Synthesis, Characterization, and Pesticidal Activity of Emamectin Benzoate Nanoformulations against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of the EMB + NFs
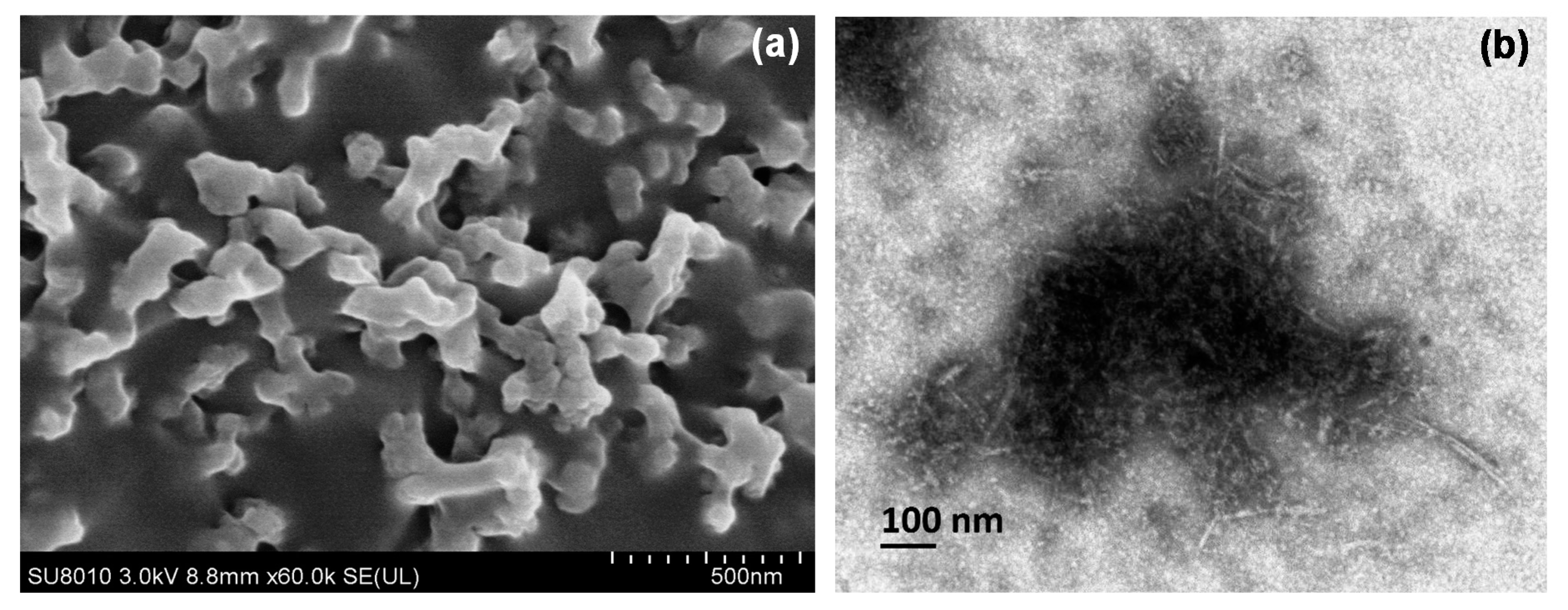
2.1.1. Morphology Analysis
2.1.2. X-ray Diffraction
2.1.3. FTIR Analysis
2.1.4. Thermogravimetric Analysis
2.1.5. Brunauer–Emmett–Teller (BET) Analysis
2.1.6. Zeta Potential
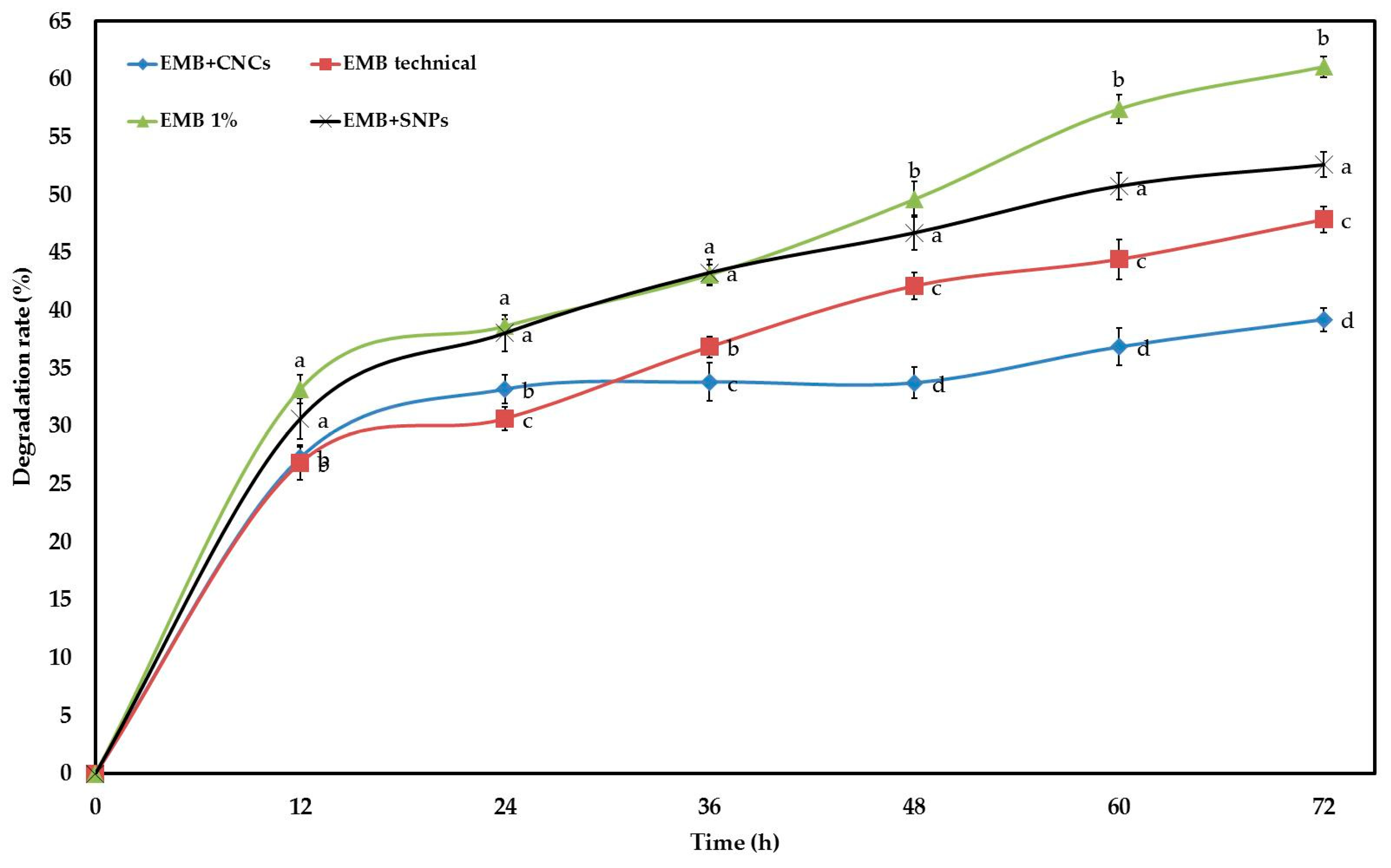
2.2. Stability Study
2.3. Bioactivity
3. Materials and Methods
3.1. Materials
Insect Culture
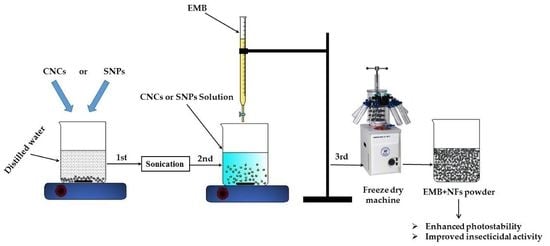
3.2. Preparation of EMB + NFs
3.2.1. Synthesis of CNCs and Loading EMB
3.2.2. Synthesis of SNPs and Loading EMB
3.3. Characterization
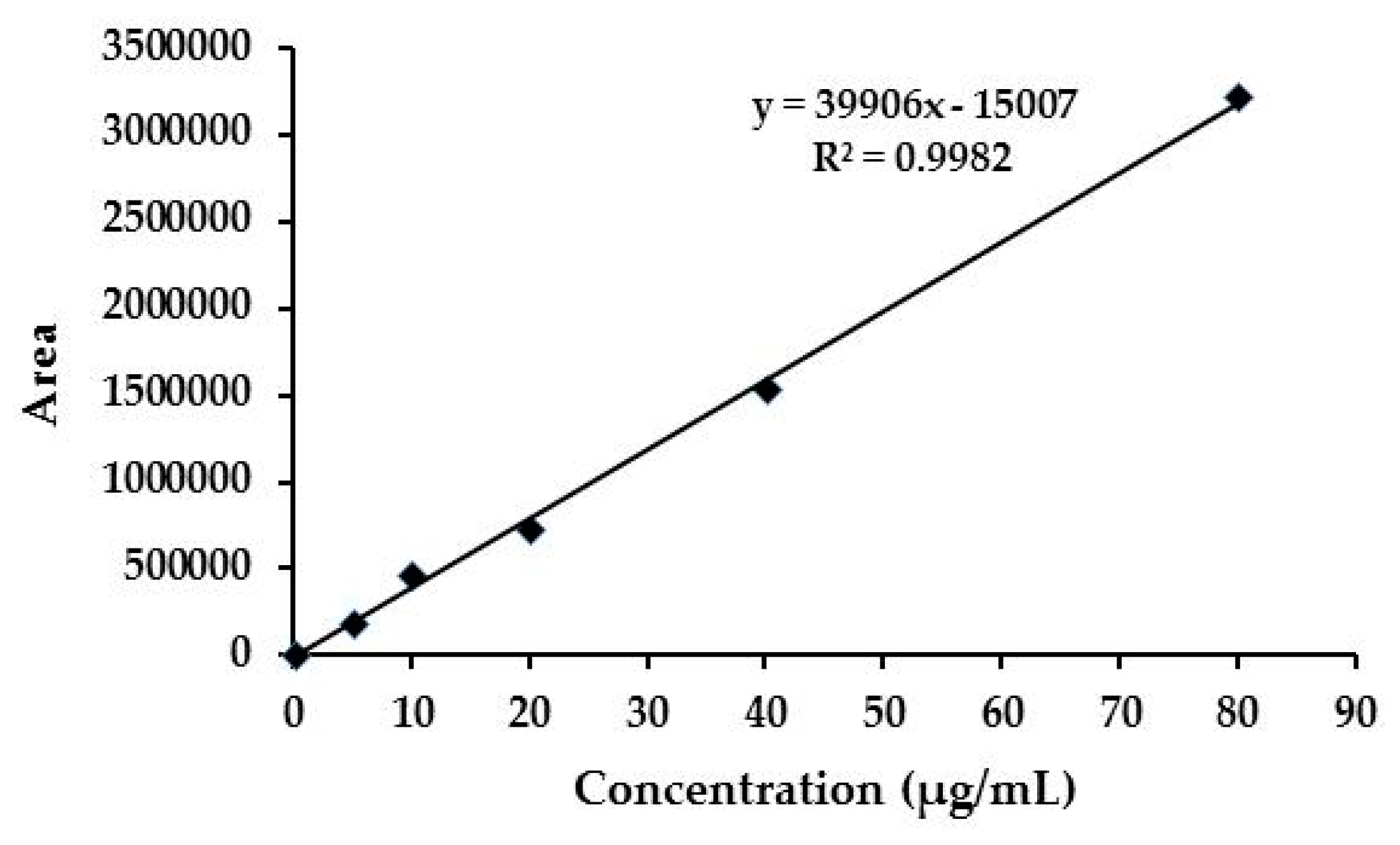
3.4. Quantitative Determination of Emamectin Benzoate in NFs
3.5. Stability Test
3.6. Bioassay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forim, M.R.; Costa, E.S.; da Silva, M.F.G.F.; Fernandes, J.B.; Mondego, J.M.; Boiça Junior, A.L. Development of a new method to prepare nano-/microparticles loaded with extracts of Azadirachta indica, their characterization and use in controlling Plutella xylostella. J. Agric. Food Chem. 2013, 61, 9131–9139. [Google Scholar] [CrossRef] [PubMed]
- Knowles, A. Recent developments of safer formulations of agrochemicals. The Environmentalist. 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Sun, C.; Wang, C.; Cui, B.; Zhao, X.; Zeng, Z.; Yao, J.; Yang, D.; Liu, G.; et al. Fabrication, characterization, and biological activity of avermectin nano-delivery systems with different particle sizes. Nanoscale Res. Lett. 2018, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Su, Y.; Yang, S.; Wu, L.; Liu, S.; Ling, C.; Yang, H. Hierarchical structure engineering of brookite TiO2 crystals for enhanced photocatalytic and external antitumor property. Sci. Bull. 2016, 61, 1818–1825. [Google Scholar] [CrossRef][Green Version]
- Patravale, V.; Date, A.A.; Kulkarni, R. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Sun, J.; Zhang, D.; Li, M.; Wang, Y.; Ling, G.; Liu, X.; Sun, Y.; Sui, X.; Luo, C. Nimodipine nanocrystals for oral bioavailability improvement: Preparation, characterization and pharmacokinetic studies. Colloids Surf. B Biointerfaces 2013, 109, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagné, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada, Minister of the Environment. Available online: http://gazette.gc.ca/rp-pr/p2/2012/2012-11-21/html/sor-dors229-eng.html#archiveda (accessed on 26 February 2019).
- Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.X.; Cahill, D.M. Mesoporous silica nanoparticles as a biomolecule delivery vehicle in plants. J. Nanopart. Res. 2013, 15, 1676. [Google Scholar] [CrossRef]
- Qian, K.; Shi, T.; He, S.; Luo, L.; Cao, Y. Release kinetics of tebuconazole from porous hollow silica nanospheres prepared by miniemulsion method. Microporous Mesoporous Mater. 2013, 169, 1–6. [Google Scholar] [CrossRef]
- Wibowo, D.; Zhao, C.-X.; Peters, B.C.; Middelberg, A.P. Sustained release of fipronil insecticide in vitro and in vivo from biocompatible silica nanocapsules. J. Agric. Food Chem. 2014, 62, 12504–12511. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-R.; Cui, S.-M.; Gao, F.; Liu, Y.-R.; Fan, C.-L.; Lei, T.-Q.; Liu, D.-C. Dispersible silica nanoparticles as carrier for enhanced bioactivity of chlorfenapyr. J. Pestic. Sci. 2012, 37, 258–260. [Google Scholar] [CrossRef]
- Shoaib, A.; Waqas, M.; Elabasy, A.; Cheng, X.; Zhang, Q.; Shi, Z. Preparation and characterization of emamectin benzoate nanoformulations based on colloidal delivery systems and use in controlling Plutella xylostella (L.)(Lepidoptera: Plutellidae). RSC Adv. 2018, 8, 15687–15697. [Google Scholar] [CrossRef]
- Jansson, R.; Brown, R.; Cartwright, B.; Cox, D.; Dunbar, D.; Dybas, R.; Eckel, C.; Lasota, J.; Mookerjee, P.; Norton, J. Emamectin benzoate: A novel avermectin derivative for control of lepidopterous pests. In Proceedings of the 3rd International Workshop on Management of Diamondback Moth and Other Crucifer Pests. MARDI: Kuala Lumpur, Kuala Lumpur, Malaysia, 29 October–1 November 1996; MARDI: Kuala Lumpur, Malaysia, 1997. [Google Scholar]
- Tomlin, C.D. The Pesticide Manual: A World Compendium; British Crop Production Council: Alton, UK, 2009. [Google Scholar]
- Zhang, S.F.; Chen, P.H.; Zhang, F.; Yang, Y.F.; Liu, D.K.; Wu, G. Preparation and physicochemical characteristics of polylactide microspheres of emamectin benzoate by modified solvent evaporation/extraction method. J. Agric. Food Chem. 2013, 61, 12219–12225. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf. B Biointerf. 2011, 85, 270–279. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. Highly pure silica nanoparticles with high adsorption capacity obtained from sugarcane waste ash. ACS Omega 2018, 3, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Berry, R.C.; Tam, K.C. Surface modification of cellulose nanocrystal with chitosan oligosaccharide for drug delivery applications. Cellulose 2013, 20, 1747–1764. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar]
- Vani, C.; Brindhaa, U. Silica nanoparticles as nanocides against Corcyra cephalonica (S.), the stored grain pest. Int. J. Pharm. Bio Sci. 2013, 4, B1108–B1118. [Google Scholar]
- Azlina, H.; Hasnidawani, J.; Norita, H.; Surip, S. Synthesis of SiO2 nanostructures using sol-gel method. Acta Phys. Pol. A 2016, 129, 842–844. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.; Shaniba, V.; Sreejith, M.; Balan, A.K.; Purushothaman, E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr. polym. 2018, 180, 13–20. [Google Scholar] [CrossRef]
- Dubey, R.; Rajesh, Y.; More, M. Synthesis and characterization of SiO2 nanoparticles via sol-gel method for industrial applications. Mater. Today: Proc. 2015, 2, 3575–3579. [Google Scholar] [CrossRef]
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Lv, L.; Li, J. Preparation and release of avermectin-loaded cellulose acetate ultrafine fibers. Polym. Eng. Sci. 2013, 53, 609–614. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for food applications: Development and characterization. Food and Bioproc. Tech. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Hwang, I.C.; Park, J.W.; Park, H.J. Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest Manag. Sci. 2012, 68, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interf. Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Torney, F.; Trewyn, B.G.; Lin, V.S.-Y.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotech. 2007, 2, 295. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M.J.N. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles–opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar]
- Meyer, W.; Gurman, P.; Stelinski, L.; Elman, N. Functional nano-dispensers (FNDs) for delivery of insecticides against phytopathogen vectors. Green Chem. 2015, 17, 4173–4177. [Google Scholar] [CrossRef]
- Saini, P.; Gopal, M.; Kumar, R.; Srivastava, C. Development of pyridalyl nanocapsule suspension for efficient management of tomato fruit and shoot borer (Helicoverpa armigera). J. Environ. Sci. Health Part B 2014, 49, 344–351. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Gao, Y.; Zhang, Y.; Qin, X.; Xiao, Y.; Zhang, Y.; You, H.; Li, J.; He, S. α-amylase triggered carriers based on cyclodextrin anchored hollow mesoporous silica for enhancing insecticidal activity of avermectin against Plutella xylostella. J. Hazard. Mater. 2018, 359, 213–221. [Google Scholar] [CrossRef]
- Shang, Q.; Shi, Y.; Zhang, Y.; Zheng, T.; Shi, H. Pesticide-conjugated polyacrylate nanoparticles: Novel opportunities for improving the photostability of emamectin benzoate. Polym. Adv. Technol. 2013, 24, 137–143. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Perlatti, B.; de Souza Bergo, P.L.; Fernandes, J.B.; Forim, M.R. Polymeric nanoparticle-based insecticides: A controlled release purpose for agrochemicals. In Insecticides-Development of Safer and More Effective Technologies; Trdan, S., Ed.; InTech: Rijeka, Croatia, 2013; Volume 20, pp. 523–550. [Google Scholar]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, W.; Ding, G.; Guo, D.; Zhu, J.; Wang, B.; Punyapitak, D.; Cao, Y. Preparation and characterization of enzyme-responsive emamectin benzoate microcapsules based on a copolymer matrix of silica–epichlorohydrin–carboxymethylcellulose. RSC Adv. 2015, 5, 93170–93179. [Google Scholar] [CrossRef]
- Afzal, M.B.S.; Shad, S.A.; Abbas, N.; Ayyaz, M.; Walker, W.B. Cross-resistance, the stability of acetamiprid resistance and its effect on the biological parameters of cotton mealybug, Phenacoccus solenopsis (Homoptera: Pseudococcidae), in Pakistan. Pest Manag. Sci. 2015, 71, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.L.; Smith, K.C.; Savin, N.; Lavigne, R.J. Effects of dose selection and sample size on the precision of lethal dose estimates in dose–mortality regression. J. Econ. Entomol. 1984, 77, 833–837. [Google Scholar] [CrossRef]
- SAS, SAS online Doc 9.2. SAS Institute Inc., Cary, NC. 2009. Available online: http://support.sas.com/documentation (accessed on 26 February 2019).
- Finney, D. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 333. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
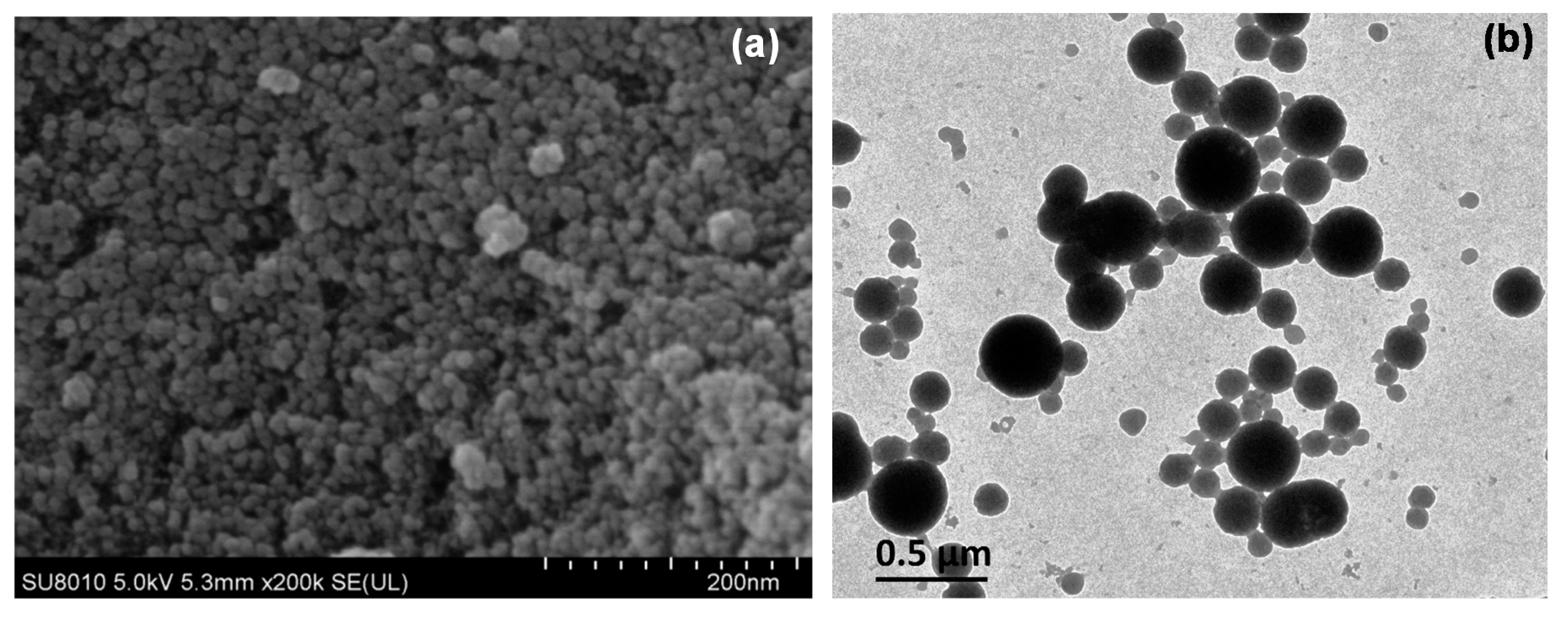
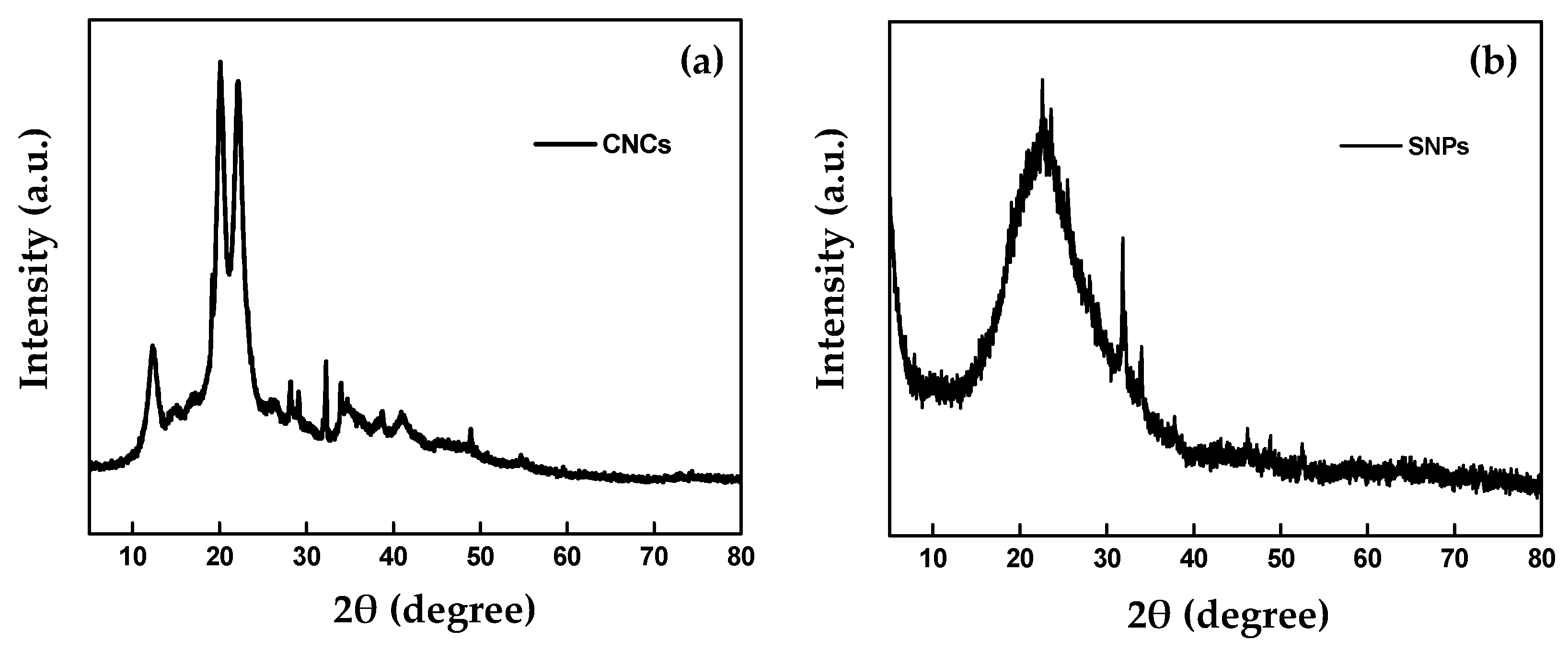
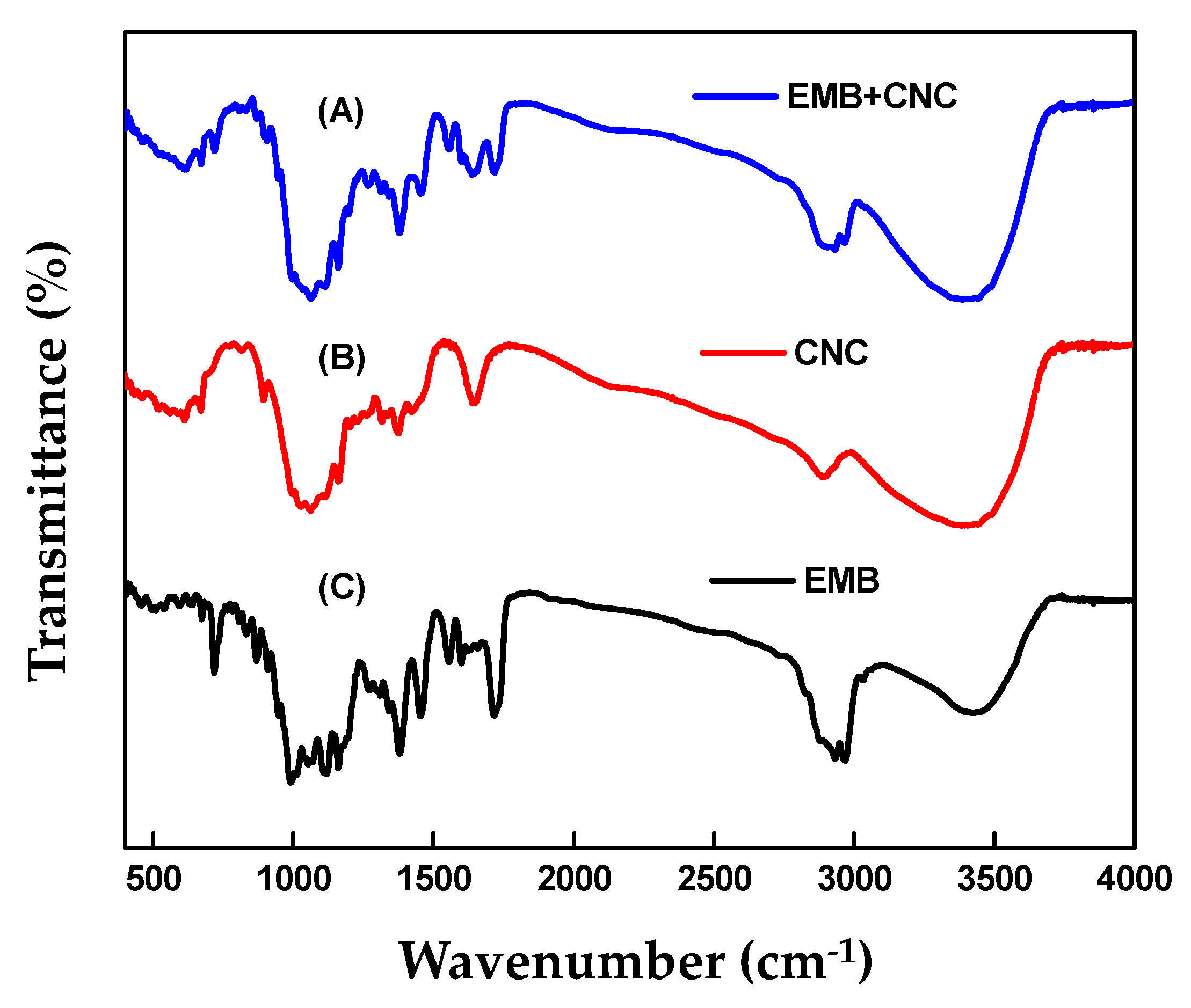
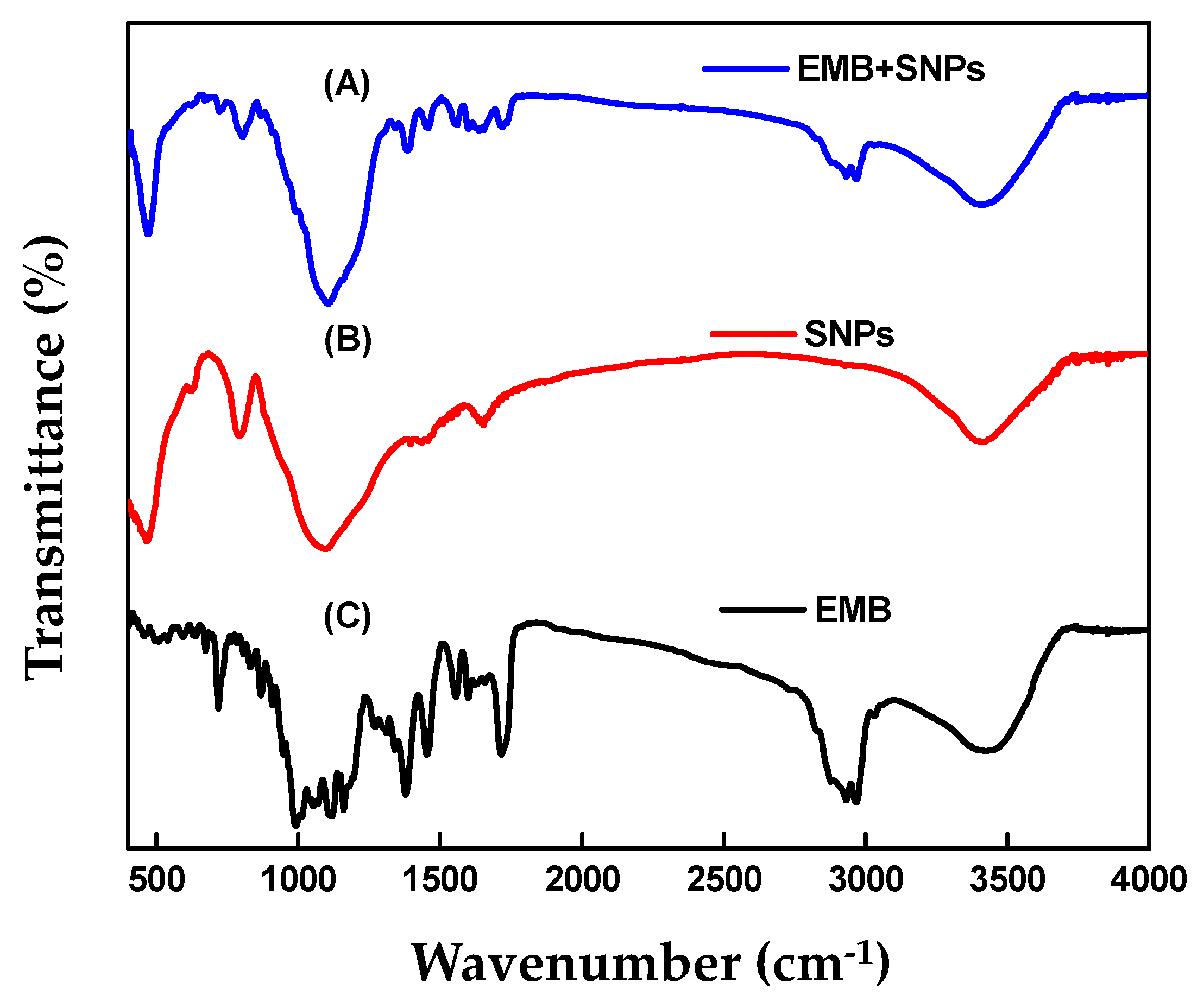


| Formulation | Zeta Potential (mV) | Particle Size | Entrapment Efficiency (%) | Absolute Recovery (%) | |
|---|---|---|---|---|---|
| Width (nm) | Length (nm) | ||||
| EMB + CNCs a | −26.4 ± 2.9 | 8.9 ± 1.7 | 78.4 ± 19.1 | 93.2 | 69.8 |
| EMB + SNPs b | −30.0 ± 0.7 | 82.5 ± 9.8 | - | 87.5 | 94.6 |
| Sample | SBET (m2 g−1) | BJH Pore Diameter (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| SNPs | 237.5 | 1.8 | 0.196 |
| EMB + SNPs | 69.4 | - | 0.157 |
| CNCs | 13.2 | 3.3 | 0.0008 |
| EMB + CNCs | 3.9 | - | 0.0001 |
| Formulation | Time (h) | LC50 (95% CL, µg/mL) | Slope ± SE | χ2 |
|---|---|---|---|---|
| EMB + SNPs | 24 | 0.05 (0.03–0.07) | 1.52 ± 0.37 | 1.27 |
| 48 | 0.01 (0.003–0.02) | 1.70 ± 0.48 | 0.81 | |
| EMB + CNCs | 24 | 0.13 (0.10–0.19) | 1.92 ± 0.38 | 1.99 |
| 48 | 0.07 (0.05–0.10) | 1.91 ± 0.39 | 2.59 | |
| 72 | 0.05 (0.03–0.07) | 2.09 ± 0.42 | 3.51 | |
| EMB 1% EC | 24 | 2.91 (1.57–7.95) | 0.81 ± 0.25 | 0.12 |
| 48 | 0.63 (0.11–1.19) | 0.86 ± 0.26 | 0.16 | |
| 72 | 0.31 (0.02–0.66) | 0.98 ± 0.29 | 0.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elabasy, A.; Shoaib, A.; Waqas, M.; Jiang, M.; Shi, Z. Synthesis, Characterization, and Pesticidal Activity of Emamectin Benzoate Nanoformulations against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Molecules 2019, 24, 2801. https://doi.org/10.3390/molecules24152801
Elabasy A, Shoaib A, Waqas M, Jiang M, Shi Z. Synthesis, Characterization, and Pesticidal Activity of Emamectin Benzoate Nanoformulations against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Molecules. 2019; 24(15):2801. https://doi.org/10.3390/molecules24152801
Chicago/Turabian StyleElabasy, Asem, Ali Shoaib, Muhammad Waqas, Mingxing Jiang, and Zuhua Shi. 2019. "Synthesis, Characterization, and Pesticidal Activity of Emamectin Benzoate Nanoformulations against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)" Molecules 24, no. 15: 2801. https://doi.org/10.3390/molecules24152801
APA StyleElabasy, A., Shoaib, A., Waqas, M., Jiang, M., & Shi, Z. (2019). Synthesis, Characterization, and Pesticidal Activity of Emamectin Benzoate Nanoformulations against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Molecules, 24(15), 2801. https://doi.org/10.3390/molecules24152801