Hypotensive Snake Venom Components—A Mini-Review
Abstract
1. Introduction
2. Overview of Hypotensive Mechanisms
3. Snake Venom Components with Hypotensive Effects
4. Snake Venom Components as Lead Molecules in Drug Discovery
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- World Health Organization. Cardiovascular diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 April 2019).
- Bloch, M.J. Worldwide prevalence of hypertension exceeds 1.3 billion. J. Am. Soc. Hypertens. 2016, 10, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J. Hypertens. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Puar, T.H.K.; Mok, Y.; Debajyoti, R.; Khoo, J.; How, C.H.; Ng, A.K.H. Secondary hypertension in adults. Singap. Med. J. 2016, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Elliott, W.J.; Peixoto, A.J.; Bakris, G.L. Primary and Secondary Hypertension. In Brenner and Rector’s The Kidney; Skorecki, K., Chertow, G.M., Marsden, P.A., Taal, M.W., Yu, A.S.L., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 1522–1566. [Google Scholar]
- World Health Organization. A Global Brief on Hypertension. Available online: https://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ (accessed on 11 April 2019).
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Brunström, M.; Carlberg, B. Association of Blood Pressure Lowering with Mortality and Cardiovascular Disease Across Blood Pressure Levels. JAMA Intern. Med. 2017, 178, 28–36. [Google Scholar] [CrossRef]
- Bryan, J. From snake venom to ACE inhibitor—The discovery and rise of captopril. Pharm. J. 2009, 282, 455. [Google Scholar]
- Boldrini-França, J.; Cologna, C.T.; Pucca, M.B.; de Bordon, K.C.F.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cerni, F.A.; Pinheiro-Junior, E.L.; et al. Minor snake venom proteins: Structure, function and potential applications. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 824–838. [Google Scholar] [CrossRef]
- Joseph, R.; Pahari, S.; Hodgson, W.; Kini, R. Hypotensive Agents from Snake Venoms. Curr. Drug Target Cardiovasc. Hematol. Disord. 2004, 4, 437–459. [Google Scholar] [CrossRef]
- Handbook of Venoms and Toxins of Reptiles, 1st ed.; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-8493-9165-1. [Google Scholar]
- Tibballs, J. The cardiovascular, coagulation and haematological effects of Tiger Snake (Notechis scutatus) venom. Anaesth. Intensive Care 1998, 26, 529–535. [Google Scholar] [CrossRef]
- Accary, C.; Hraoui-Bloquet, S.; Sadek, R.; Alameddine, A.; Fajloun, Z.; Desfontis, J.-C.; Mallem, Y. The relaxant effect of the Montivipera bornmuelleri snake venom on vascular contractility. J. Venom Res. 2016, 7, 10–15. [Google Scholar]
- Takacs, Z.; Nathan, S. Animal Venoms in Medicine. In Encyclopedia of Toxicology; Wexler, P., Ed.; Academic Press: Cambridge, UK, 2014; pp. 252–259. ISBN 9780123864543. [Google Scholar]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake Venom: From Deadly Toxins to Life-saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, M.; Mostafa, A.; Elbehiry, S.E. Intersexual Variation in Tail Length, Venom Composition Toxicity, and Anticancer Activity of Cerastes Cerastes (Viperidae). Egypt. J. Hosp. Med. 2017, 66, 81–90. [Google Scholar] [CrossRef]
- Amorim, F.G.; Costa, T.R.; Baiwir, D.; De Pauw, E.; Quinton, L.; Sampaio, S.V. Proteopeptidomic, functional and immunoreactivity characterization of Bothrops moojeni snake venom: Influence of snake gender on venom composition. Toxins 2018, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wagstaff, S.C.; Wuster, W.; Cook, D.A.N.; Bolton, F.M.S.; King, S.I.; Pla, D.; Sanz, L.; Calvete, J.J.; Harrison, R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef] [PubMed]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef]
- Mtewa, A.G.; Bekele, T.; Amanjot, A. From Toxins to Drugs: Chemistry and Pharmacology of Animal Venom and other Secretions. Online J. Compliment. Altern. Med. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical Renin-Angiotensin system in kidney physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [CrossRef]
- Fountain, J.H.; Lappin, S.L. Physiology, Renin Angiotensin System. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470410/ (accessed on 9 June 2019).
- Lameu, C.; Neiva, M.; FHayashi, M.A. Venom Bradykinin-Related Peptides (BRPs) and Its Multiple Biological Roles. In An Integrated View of the Molecular Recognition and Toxinology—From Analytical Procedures to Biomedical Applications; Radis-Baptista, G., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Taddei, S.; Bortolotto, L. Unraveling the Pivotal Role of Bradykinin in ACE Inhibitor Activity. Am. J. Cardiovasc. Drugs 2016, 16, 309–321. [Google Scholar] [CrossRef]
- Putney, J.W.; Tomita, T. Phospholipase C signaling and calcium influx. Adv. Biol. Regul. 2012, 52, 152–164. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor–Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef]
- Haines, R.J.; Pendleton, L.C.; Eichler, D.C. Argininosuccinate synthase: At the center of arginine metabolism. Int. J. Biochem. Mol. Biol. 2011, 2, 8–23. [Google Scholar]
- Yang, J. Mathematical modeling of the nitric oxide/cGMP pathway in the vascular smooth muscle cell. AJP Heart Circ. Physiol. 2005, 289, 886–897. [Google Scholar] [CrossRef]
- Chen, H.H.; Burnett, J.C. Clinical application of the natriuretic peptides in heart failure. Eur. Heart J. Suppl. 2006, 8, E18–E25. [Google Scholar] [CrossRef]
- Pluchart, H.; Khouri, C.; Blaise, S.; Roustit, M.; Cracowski, J.L. Targeting the Prostacyclin Pathway: Beyond Pulmonary Arterial Hypertension. Trends Pharmacol. Sci. 2017, 38, 512–523. [Google Scholar] [CrossRef]
- Manni, S.; Mauban, J.H.; Ward, C.W.; Bond, M. Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells. J. Biol. Chem. 2008, 283, 24145–24154. [Google Scholar] [CrossRef]
- Xavier, C.H.; Miranda, J.R.R.; Yamaguchi, J.; da Silveira, K.D.; Teixeira, M.M.; Chianca, D.A., Jr.; Simões e Silva, A.C.; Santos, R.A.S.; Camargo, A.C.M.; Ianzer, D. Bj-PRO-5a and Bj-PRO 10c Found at C-Type Natriuretic Peptide Precursor of Bothrops jararaca Change Renal Function of Hypertensive Rats. Int. J. Pept. Res. Ther. 2017, 23, 381–385. [Google Scholar] [CrossRef]
- Morais, K.L.P.; Hayashi, M.A.F.; Bruni, F.M.; Lopes-Ferreira, M.; Camargo, A.C.M.; Ulrich, H.; Lameu, C. Bj-PRO-5a, a natural angiotensin-converting enzyme inhibitor, promotes vasodilatation mediated by both bradykinin B2 and M1 muscarinic acetylcholine receptors. Biochem. Pharmacol. 2011, 81, 736–742. [Google Scholar] [CrossRef]
- Negraes, P.D.; Lameu, C.; Hayashi, M.A.F.; Melo, R.L.; Camargo, A.C.M.; Ulrich, H. The snake venom peptide Bj-PRO-7a is a M1 muscarinic acetylcholine receptor agonist. Cytom. Part A 2011, 79, 77–83. [Google Scholar] [CrossRef]
- Morais, K.L.P.; Ianzer, D.; Miranda, J.R.R.; Melo, R.L.; Guerreiro, J.R.; Santos, R.A.S.; Ulrich, H.; Lameu, C. Proline rich-oligopeptides: Diverse mechanisms for antihypertensive action. Peptides 2013, 48, 124–133. [Google Scholar] [CrossRef]
- Guerreiro, J.R.; Lameu, C.; Oliveira, E.F.; Klitzke, C.F.; Melo, R.L.; Linares, E.; Augusto, O.; Fox, J.W.; Lebrun, I.; Serrano, S.M.T.; et al. Argininosuccinate synthetase is a functional target for a snake venom anti-hypertensive peptide. Role in arginine and nitrix oxide production. J. Biol. Chem. 2009, 284, 20022–20033. [Google Scholar] [CrossRef]
- Lameu, C.; Pontieri, V.; Guerreiro, J.R.; Oliveira, E.F.; Da Silva, C.A.; Giglio, J.M.; Melo, R.L.; Campos, R.R.; De Camargo, A.C.M.; Ulrich, H. Brain nitric oxide production by a proline-rich decapeptide from Bothrops jararaca venom improves baroreflex sensitivity of spontaneously hypertensive rats. Hypertens. Res. 2010, 33, 1283–1288. [Google Scholar] [CrossRef]
- Paschoal, J.F.B.; Yamaguchi, J.; Miranda, J.R.R.; Carretero, G.; Melo, R.L.; Santos, R.A.S.; Xavier, C.H.; Schreier, S.; Camargo, A.C.M.; Ianzer, D. Insights into cardiovascular effects of proline-rich oligopeptide (Bj-PRO-10c) revealed by structure-activity analyses: Dissociation of antihypertensive and bradycardic effects. Amino Acids 2014, 2014, 401–413. [Google Scholar] [CrossRef]
- Kodama, R.T.; Cajado-Carvalho, D.; Kuniyoshi, A.K.; Kitano, E.S.; Tashima, A.K.; Barna, B.F.; Takakura, A.C.; Serrano, S.M.T.; Dias-Da-Silva, W.; Tambourgi, D.V.; et al. New proline-rich oligopeptides from the venom of African adders: Insights into the hypotensive effect of the venoms. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1180–1187. [Google Scholar] [CrossRef]
- Lopes, D.M.; Junior, N.E.G.; Costa, P.P.C.; Martins, P.L.; Santos, C.F.; Carvalho, E.D.F.; Carvalho, M.D.F.; Pimenta, D.C.; Cardi, B.A.; Fonteles, M.C.; et al. A new structurally atypical bradykinin-potentiating peptide isolated from Crotalus durissus cascavella venom (South American rattlesnake). Toxicon 2014, 90, 36–44. [Google Scholar] [CrossRef]
- Pinheiro-Júnior, E.L.; Boldrini-França, J.; de Campos Araújo, L.M.P.; Santos-Filho, N.A.; Bendhack, L.M.; Cilli, E.M.; Arantes, E.C. LmrBPP9: A synthetic bradykinin-potentiating peptide from Lachesis muta rhombeata venom that inhibits the angiotensin-converting enzyme activity in vitro and reduces the blood pressure of hypertensive rats. Peptides 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Munawar, A.; Zahid, A.; Negm, A.; Akrem, A.; Spencer, P.; Betzel, C. Isolation and characterization of Bradykinin potentiating peptides from Agkistrodon bilineatus venom. Proteome Sci. 2016, 14, 1–9. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Almeida, J.R.; Resende, L.M.; Martins, W.; Henriques, F.A.F.A.; Baldasso, P.A.; Soares, A.M.; Taranto, A.G.; Resende, R.R.; Marangoni, S.; et al. Isolation and Characterization of a Natriuretic Peptide from Crotalus oreganus abyssus (Grand Canyon Rattlesnake) and its Effects on Systemic Blood Pressure and Nitrite Levels. Int. J. Pept. Res. Ther. 2011, 17, 165–173. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Dias-Junior, C.A.; Baldasso, P.A.; Damico, D.C.S.; Carvalho, B.M.A.; Garanto, A.; Acosta, G.; Oliveira, E.; Albericio, F.; Soares, A.M.; et al. Vascular effects and electrolyte homeostasis of the natriuretic peptide isolated from Crotalus oreganus abyssus (North American Grand Canyon rattlesnake) venom. Peptides 2012, 36, 206–212. [Google Scholar] [CrossRef]
- Park, S.A.; Kim, T.G.; Han, M.K.; Ha, K.C.; Kim, S.Z.; Kwak, Y.G. Dendroaspis natriuretic peptide regulates the cardiac l-type ca2+ channel activity by the phosphorylation of α1c proteins. Exp. Mol. Med. 2012, 44, 363–368. [Google Scholar] [CrossRef]
- Evangelista, J.S.A.M.; Martins, A.M.C.; Nascimento, N.R.F.; Sousa, C.M.; Alves, R.S.; Toyama, D.O.; Toyama, M.H.; Evangelista, J.J.F.; Menezes DB, D.; Fonteles, M.C.; et al. Renal and vascular effects of the natriuretic peptide isolated from Crotalus durissus cascavella venom. Toxicon 2008, 52, 737–744. [Google Scholar] [CrossRef]
- Amininasab, M.; Elmi, M.M.; Endlich, N.; Endlich, K.; Parekh, N.; Naderi-Manesh, H.; Schaller, J.; Mostafavi, H.; Sattler, M.; Sarbolouki, M.N.; et al. Functional and structural characterization of a novel member of the natriuretic family of peptides from the venom of Pseudocerastes persicus. FEBS Lett. 2004, 557, 104–108. [Google Scholar] [CrossRef]
- St Pierre, L.; Flight, S.; Masci, P.P.; Hanchard, K.J.; Lewis, R.J.; Alewood, P.F.; de Jersey, J.; Lavin, M.F. Cloning and characterisation of natriuretic peptides from the venom glands of Australian elapids. Biochimie 2006, 88, 1923–1931. [Google Scholar] [CrossRef]
- Silveira, L.B.; Marchi-Salvador, D.P.; Santos-Filho, N.A.; Silva, F.P.; Marcussi, S.; Fuly, A.L.; Nomizo, A.; da Silva, S.L.; Stábeli, R.G.; Arantes, E.C.; et al. Isolation and expression of a hypotensive and anti-platelet acidic phospholipase A2 from Bothrops moojeni snake venom. J. Pharm. Biomed. Anal. 2013, 73, 35–43. [Google Scholar] [CrossRef]
- Andrião-Escarso, S.H.; Soares, A.M.; Fontes, M.R.M.; Fuly, A.L.; Corrêa, F.M.A.; Rosa, J.C.; Greene, L.J.; Giglio, J.R. Structural and functional characterization of an acidic platelet aggregation inhibitor and hypotensive phospholipase A2 from Bothrops jararacussu snake venom. Biochem. Pharmacol. 2002, 64, 723–732. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Ma, B.; Huang, K.; Song, M.; Zhang, N.; Zhang, Y.; Wang, Y.; Dai, Y.; Luo, Y. Isolation and characterisation of a kallikrein-like enzyme from Agkistrodon halys pallas snake venom. J. Sci. Food Agric. 2012, 92, 1497–1503. [Google Scholar] [CrossRef]
- He, J.; Chen, S.; Gu, J. Identification and characterization of Harobin, a novel fibrino(geno)lytic serine protease from a sea snake (Lapemis hardwickii). FEBS Lett. 2007, 581, 2965–2973. [Google Scholar] [CrossRef]
- Megale, Â.A.A.; Magnoli, F.C.; Kuniyoshi, A.K.; Iwai, L.K.; Tambourgi, D.V.; Portaro, F.C.V.; Da Silva, W.D. Kn-Ba: A novel serine protease isolated from Bitis arietans snake venom with fibrinogenolytic and kinin-releasing activities. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 38. [Google Scholar] [CrossRef]
- Felicori, L.F.; Souza, C.T.; Velarde, D.T.; Magalhaes, A.; Almeida, A.P.; Figueiredo, S.; Richardson, M.; Diniz, C.R.; Sanchez, E.F. Kallikrein-like proteinase from bushmaster snake venom. Protein Expr. Purif. 2003, 30, 32–42. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Harrison, R.A.; Bicknell, A.B.; Gibbins, J.M.; Hutchinson, G. Purification and functional characterisation of rhinocerase, a novel serine protease from the venom of Bitis gabonica rhinoceros. PLoS ONE 2010, 5, e9687. [Google Scholar] [CrossRef]
- Hung, C.C.; Chiou, S.H. Fibrinogenolytic proteases isolated from the snake venom of Taiwan Habu: Serine proteases with kallikrein-like and angiotensin-degrading activities. Biochem. Biophys. Res. Commun. 2001, 281, 1012–1018. [Google Scholar] [CrossRef]
- Takahashi, H.; Hattori, S.; Iwamatsu, A.; Takizawa, H.; Shibuya, M. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J. Biol. Chem. 2004, 279, 46304–46314. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Yamazaki, Y.; Morita, T. Specific distribution of VEGF-F in Viperinae snake venoms: Isolation and characterization of a VEGF-F from the venom of Daboia russelli siamensis. Arch. Biochem. Biophys. 2005, 439, 241–247. [Google Scholar] [CrossRef]
- Yasuda, O.; Morimoto, S.; Jiang, B.; Kuroda, H.; Kimura, T.; Sakakibara, S.; Fukuo, K.; Chen, S.; Tamatani, M.; Ogihara, T. FS2, a mamba venom toxin, is a specific blocker of the L-type calcium channels. Artery 1994, 21, 287–302. [Google Scholar]
- Watanabe, T.X.; Itahara, Y.; Kuroda, H.; Chen, Y.-N.; Kimura, T.; Sakakibara, S. Smooth Muscle Relaxing and Hypotensive Activities of Synthetic Calciseptine and the Homologous Snake Venom Peptide FS2. Jpn. J. Pharmacol. 2008, 68, 305–313. [Google Scholar] [CrossRef]
- Komori, Y.; Nikai, T.; Taniguchi, K.; Masuda, K.; Sugihara, H. Vascular endothelial growth factor VEGF-like heparin-binding protein from the venom of Vipera aspis aspis (Aspic viper). Biochemistry 1999, 38, 11796–11803. [Google Scholar] [CrossRef]
- Caccin, P.; Pellegatti, P.; Fernandez, J.; Vono, M.; Cintra-Francischinelli, M.; Lomonte, B.; Gutiérrez, J.M.; Di Virgilio, F.; Montecucco, C. Why myotoxin-containing snake venoms possess powerful nucleotidases? Biochem. Biophys. Res. Commun. 2013, 430, 1289–1293. [Google Scholar] [CrossRef]
- Ferreira, S.H. A Bradykinin Potentiating Factor (BPF) Present in the Venom of Bothrops Jararaca. Br. J. Pharmacol. Chemother. 1965, 24, 163–169. [Google Scholar] [CrossRef]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef]
- Golias, C.; Charalabopoulos, A.; Stagikas, D.; Charalabopoulos, K.A.; Batistatou, A. The kinin system-bradykinin: Biological effects and clinical implications. Multiple role of the kinin system-bradykinin. Hippokratia 2007, 11, 124–128. [Google Scholar]
- Soares De Moura, R.; Resende, A.C.; Emiliano, A.F.; Tano, T.; Mendes-Ribeiro, A.C.; Correia, M.L.G.; Marins De Carvalho, L.C.R. The role of bradykinin, AT 2 and angiotensin 1–7 receptors in the EDRF-dependent vasodilator effect of angiotensin II on the isolated mesenteric vascular bed of the rat. Br. J. Pharmacol. 2004, 141, 860–866. [Google Scholar] [CrossRef][Green Version]
- Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake venom peptides: Tools of biodiscovery. Toxins 2018, 10, 474. [Google Scholar] [CrossRef]
- Lameu, C.; Ulrich, H. Applications of Snake Venom Proline-Rich Oligopeptides (Bj-PROs) in Disease Conditions Resulting from Deficient Nitric Oxide Production. In Drug Discovery; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Suzuki, T.; Yamazaki, T.; Yazaki, Y. The role of the natriuretic peptides in the cardiovascular system. Cardiovasc. Res. 2001, 51, 489–494. [Google Scholar] [CrossRef]
- Wong, P.C.Y.; Guo, J.; Zhang, A. The renal and cardiovascular effects of natriuretic peptides. Adv. Physiol. Educ. 2017, 41, 179–185. [Google Scholar] [CrossRef]
- Koh, C.Y.; Kini, R.M. From snake venom toxins to therapeutics—Cardiovascular examples. Toxicon 2012, 59, 497–506. [Google Scholar] [CrossRef]
- McCarthy, R.T.; Isales, C.M.; Bollag, W.B.; Rasmussen, H.; Barrett, P.Q. Atrial natriuretic peptide differentially modulates T- and L-type calcium channels. Am. J. Physiol. Physiol. 2017, 258, F473–F478. [Google Scholar] [CrossRef]
- Sun, J.B.; Huang, X.; Xu, H.Y.; Li, X.L.; Gao, L.; Kim, Y.C.; Xu, W.X. Inhibitory effect of C-type natriuretic peptide on L-type calcium channel currents in gastric antral myocytes of guinea pigs. Gen. Physiol. Biophys. 2006, 25, 365–377. [Google Scholar]
- Sodi, R.; Dubuis, E.; Shenkin, A.; Hart, G. B-type natriuretic peptide (BNP) attenuates the L-type calcium current and regulates ventricular myocyte function. Regul. Pept. 2008, 151, 95–105. [Google Scholar] [CrossRef]
- Nishikimi, T.; Maeda, N.; Matsuoka, H. The role of natriuretic peptides in cardioprotection. Cardiovasc. Res. 2006, 69, 318–328. [Google Scholar] [CrossRef]
- Lumsden, N.G.; Khambata, R.S.; Hobbs, A.J. C-type Natriuretic Peptide (CNP): Cardiovascular Roles and Potential as a Therapeutic Target. Curr. Pharm. Des. 2011, 16, 4080–4088. [Google Scholar] [CrossRef]
- Pandit, K.; Ghosh, S.; Mukhopadhyay, P.; Chowdhury, S. Natriuretic peptides: Diagnostic and therapeutic use. Indian J. Endocrinol. Metab. 2011, 15, S345–S353. [Google Scholar] [CrossRef]
- Schweitz, H.; Vigne, P.; Moinier, D.; Frelin, C.; Lazdunski, M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J. Biol. Chem. 1992, 267, 13928–13932. [Google Scholar] [CrossRef]
- Lisy, O.; Jougasaki, M.; Heublein, D.M.; Schirger, J.A.; Chen, H.H.; Wennberg, P.W.; Burnett, J.C. Renal actions of synthetic Dendroaspis natriuretic peptide. Kidney Int. 1999, 56, 502–508. [Google Scholar] [CrossRef]
- Lisy, O.; Huntley, B.K.; McCormick, D.J.; Kurlansky, P.A.; Burnett, J.C. Design, Synthesis, and Actions of a Novel Chimeric Natriuretic Peptide: CD-NP. J. Am. Coll. Cardiol. 2008, 52, 60–68. [Google Scholar] [CrossRef]
- Vink, S.; Jin, A.H.; Poth, K.J.; Head, G.A.; Alewood, P.F. Natriuretic peptide drug leads from snake venom. Toxicon 2012, 59, 434–445. [Google Scholar] [CrossRef]
- Meems, L.M.G.; Burnett, J.C. Innovative Therapeutics: Designer Natriuretic Peptides. JACC Basic Transl. Sci. 2016, 1, 557–567. [Google Scholar] [CrossRef]
- Kawakami, R.; Lee, C.Y.W.; Scott, C.; Bailey, K.R.; Schirger, J.A.; Chen, H.H.; Benike, S.L.; Cannone, V.; Martin, F.L.; Sangaralingham, S.J.; et al. A Human Study to Evaluate Safety, Tolerability, and Cyclic GMP Activating Properties of Cenderitide in Subjects with Stable Chronic Heart Failure. Clin. Pharmacol. Ther. 2018, 104, 546–552. [Google Scholar] [CrossRef]
- Ichiki, T.; Dzhoyashvili, N.; Burnett, J.C. Natriuretic peptide based therapeutics for heart failure: Cenderitide: A novel first-in-class designer natriuretic peptide. Int. J. Cardiol. 2019, 281, 166–171. [Google Scholar] [CrossRef]
- Siang, A.S.; Doley, R.; Vonk, F.J.; Kini, R.M. Transcriptomic analysis of the venom gland of the red-headed krait (Bungarus flaviceps) using expressed sequence tags. BMC Mol. Biol. 2010, 11, 24. [Google Scholar] [CrossRef]
- Sridharan, S.; Kini, R.M. Snake venom natriuretic peptides: Potential molecular probes. BMC Pharmacol. Toxicol. 2015, 16, A87. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Lee, W.; Xu, X.; Zhang, Y.; Zhao, R.; Zhang, Y.; Wang, W. Venom gland transcriptomes of two elapid snakes (Bungarus multicinctus and Naja atra) and evolution of toxin genes. BMC Genom. 2011, 12, 1. [Google Scholar] [CrossRef]
- Soares, M.R.; Oliveira-Carvalho, A.L.; Wermelinger, L.S.; Zingali, R.B.; Ho, P.L.; Junqueira-De-Azevedo, I.D.L.M.; Diniz, M.R.V. Identification of novel bradykinin-potentiating peptides and C-type natriuretic peptide from Lachesis muta venom. Toxicon 2005, 46, 31–38. [Google Scholar] [CrossRef]
- Fry, B.G.; Wickramaratana, J.C.; Lemme, S.; Beuve, A.; Garbers, D.; Hodgson, W.C.; Alewood, P. Novel natriuretic peptides from the venom of the inland taipan (Oxyuranus microlepidotus): Isolation, chemical and biological characterisation. Biochem. Biophys. Res. Commun. 2005, 327, 1011–1015. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A 2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake Venom PLA 2, a Promising Target for Broad-Spectrum Antivenom Drug Development. BioMed Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef]
- Serrano, S.M.T.; Maroun, R.C. Snake venom serine proteinases: Sequence homology vs. substrate specificity, a paradox to be solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef]
- Serrano, S.M.T. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef]
- Xiong, S.; Huang, C. Synergistic strategies of predominant toxins in snake venoms. Toxicol. Lett. 2018, 287, 142–154. [Google Scholar] [CrossRef]
- Tsai, I.-H. Snake Venom Phospholipase A2: Evolution and Diversity. In Venom Genomics and Proteomics; Gopalakrishnakone, P., Calvete, J., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 291–306. [Google Scholar]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 437, 169–183. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Yang, R.; Ogasawara, A.K.; Zioncheck, T.F.; Ren, Z.; He, G.W.; DeGuzman, G.G.; Pelletier, N.; Shen, B.Q.; Bunting, S.; Jin, H. Exaggerated hypotensive effect of vascular endothelial growth factor in spontaneously hypertensive rats. Hypertension 2002, 39, 815–820. [Google Scholar] [CrossRef][Green Version]
- Liu, M.H.; Jin, H.K.; Floten, H.S.; Yang, Q.; Yim, A.P.; Furnary, A.; Zioncheck, T.F.; Bunting, S.; He, G.W. Vascular endothelial growth factor-mediated endothelium-dependent relaxation is blunted in spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 2001, 296, 473–477. [Google Scholar]
- Quan, R.; Du, W.; Zheng, X.; Xu, S.; Li, Q.; Ji, X.; Wu, X.; Shao, R.; Yang, D. VEGF165 induces differentiation of hair follicle stem cells into endothelial cells and plays a role in in vivo angiogenesis. J. Cell. Mol. Med. 2017, 21, 1593–1604. [Google Scholar] [CrossRef]
- Kaji, T.; Yamamoto, C.; Oh-i, M.; Fujiwara, Y.; Yamazaki, Y.; Morita, T.; Plaas, A.H.; Wight, T.N. The vascular endothelial growth factor VEGF165 induces perlecan synthesis via VEGF receptor-2 in cultured human brain microvascular endothelial cells. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1465–1474. [Google Scholar] [CrossRef]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1094–1096. [Google Scholar] [CrossRef]
- Paredes-Gamero, E.J.; Medeiros, V.P.; Farias, E.H.C.; Justo, G.Z.; Trindade, E.S.; Andrade-Lopes, A.L.; Godinho, R.O.; De Miranda, A.; Ferreira, A.T.; Tersariol, I.L.S.; et al. Heparin induces rat aorta relaxation via integrin-dependent activation of muscarinic M3 receptors. Hypertension 2010, 56, 713–721. [Google Scholar] [CrossRef]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol. Chem. 2018, 10, 17–27. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Striessnig, J.; Ortner, N.; Pinggera, A. Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets? Curr. Mol. Pharmacol. 2015, 8, 110–122. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef]
- Feng, T.; Kalyaanamoorthy, S.; Barakat, K. L-Type Calcium Channels: Structure and Functions. In Ion Channels in Health and Sickness; Kaneez, F.S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Imanishi, T.; Matsushima, K.; Kawaguchi, A.; Wada, T.; Yoshida, S.; Ichida, S. Increased response to high KCl-induced elevation in the intracellular-Ca2+ concentration in differentiated NG108-15 cell and the inhibitory effect of the L-Type Ca2+ channel blocker, calciseptine. Neurochem. Res. 2006, 31, 33–40. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; Nataraju, A.; Raghavendra Gowda, C.D.; Sharath, B.K.; D’souza, C.J.M. Vanillic acid as a novel specific inhibitor of snake venom 5′-nucleotidase: A pharmacological tool in evaluating the role of the enzyme in snake envenomation. Biochemistry 2009, 74, 1315–1319. [Google Scholar] [CrossRef]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Robinson, S.D.; Undheim, E.A.B.; Ueberheide, B.; King, G.F. Venom peptides as therapeutics: Advances, challenges and the future of venom-peptide discovery. Expert Rev. Proteom. 2017, 14, 931–939. [Google Scholar] [CrossRef]
- Zelanis, A.; Huesgen, P.F.; Oliveira, A.K.; Tashima, A.K.; Serrano, S.M.T.; Overall, C.M. Snake venom serine proteinases specificity mapping by proteomic identification of cleavage sites. J. Proteom. 2015, 113, 260–267. [Google Scholar] [CrossRef]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of snake venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Camargo, A.C.M. The Bradykinin-potentiating peptides from venom gland and brain of Bothrops jararaca contain highly site specific inhibitors of the somatic angiotensin-converting enzyme. Toxicon 2005, 45, 1163–1170. [Google Scholar] [CrossRef]
- Cushman, D.W.; Ondetti, M.A. Design of angiotensin converting enzyme inhibitors. Nat. Med. 1999, 5, 1110–1112. [Google Scholar] [CrossRef]
- Cushman, D.W.; Ondetti, M.A. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 1991, 17, 589–592. [Google Scholar] [CrossRef]
- Lee, C.Y.W.; Chen, H.H.; Lisy, O.; Swan, S.; Cannon, C.; Lieu, H.D.; Burnett, J.C. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J. Clin. Pharmacol. 2009, 49, 668–673. [Google Scholar] [CrossRef]
- Neutel, J.; Rolston, W.; Maddock, S.; Goldsmith, S.; Koren, M.; Bill, V.A.; Burnett, J.; Lieu, H.D. Initial Experience With Subcutaneous Infusion of Cenderitide in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2012, 59, E1037. [Google Scholar] [CrossRef]
- Avila, A.; Vidal, P.M.; Dear, T.N.; Harvey, R.J.; Rigo, J.M.; Nguyen, L. Glycine receptor α2 subunit activation promotes cortical interneuron migration. Cell Rep. 2013, 4, 738–750. [Google Scholar] [CrossRef]
- Goyal, A.; Bhattacharyya, S.; Majumdar, S.; Narang, A.; Ghosh, S. Cellular response induced by a galactose-specific adhesin of enteroaggregative Escherichia coli in INT-407 cells. FEMS Immunol. Med. Microbiol. 2009, 55, 378–387. [Google Scholar] [CrossRef]
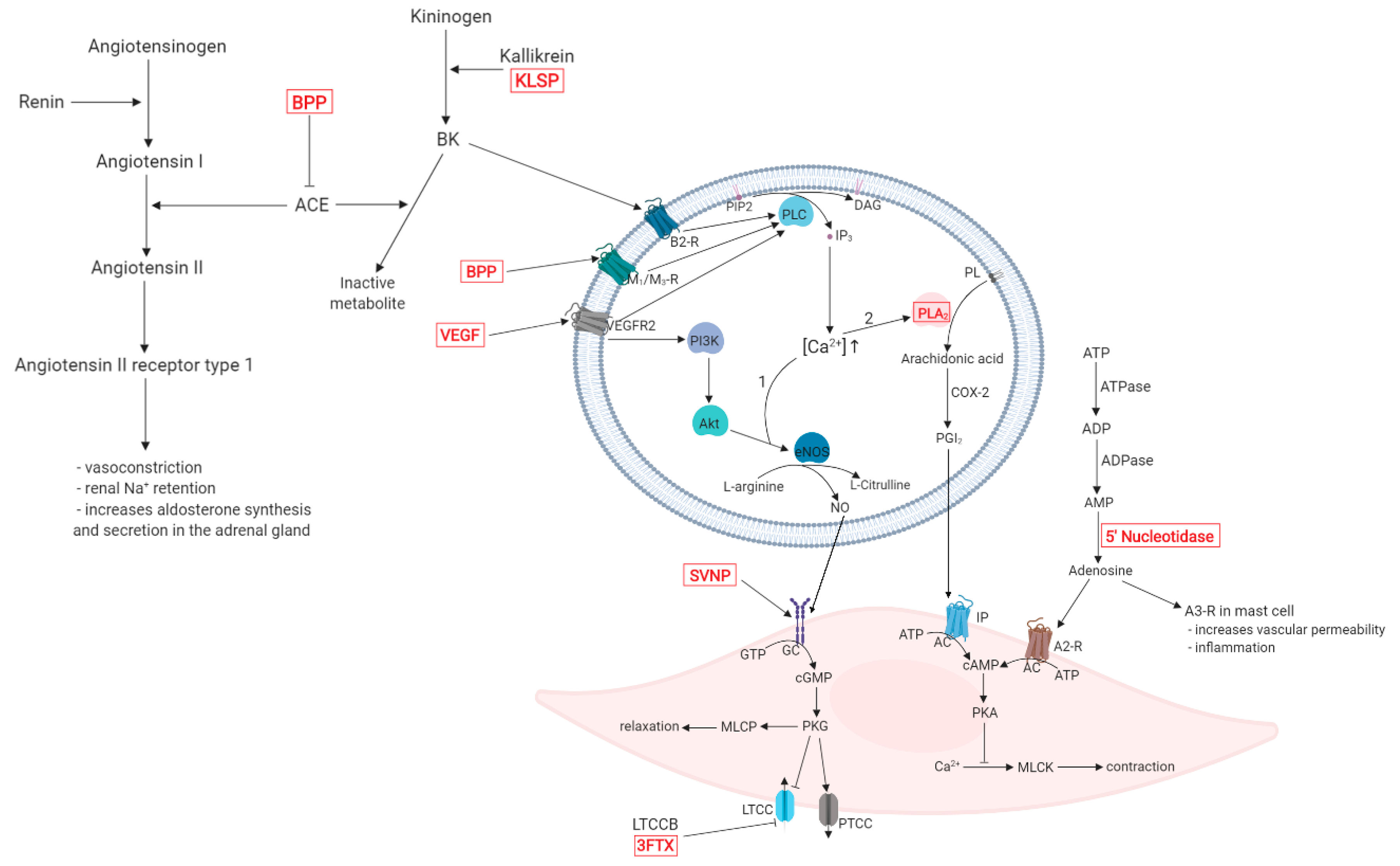
| Protein/Peptide | Source | Mechanism of Hypotensive Effect | Ref. |
|---|---|---|---|
| Bradykinin Potentiating Peptides (BPP)/Proline-Rich Oligopeptides (PRO) * | |||
| Bj-PRO-5a | Bothrops jararaca | Increases urinary flow rate and sodium excretion. Vasodilation is achieved through the inhibition of the angiotensin-converting enzyme (ACE), along with the activation of bradykinin B2 and muscarinic M1 receptors. Lowers cardiac output by inducing bradycardia. | [34,35] |
| Bj-PRO-7a | Bothrops jararaca | Acts as an M1 muscarinic receptor agonist, thus mobilizes intracellular Ca2+ in various cell types and induces vasodilation through vascular endothelial cells. | [36] |
| Bj-PRO-9a | Bothrops jararaca | Inhibits ACE and increases the effect of endogenous bradykinin (BK). | [37] |
| Bj-PRO-10c (Bj-BPP-10c) | Bothrops jararaca | Activates argininosuccinate synthetase (AsS) at kidney level, increasing l-arginine and consecutive nitric oxide (NO) production. Increases production of NO through gene expression of AsS and nitric oxide synthase (NOS) in the endothelium. Improves baroreflex sensitivity in the central nervous system and increases the release of gamma-aminobutyric acid (GABA) and glutamate mediators, involved in the regulation of the autonomic nervous system. Lowers cardiac output by inducing bradycardia. Increases urinary flow rate and sodium excretion. Inhibits the angiotensin-converting enzyme (ACE). | [34,38,39,40] |
| Bj-PRO-11e Bj-PRO-12b | Bothrops jararaca | Lowers cardiac output by inducing bradycardia. Induces Ca2+ mobilization in different tissues, possibly interacting with regulators of the cardiovascular system, such as the Ca2+/calmodulin-dependent kinase II (CaMK-II). Increases the effect of endogenous BK. | [37] |
| Bj-PRO-13a | Bothrops jararaca | Increases AsS activity, NO production and Ca2+ mobilization. Agonist on M3 muscarinic receptors (mAChR), possibly inducing smooth muscle relaxation and negative cardiac chronotropy. | [37] |
| Bn-PRO-10a, Bn-PRO-10a-MK, Bn-PRO-10b-MK, Br-PRO-10a, Bg-PRO-11a, Bn-PRO-10c | Bitis spp. | Inhibits the enzymatic activity of ACE with some minor differences regarding modulation of angiotensin I conversion and BK degradation. | [41] |
| BPP-Cdc | Crotalus durissus cascavella | Inhibits BK degradation and conversion of AT I into AT II by inhibiting the enzymatic activity of ACE | [42] |
| LmrBPP9 | Lachesis muta rhombeata | Inhibits the enzymatic activity of ACE. | [43] |
| PRO synthetic analogues | Agkistrodon bilineatus | Inhibits the enzymatic activity of ACE. | [44] |
| Natriuretic Peptides (NP) | |||
| Coa_NP | Crotalus oreganus abyssus | Induces endothelium dependent vasodilatation through NO formation. | [45,46] |
| DNP | Dendroaspis angusticeps | Phosphorylates Ca2+ channel proteins via protein kinase G (PKG) activation, inhibiting L-type Ca2+ channel activity in the hearth, modulating contractility. | [47] |
| NP2_Casca | Crotalus durissus cascavella | Increases NO production and consequent vasodilation, also causes increase in urinary flow, glomerular filtration rate and sodium excretion, thus resulting a strong diuretic effect. | [48] |
| PNP | Pseudocerastes persicus | Increases urine flow and sodium excretion thus decreasing blood pressure. It exerts ANP-like activity as it induces cGMP activity and binds to natriuretic peptide receptor (NPR)-A. | [49] |
| PtNP-a | Pseudonaja textilis | Increases intracellular cGMP levels similarly to ANP and BNP while inhibiting ACE activity. | [50] |
| Phospholipases A2 (PLA2) | |||
| BmooPLA2-I | Bothrops moojeni | Decreases blood pressure through an unreported mechanism. | [51] |
| BthA-I-PLA2 | Bothrops jararacussu | Decreases blood pressure due to its phospholipase activity. | [52] |
| OSC3a, OSC3b | Oxyuranus scutellatus | Hypotensive effect induced through cyclooxygenase metabolites (dilator prostaglandins or prostacyclin). Possible involvement in the release of endogenous mediators, such as histamine and bradykinin. Both direct (OSC3a) and endothelium-dependent (OSC3a, OSC3b) vasodilator effect. | [53] |
| Snake Venom Serine-Proteases (SVSP) | |||
| AHP-Ka | Agkistrodon halys pallas | Possible hypotensive effect, due to its kallikrein-like activity. | [54] |
| Harobin | Lapemis hardwickii | Degrades angiotensin I to angiotensin II and angiotensin II to tetrapeptides lacking hypertensive activity. Releases BK with vasodilator effect, due to its kallikrein-like activity. Decreases blood fibrinogen levels, altering the blood rheology. | [55] |
| Kn-Ba | Bitis arietans | Releases BK and Met-Lys-bradykinin from kininogen, the latter has equivalent biological activity with BK at B1 and B2 receptors causing vasodilation. | [56] |
| LV-Ka | Lachesis muta | Decreases blood pressure through its kallikrein-like activity. | [57] |
| Rhinocerase | Bitis gabonica rhinoceros | Possible hypotensive effect, due to its kallikrein-like activity. | [58] |
| Tm-VIG and Tm-IIG | Trimeresurus mucrosquamatus | Degrades angiotensin I and releases bradykinin from plasma kininogen with potent vasodilator effect. | [59] |
| Vascular endothelial Growth Factor Like (VEGF-like) Peptides | |||
| TfsvVEGF | Trimeresurus flavoviridis | Possible hypotensive effect, due to VEGF-like mechanism of action. | [60] |
| VEGF-F (VR-1’) | Daboia russelli siamensis | Possible hypotensive effect, due to VEGF-like mechanism of action. | [61] |
| Other Hypotensive Snake Venom Components | |||
| Calciseptine FS-2 toxin | Dendroaspis polylepis | Act as l-Type Ca2+ channel blockers. | [62,63] |
| Heparin-binding dimeric hypotensive factor (HF) | Vipera aspis | Exhibits potent hypotensive effect, due to VEGF-like mechanism of action (vasodilation and hyperpermeability). | [64] |
| Nucleotidases | Bothrops asper | Degrades adenosine triphosphate (ATP) to adenosine, which exerts hypotensive activity through vasodilation. | [65] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péterfi, O.; Boda, F.; Szabó, Z.; Ferencz, E.; Bába, L. Hypotensive Snake Venom Components—A Mini-Review. Molecules 2019, 24, 2778. https://doi.org/10.3390/molecules24152778
Péterfi O, Boda F, Szabó Z, Ferencz E, Bába L. Hypotensive Snake Venom Components—A Mini-Review. Molecules. 2019; 24(15):2778. https://doi.org/10.3390/molecules24152778
Chicago/Turabian StylePéterfi, Orsolya, Francisc Boda, Zoltán Szabó, Elek Ferencz, and László Bába. 2019. "Hypotensive Snake Venom Components—A Mini-Review" Molecules 24, no. 15: 2778. https://doi.org/10.3390/molecules24152778
APA StylePéterfi, O., Boda, F., Szabó, Z., Ferencz, E., & Bába, L. (2019). Hypotensive Snake Venom Components—A Mini-Review. Molecules, 24(15), 2778. https://doi.org/10.3390/molecules24152778






