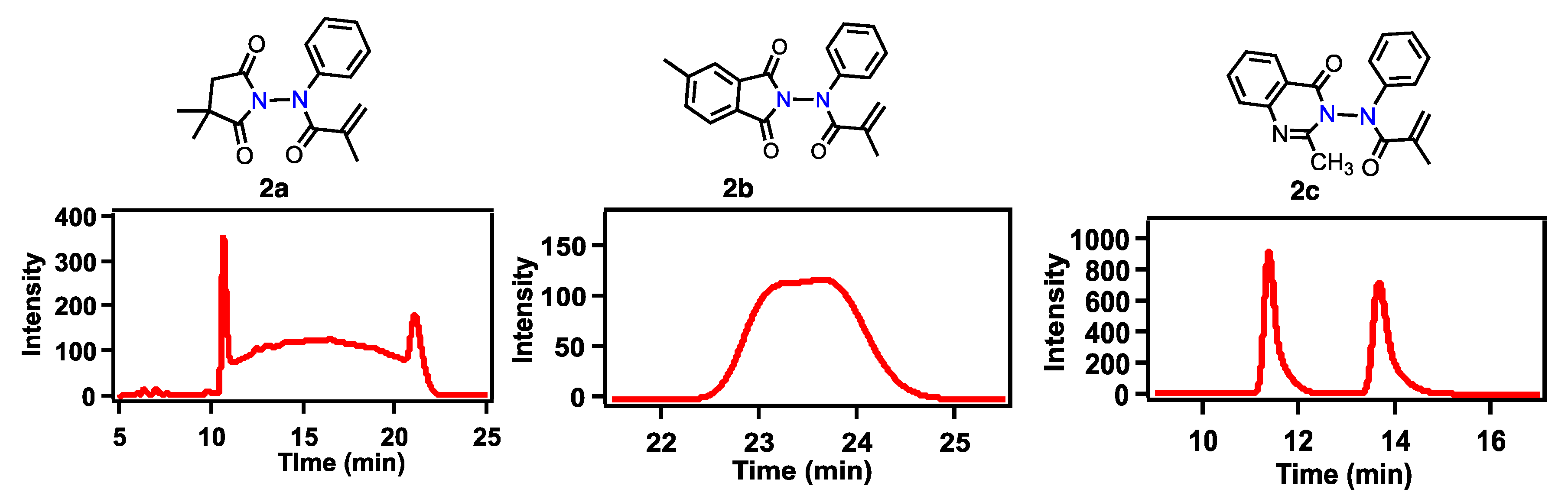
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Jong, J.J.D.; Tiemersma-Wegman, T.D.; Van Esch, J.H.; Feringa, B.L. Dynamic Chiral Selection and Amplification Using Photoresponsive Organogelators. J. Am. Chem. Soc. 2005, 127, 13804–13805. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.J.; Schafmeister, C.; Bird, G.; Paul, A.; Naaman, R.; Waldeck, D.H. Molecular Chirality and Charge Transfer through Self-Assembled Scaffold Monolayers. J. Phys. Chem. B 2006, 110, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V.; Sivaguru, J. Supramolecular Photochemistry as a Potential Synthetic Tool: Photocycloaddition. Chem. Rev. 2016, 116, 9914–9993. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Ahuja, S.; Jockusch, S.; Ugrinov, A.; Sivaguru, J. Conjugate addition from the excited state. Chem. Commun. 2018, 54, 11021–11024. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Jockusch, S.; Sivaguru, J. A photo-auxiliary approach–enabling excited state classical phototransformations with metal free visible light irradiation. Chem. Commun. 2017, 53, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.W.; Gilchrist, T.L.; Stanton, E. Reactives intermediates. XIV. Photochemistry of phthalimidoaziridines and related systems. J. Chem. Soc. C 1971, 988–993. [Google Scholar] [CrossRef]
- Couture, A.; Lebrun, S.; Deniau, E.; Grandclaudon, P. Synthesis of Cyclic Enehydrazides by Ring-Closing Metathesis. Synthesis 2006, 2006, 3490–3494. [Google Scholar] [CrossRef]
- Lebrun, S.; Couture, A.; Deniau, E.; Grandclaudon, P. A Practical Photochemically Induced Method for N-N Bond Cleavage of N,N-Disubstituted Hydrazides. Synlett 2009, 2009, 2621–2624. [Google Scholar]
- Watterson, A.C.; Shama, S.A. Photochemistry of acid hydrazides. Determination of modes of reaction and identification of photoproducts. J. Org. Chem. 1975, 40, 19–24. [Google Scholar] [CrossRef]
- Breliere, J.C.; Lehn, J.M. N.m.r. studies of rate processes and conformation. A double rate process. Chem. Commun. 1965, 426–427. [Google Scholar] [CrossRef]
- King, G.S.D. The crystal and molecular structure of NN-prime or minute]-bisuccinimidyl. J. Chem. Soc. B Phys. Org. 1966, 1224–1229. [Google Scholar] [CrossRef]
- Korsch, B.H.; Riggs, N.V. Restricted rotation about N-N single bonds. The conformation of tetrahydropyridazine rings. Tetrahedron Lett. 1966, 7, 5897–5903. [Google Scholar] [CrossRef]
- Price, B.; Sutherland, I.O.; Williamson, F.G. Conformational changes in diacyl-tetrahydropyridazine and piperidazine systems. Tetrahedron 1966, 22, 3477–3490. [Google Scholar] [CrossRef]
- Bishop, G.J.; Price, B.J.; Sutherland, I.O. Torsional barriers in NN′-diacylhydrazines. Chem. Commun. 1967, 14, 672–674. [Google Scholar] [CrossRef]
- Moriarty, R.M., Sr.; Murphy, M.R.; Druck, S.J.; May, L. Conformational studies on the amido group. Hindered internal rotation in N,N′-dialkyl hydrazocarboxylates. Tetrahedron Lett. 1967, 8, 1603–1609. [Google Scholar] [CrossRef]
- Atkinson, R.S.; Judkins, B.D.; Patwardhan, B. Rotational isomerism in N-(N-heteroaryl)arenesulphenamides. J. Chem. Soc. Perkin Trans. 2 1979, 1490–1495. [Google Scholar] [CrossRef]
- Atkinson, R.S.; Kelly, B.J.; Williams, J. Amination with 3-acetoxyaminoquinazolin-4-(3h)ones: Preparation of α-aminoacid esters by reaction with silyl ketene acetals followed by N N bond cleavage. Tetrahedron 1992, 48, 7713–7730. [Google Scholar] [CrossRef]
- Atkinson, R.S.; Barker, E.; Price, C.J.; Russell, D.R. The N-N bond as a chiral axis: 3-(diacylamino)quinazolin-4(3H)ones as chiral acylating agents. Chem. Commun. 1994, 1159–1160. [Google Scholar] [CrossRef]
- Atkinson, R.S.; Barker, E.; Edwards, P.J.; Thomson, G.A. The N-N bond as a chiral axis: 3-diacylaminoquinazolinones as chiral acylating agents. J. Chem. Soc. Perkin Trans. 1 1996, 1047–1055. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Atkinson, R.S.; Fawcett, J.; Russell, D.R. Stereoisomerism in 3-[N-(2-acetoxypropanoyl)-N-acylamino]quinazolin-4(3H )-ones, enantioselective acylating agents. J. Chem. Soc. Perkin Trans. 1 2000, 4413–4421. [Google Scholar] [CrossRef]
- Arthur, R.J.; Coogan, M.P.; Casadesus, M.; Haigh, R.; Headspith, D.A.; Francesconi, M.G.; Laye, R.H. Stereostructural behaviour of N–N atropisomers: Two conglomerate crystallisations and a crystallisation-induced deracemisation. CrystEngComm 2009, 11, 610–619. [Google Scholar] [CrossRef]
- Aitken, K.M.; Aitken, R.A.; Slawin, A.M.Z. The X-ray Structure of N-(Acetylamino)phthalimide, an Atypical Triacylhydrazine. J. Chem. Crystallogr. 2013, 44, 25–29. [Google Scholar] [CrossRef]
- Iyer, A. Visible Light Mediated Photocatalysis of N-N Bond Based Compounds; North Dakota State Univeristy: Fargo, ND, USA, 2016. [Google Scholar]
- Fletcher, J.R.; Sutherland, I.O. Conformational changes in hydrazine and hydroxylamine derivatives studied by nuclear magnetic resonance spectroscopy. Chem. Commun. 1970, 687–688. [Google Scholar] [CrossRef]
- Verma, S.M.; Rao, S.O.; Sinha, K.O.P. Conformational analysis about N-N bond by NMR spectroscopy: N’-sulphyl derivatives of N-aminoimides of anthracene-citraconic anhydride and naphthalene-maleic anhydride adducts. Bull. Chem. Soc. Jap. 1974, 47, 2311–2314. [Google Scholar] [CrossRef]
- Verma, S.M.; Prasad, R. Conformational analysis by nuclear magnetic resonance spectroscopy. N’-derivatives of N-aminocamphorimides. J. Org. Chem. 1973, 38, 1004–1010. [Google Scholar] [CrossRef]
- Verma, S.M.; Sinha, K.O.P.; Rao, C.K. Studies on N,N′-Diimidyl Systems by Nuclear Magnetic Resonance Spectroscopy. Can. J. Chem. 1974, 52, 2399–2402. [Google Scholar] [CrossRef]
- Verma, S.M.; Singh, R.M. Assignment of Configurations to Adducts of 2-Substituted Anthracene with Maleic Anhydride by N.M.R spectroscopy. Aus. J. Chem. 1976, 29, 1215–1222. [Google Scholar] [CrossRef]
- Verma, S.M.; Singh, M.D. Structural elucidation with nuclear magnetic resonance spectroscopy. Diels-Alder adducts of 1-aminoanthracene and maleic anhydride: Restricted rotation about the aryl C(1)-N bond and intrinsic asymmetry about the imide (Nsp2-Csp3) system. J. Org. Chem. 1977, 42, 3736–3740. [Google Scholar] [CrossRef]
- Verma, S.M.; Prasad, R. Conformational analysis about the nitrogen -nitrogen bond by nuclear magnetic resonance spectroscopy. N’-Sulfonyl derivatives of N-aminocamphorimide. J. Org. Chem. 1973, 38, 3745–3749. [Google Scholar] [CrossRef]
- Srivastava, A.; Samtani, S.; Verma, S.M. Role of Lone Electron Pairs of Heterocyclic Moiety in Controlling the Conformation about N-N Bond. Proc. Indian Natn. Sci. Acad. 1994, 60, 447–455. [Google Scholar]
- Verma, S.M.; Singh, R.M. Structural Assignment by NMR analalysis of N-(Diacylamino)imide Derivatives. Diels-Alder Adducts of 2,3-Dimethylnaphthalene and 6,6-Diphethylfulvene with Maleic Anhydride. Bull. Chem. Soc. Jpn. 1978, 51, 516–519. [Google Scholar] [CrossRef]
- Srivastava, V. Preparation and stereochemistry of some hydrazones derived from cyclic ketones. J. Sci. Res. 2011, 55, 111–117. [Google Scholar]
- Trapp, O. Interconversion of stereochemically labile enantiomers (enantiomerization). Top. Curr. Chem. 2013, 341, 231–269. [Google Scholar]
- D’Acquarica, I.; Gasparrini, F.; Pierini, M.; Villani, C.; Zappia, G. Dynamic HPLC on chiral stationary phases: A powerful tool for the investigation of stereomutation processes. J. Sep. Sci. 2006, 29, 1508–1516. [Google Scholar] [CrossRef]
- Atkinson, R.S.; Judkins, B.D. Resolution of N-benzyl-N-(1,2-dihydro-2-oxoquinolin-1-yl)glycine. Tetrahedron Lett. 1979, 20, 4001–4002. [Google Scholar] [CrossRef]
- Atkinson, R.S.; Edwards, P.J.; Thomson, G.A. Asymmetric induction mediated by an N-N chiral axis. Chem. Commun. 1992, 1256–1257. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Atkinson, R.S.; Fawcett, J.; Russell, D.R. 3-Di-[(S)-2-acetoxypropanoyl]aminoquinazolin-4(3H)-ones: Stereostructure and application in kinetic resolution of amines. Tetrahedron Lett. 2000, 41, 2243–2246. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Atkinson, R.S.; Fawcett, J.; Russell, D.R. 3-(N,N-Diacylamino)quinazolin-4(3H)-ones as enantioselective acylating agents for amines. Tetrahedron Lett. 2000, 41, 2239–2242. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Atkinson, R.S.; Fawcett, J.; Russell, D.R. Kinetic resolution of amines by acylation using 3-diacylaminoquinazolin-4(3H)-ones. Chem. Commun. 2000, 43–44. [Google Scholar] [CrossRef]
- Atkinson, R.S.; Draycott, R.D.; Hirst, D.J.; Parratt, M.J.; Raynham, T.M. Completely diastereoselective aziridination of α,β-unsaturated acids via intramolecular reaction of 3-acetoxyaminoquinazolin-4(3H)-ones. Tetrahedron Lett. 2002, 43, 2083–2085. [Google Scholar] [CrossRef]
- Kamiński, K.; Obniska, J. Design, synthesis, and anticonvulsant activity of N-phenylamino derivatives of 3,3-dialkyl-pyrrolidine-2,5-diones and hexahydro-isoindole-1,3-diones. Bioorg. Med. Chem. 2008, 16, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors as per federal and university protocols. |





| Entry | Hydrazide | Torsional Angle (θ) |
|---|---|---|
| 1 | 1 | 76.9° |
| 2 | 2a | 72.4° |
| 3 | 2b | 72.8° |
| 4 | 2c | 82.8° |
| Entry | Solvent | Kinetic Parameters | ||
|---|---|---|---|---|
| τ1/2 (h) | krac (s−1) | ΔG‡rac (kcal mol−1) | ||
| 1 | Benzene | 0.89 | 2.15 × 10−4 | 23.9 |
| 2 | Ethyl acetate | 1.20 | 16.4 × 10−5 | 24.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, A.; Ugrinov, A.; Sivaguru, J. Understanding Conformational Preferences of Atropisomeric Hydrazides and Its Influence on Excited State Transformations in Crystalline Media. Molecules 2019, 24, 3001. https://doi.org/10.3390/molecules24163001
Iyer A, Ugrinov A, Sivaguru J. Understanding Conformational Preferences of Atropisomeric Hydrazides and Its Influence on Excited State Transformations in Crystalline Media. Molecules. 2019; 24(16):3001. https://doi.org/10.3390/molecules24163001
Chicago/Turabian StyleIyer, Akila, Angel Ugrinov, and J. Sivaguru. 2019. "Understanding Conformational Preferences of Atropisomeric Hydrazides and Its Influence on Excited State Transformations in Crystalline Media" Molecules 24, no. 16: 3001. https://doi.org/10.3390/molecules24163001
APA StyleIyer, A., Ugrinov, A., & Sivaguru, J. (2019). Understanding Conformational Preferences of Atropisomeric Hydrazides and Its Influence on Excited State Transformations in Crystalline Media. Molecules, 24(16), 3001. https://doi.org/10.3390/molecules24163001








